Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 14
Pediatric Diabetes and Diabetic Ketoacidosis After COVID-19: Challenges Faced and Lessons Learnt
Authors Agarwal A, Bansal D, Nallasamy K, Jayashree M , William V
Received 22 March 2023
Accepted for publication 24 August 2023
Published 4 September 2023 Volume 2023:14 Pages 281—288
DOI https://doi.org/10.2147/PHMT.S384104
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Ashish Agarwal,1 Deepankar Bansal,1 Karthi Nallasamy,1 Muralidharan Jayashree,1 Vijai William2
1Division of Pediatric Emergency and Intensive Care, Department of Pediatrics, Advanced Pediatrics Centre, Post Graduate Institute of Medical Education & Research, Chandigarh, India; 2Division of Pediatric Critical Care, Department of Critical Care, Sheikh Khalifa Medical City, Abu Dhabi, United Arab Emirates
Correspondence: Vijai William, Division of Pediatric Critical Care, Department of Critical Care, Sheikh Khalifa Medical City, Abu Dhabi, United Arab Emirates, Tel +971502988149, Email [email protected]
Abstract: The coronavirus disease (COVID-19) pandemic affected the management and follow-up of several chronic ailments, including pediatric type 1 diabetes mellitus (T1DM). Restricted access to healthcare and fear of contracting the virus during medical facility visits resulted in poor compliance, irregular follow-up visits, treatment, and delayed diagnosis of complications in pediatric diabetes such as diabetic ketoacidosis (DKA). As such, the incidence of complicated DKA in resource-limited settings is high due to delayed presentation, poor compliance with therapy, and associated comorbidities such as malnutrition and sepsis. The pandemic had only added to the woes. The increased surge in DKA, in the face of limited resources, prompted clinicians to find alternative solutions to manage these children effectively. In this narrative review, we discuss the key challenges faced globally while caring for children with T1DM and DKA during the COVID-19 pandemic, and the lessons learned thereof.
Keywords: SARS-CoV-2, COVID-19, diabetes, ketoacidosis, pediatrics
Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic had a significant impact on all facets of the health-care sector, causing tremendous strain on both people and resources. Modern medicine experienced several trials and tribulations during this time. Besides the direct effect on morbidity and mortality, the pandemic caused limitations to effective healthcare delivery due to restricted movement and lack of transportation. In addition, hesitation of parents to visit a medical facility owing to fear of contracting the virus resulted in delayed diagnosis and irregular treatment of many chronic ailments, especially malignancies.1,2 On the other hand, isolation and use of personal protective measures had some positive impacts, which led to decreased hospitalizations due to other respiratory viruses. Efforts were made to strengthen health-care systems by increasing the number of intensive care beds and improving telehealth services. While the health-care systems had witnessed transformations like never before, it was important to strike a balance between the acute care needs of coronavirus disease (COVID-19) patients, judicious use of hospital resources, and at the same time protection of both patients and health-care workers from exposure to the contagious virus. In this exercise, the quality of treatment provided to children with chronic ailments took a back seat.
Among several chronic ailments, the pandemic had both directly and indirectly impacted the epidemiology, clinical manifestation, and therapy of Type 1 Diabetes Mellitus (T1DM) and Diabetic Ketoacidosis (DKA) in children. Although the morbidity and mortality of T1DM and DKA in children have decreased significantly over the years,3 the economic burden of this disease remains considerable. The pandemic adversely affected the ready availability of resources, posing challenges to both the diagnosis and management of these children. Not only was the timely follow-up disrupted but the supply of insulin and self-monitoring tools was halted due to lockdown and restrictions. Rapid lifestyle changes resulted in substantially increased screen time, reduced physical activity, loss of a structured routine and psychological stress due to lack of social interactions.
Challenges Faced in the Management of Pediatric Diabetes
1. During the pandemic, the moot point was whether SARS-CoV-2 increased the prevalence of new T1DM, and if so, what could be the possible mechanism?
After initial reports suggested an increase in T1DM incidence attributable to SARS-CoV-2, considerable research was conducted to compare the incidence of new TIDM between the pre-pandemic and pandemic phases. Initial reports by Tittel et al from Germany4 and Ho et al from Canada5 showed that the incidence of new T1DM during the first wave was not different from the pre-pandemic phase. However, after the second and third waves, the same group reported an increased incidence of new T1DM associated with SARS-CoV-2 infection.6 Subsequently, several reports from the United States, Germany, Italy, and Finland, including recent systematic reviews and meta-analyses, substantiated that the COVID-19 pandemic led to an increased incidence of new T1DM.7,8
A large study that included more than a million children with respiratory viral infections compared the incidence of new T1DM between SARS-CoV-2 and non-SARS-CoV-2 respiratory viral infections. They found that incidence of new T1DM was significantly higher among the SARS-CoV-2 group.9 Another very large study by Qeadan et al demonstrated that, in children infected with SARS-CoV-2, the incidence of new T1DM was high.10 A well-established hypothesis for the development of T1DM proposes an interplay between genetic susceptibility to human leukocyte antigens (HLA) and secondary hits in the form of viral infections, diet, and environmental and/or autoimmune factors. Coxsackie virus-B (CBV), mumps, rubella, cytomegalovirus, and rotaviruses are among the viruses linked to the development of T1DM. Viruses either directly harm beta cells through proliferation and destruction or cause autoimmunity and inflammation through “molecular mimicry.”11 Molecular research has demonstrated that Angiotensin converting enzyme-2 (ACE-2) receptors expressed on the surface of pancreatic beta cells are utilized by the SARS-CoV-2 virus to infect and cause destruction of the endocrine pancreatic cells.12,13 However, the outcome of this host–virus interaction depends on the epigenetic modifications brought about by the virus and the response of the immune system to those alterations. The role of genetic and epigenetic factors (DNA methylation, histone modification, microRNA formation, ubiquitination, phosphorylation, and acetylation) came to the fore as increased incidence of new T1DM and DKA in children with SARS-CoV-2 infection differed by race. The highest risk of new T1DM and DKA was noted among American-Indians, followed by Asian, Black and White children10,14 (Figure 1).
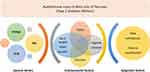 |
Figure 1 Role of genetic and epigenetic factors in type 1 diabetes mellitus. PTPN2: protein tyrosine phosphatase non-receptor type 2, INS: insulin gene, HLA: human leukocyte antigen. |
Reports showed a more than usual annual rise (3–4%)15 in the incidence of TIDM during the COVID 19 pandemic compared to the pre-pandemic period. However, whether this increase is the result of a direct effect of SARS-CoV-2 infection on the pancreatic beta cells or autoimmunity triggered by the virus is a matter of ongoing discourse. SARS-CoV-2 infection could have caused epigenetic changes in gene regulation and provided a favorable environment for autoimmune destruction of pancreatic beta cells.14
2. Did the pandemic lead to increased incidence and severity of pediatric DKA, along with an increase in the incidence of new-onset pediatric diabetes?
The incidence of DKA increased during the pandemic, commensurate with the increase in the incidence of new T1DM. Some of the possible explanations include the increased incidence of childhood diabetes itself, delayed initiation of insulin following the diagnosis of T1DM, delayed presentation to a medical facility due to containment measures, COVID-19 induced cytokine storm, use of steroids for COVID-19 treatment, and changes in lifestyle during the pandemic. Data have unanimously shown an increase in the incidence of new-onset DKA in children.5,7,16–18
In the pre-pandemic era, the frequency estimates of DKA at the diagnosis of T1DM reported from several nations spanning three continents, comprising close to 60,000 children with T1DM, was approximately 30%.19 When compared to the pre-pandemic period, the reported estimates of DKA at the diagnosis of T1DM were as high as 60% according to some studies.5 An international multicenter study from 13 national diabetes registries compared the rates of DKA at the diagnosis of T1DM in the pre-pandemic period (870,000 children between 2006 and 2019) and the pandemic period (16,800 in 2020–2021).20 Around 27% of children in the pre-pandemic period presented with DKA at diagnosis of T1DM. With a mean annual increase of approximately 1.6%, the predicted rates in 2020 and 2021 should have been approximately 32–33%.20 However, the observed prevalence of DKA at the time of T1DM diagnosis was significantly higher (38–39%), suggesting that the COVID-19 pandemic resulted in a significant increase in the incidence of DKA at the time of T1DM diagnosis. In addition to the increased incidence, several studies across the globe have also reported delayed presentation of children with DKA to health-care facilities.21–23 Suspension of outpatient and follow-up services, difficulty adapting to virtual follow-up, and hesitancy to seek hospital care during acute sickness were some of the factors attributed to this delay.
The pandemic resulted in an increased incidence of new-onset DKA, possibly related to delayed recognition and referral.
3. Did the clinical presentation of children with T1DM during and after the pandemic differ from the pre-pandemic period?
Studies have compared clinical parameters such as duration of osmotic symptoms, severity of DKA, median blood glucose and HbA1C levels at presentation, ICU needs, and concomitant autoimmune disorders such as celiac disease and thyroiditis between the pre-pandemic and pandemic phases. The median duration of osmotic symptoms prior to presentation to a health-care facility was much longer during the pandemic period than during the pre-pandemic period, underscoring the delay in diagnosis.5,24 The considerably higher HbA1C at presentation during the pandemic compared to the pre-pandemic time also confirms delayed presentation.8,25,26 Similarly, the proportion of children requiring intensive care was also reported to be higher during the pandemic period.27,28
Presentation of DKA at diagnosis of T1DM may be misdiagnosed as pneumonia (mistakenly for acidotic breathing), acute abdomen, or in late cases such as encephalopathy.29 This is more of a concern in resource-limited settings where the proportion of children with DKA at diagnosis of T1DM is very high (50–80%),30,31 as compared to developed nations (around 30% in Europe, Australia, USA).19 Virtual health-care visits during the pandemic, lack of community awareness and screening programs to recognize early symptoms, and virtual contact with physicians further amplified the problem during the lockdown.
The clinical presentation of children with T1DM differed during the pandemic. A greater proportion of children presented with DKA at T1DM diagnosis had a higher severity of DKA and need for PICU care.
4. What was the effect of T1DM on COVID-19 severity?
According to the Centers for Disease Control and Prevention, adults with T2DM and COVID-19 had worse clinical outcomes. However, data are limited to support poor clinical outcomes in children with T1DM and COVID-19.32 In both T1DM and T2DM with COVID-19, the degree of hyperglycemia and HbA1C levels were found to be independent predictors of mortality.33 Impaired adaptive and innate immune responses noted in children with T1DM, proinflammatory state during DKA, endothelial and microcirculatory dysfunction, and hypercoagulable state contributed to the increased severity of illness and organ failure in children with T1DM and COVID-19.33
T1DM exacerbated the severity of SARS-CoV-2 infection in children, similar to adults with T2DM and COVID-19.
5. Did mortality rates in children with DKA differ between the pandemic and pre-pandemic periods?
DKA-related mortality in children is reported to be very low (less than 1%), of which cerebral edema is the major contributor.34 In contrast, mortality rates in adults with DKA are reported to be higher in those with COVID-19 disease.35,36 However, it is unclear whether the mortality in these studies was attributable to DKA or COVID-19. A study comparing DKA cases from the pre-pandemic period to DKA with COVID-19 cases concluded that COVID-19 severity was the driving factor for increased mortality rates.37
Although morbidity in children with T1DM and DKA increased during the pandemic, mortality attributable to DKA in children with T1DM continued to remain low.
Lessons Learnt
1. Did the pandemic enforce any modifications to the existing treatment strategies for pediatric DKA?
Fluids and insulin remain the cornerstones of DKA management in the backdrop of stringent clinical and laboratory monitoring. Limited ICU beds and infusion pumps, shortage of laboratory resources such as point-of-care (POC) blood glucose, and blood ketone testing compounded by the need to prevent exposure of patients and health-care providers to SARS-CoV-2 necessitated the adoption of alternative management strategies for the management of DKA in children during the pandemic. Health-care providers have adapted to the more frequent use of subcutaneous insulin in the management of mild-to-moderate DKA. The modified protocols recommended 2–4 hourly short acting subcutaneous insulin with or without long-acting basal insulin.38 Insulin Aspart, Lispro, and Glulisine, which are rapidly acting insulin analogs that were administered subcutaneously. This adaptation in the treatment of mild-to-moderate DKA lowered the demand for infusion pumps, other monitoring tools, and the need for ICU care.
During the pre-pandemic period, subcutaneous insulin therapy was less preferred in the treatment of DKA owing to erratic absorption from subcutaneous tissue in the presence of dehydration and shock. However, small prospective trials have demonstrated that the use of subcutaneous short-acting insulin analogs is safe and cost-effective for the treatment of uncomplicated mild-to-moderate DKA. In a randomized trial conducted in adults with uncomplicated DKA, Umpierrez et al showed that rapid-acting subcutaneous insulin (Lispro) in non-intensive care settings was safer and more cost-effective than intravenous insulin in intensive care settings.39 The same group also confirmed the safety and efficacy of a similar insulin analog (Aspart) for the treatment of uncomplicated DKA.40 In a Turkish study, Ersoz et al compared short-acting insulin lispro with regular insulin infusion, and demonstrated that the DKA resolution time was similar in both groups.41 In contrast, a study from Brazil in children with DKA comparing subcutaneous insulin lispro with intravenous regular insulin found that although the blood glucose reduction to 250 mg/dl was similar between the two insulin preparations (approximately 6 hr), the time taken for resolution of DKA was longer in the lispro group. No iatrogenic complications occurred in either group. This study concluded that fast-acting insulin analogs were cost-effective and technically simplified alternative to decrease need of intensive care unit admission.42
Use of subcutaneous insulin instead of intravenous insulin for management of mild-moderate DKA in a non-ICU setting worked as an effective adaptation in the face of constrained resources during the pandemic.
2. Was there any change in the monitoring tools for glycemic control in children with T1DM during the pandemic?
Self-monitoring of blood glucose (SMBG) and HbA1C remains the most widely used method for monitoring glycemic control and titrating insulin in children with T1DM. However, the recognition of interval episodes of hypoglycemia or postprandial hyperglycemia, which are linked to micro- and macrovascular complications, remains a major limitation of this method. Continuous glucose monitoring (CGM) provides minute-to-minute blood glucose readings, mitigating the limitations of SMBG.43 A CGM device consists of an electrochemical trans-dermally placed sensor that detects glucose levels of interstitial fluid and alerts the individual about acute glycemic events based on pre-set high and low threshold values.44 CGM has been approved by the Food and Drug Administration (FDA) for outpatient settings and has led to improved glycemic control in patients on intermittent subcutaneous insulin.45,46 The current International Society for Pediatric and Adolescent Diabetes (ISPAD)-2022 guidelines strongly recommend the use of CGM in children, adolescents, and young adults with T1DM and its initiation at the earliest possible time after initial diagnosis in order to improve glycemic metrics.47 In a longitudinal analysis of data from two international Type 1 Diabetes registries, it was reported that mean Hb1Ac values were significantly lower in individuals using CGM, regardless of the insulin delivery system used. CGM users were noted to be more likely to achieve target Hb1Ac levels.48
Although bedside point-of-care blood glucose monitoring remains the standard of care for titrating intravenous insulin infusion in DKA management, to minimize patient contact time and prevent the risk of SARS-CoV-2 exposure, CGM was temporarily extended by the FDA for in-patient management of DKA during the pandemic. Until there is more evidence on the efficacy and safety of CGM in in-patient settings, these devices should be best used to supplement and not replace POC blood glucose testing in patients on insulin infusion. In resource-limited settings with other urgent health-care priorities, CGM with its associated cost, complexity, and need for expertise may pose additional burdens that hinder its widespread use at the current stage.49
The use of continuous glucose monitors (CGM) increased during the pandemic and reduced the exposure of health-care providers to the virus, but their use is not well established in resource-limited settings.
3. Were there any modifications to insulin delivery systems to prevent exposure?
The use of insulin pump devices with sensor-based feedback (closed loop) helps patients deliver the required dose of insulin, depending on their blood glucose values. A selected patient population who can titrate the insulin dose with the help of insulin pumps may be allowed to do so; however, a support system from health-care providers should be made available for them. Closed-loop technology may help save resources, decrease the need of nursing personnels for glycemic monitoring and prevent unnecessary health-care visits and thereby exposure to transmissible infections.50 A study conducted during the pandemic also showed that glycemic control in children treated with insulin pumps was better as compared to those using insulin pens.51
The use of insulin pumps fared better than insulin pens in patient compliance and glycemic control.
4. What was the role of telehealth in the follow-up of T1DM children during the pandemic?
Telehealth has remained an underutilized tool in managing chronic diseases until the COVID-19 pandemic struck. The use of telehealth for both acute and chronic ailments had increased exponentially during the pandemic. Telehealth has been shown to be an efficient, popular, and practical method of providing diabetes education and insulin titration, in addition to the digital revolution in diabetic monitoring and insulin administration. Telemedicine was utilized to recall sick day schedules in addition to promoting diet and exercise. In all the studies, it has been seen that regular follow-up via telemedicine led to improvement in glycemic metrics.
During the COVID-19 pandemic, a global survey conducted across 89 countries to analyze the perception and use of telemedicine in patients with TIDM yielded encouraging results.52 About 86% of those who received remote treatment regarded these appointments as helpful, and 75% said they planned to schedule similar appointments in the future. Among T1DM participants with telemedicine visits, younger age, past virtual platform experience, and trust in being able to download data were associated with a preference to use telemedicine in the future. About 49% preferred virtual platform for future use, while the rest preferred a hybrid mode.53
Rapid adoption of telemedicine significantly improved the follow-up needs and glycemic metrics in pediatric Type 1 Diabetes.
Future Directions
Was there any association between long COVID syndrome and diabetic ketoacidosis?
Long COVID syndrome (Post-acute COVID-19 syndrome) with involvement of multiple organ systems until 6 months after infection with SARS-CoV-2 is a well-described entity.54 The expression of ACE2, an entry receptor of SARS-CoV-2, in multiple tissues of the body makes it a plausible biological association. Endocrine manifestations of long COVID syndrome include new-onset diabetes, transient loss of glycemic control, and/or progression to DKA. Studies have shown that ACE2 and transmembrane serine protease (TMPRSS2), involved in SARS-CoV-2 entry, are also expressed in pancreatic beta cells.55 Binding of the virus causes inflammation and immunological aberrations resulting in cell damage. However, the duration of this predisposition following SARS-CoV-2 infection remains unknown. The bulk of evidence on the increased incidence of new diabetes and DKA at presentation during the pandemic period does not specify the status of SARS-CoV-2 infection or the duration after the infection. Most studies have compared the overall incidence of T1DM and DKA between the pre-pandemic and pandemic periods, irrespective of SARS-CoV-2 infection status. However, studies on children infected with SARS-CoV-2 with follow-up data on the incidence of new T1DM in children over a period of time are limited. A clear temporal association between the COVID peak and the onset of diabetes, as described for MISC, has not been established. Answers to these questions might be addressed through data from international registries like CoviDiab registry.56
Prospective studies to evaluate various organ system involvement in children exposed to SARS-CoV-2 and those with long COVID syndrome are needed to establish possible endocrine association like new onset diabetes and DKA in children infected with SARS-CoV-2.
Conclusions
The direct effect of the virus and the indirect effects of virus containment measures created various challenges to the healthcare system in the field of pediatric diabetes. The increased incidence of TIDM, new-onset DKA presenting in severe forms, limited resources for monitoring and insulin delivery, and poor glycemic metrics due to loss of follow-up were some of the challenges that needed different adaptive strategies. Revised management protocols for treatment, monitoring, and follow-up were devised to balance patient care needs within available resources and reduce the risk of virus transmission to both patients and health-care providers. Telehealth was used to its maximum potential. However, follow-up studies on SARS-CoV-2 infected children are desirable for assessing long-term consequences.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Park JY, Lee YJ, Kim T, et al. Collateral effects of the coronavirus disease 2019 pandemic on lung cancer diagnosis in Korea. BMC Cancer. 2020;20(1):1040. doi:10.1186/s12885-020-07544-3
2. Hrynick TA, Ripoll Lorenzo S, Carter SE. COVID-19 response: mitigating negative impacts on other areas of health. BMJ Glob Health. 2021;6(4):e004110. doi:10.1136/bmjgh-2020-004110
3. Jayashree M, Williams V, Iyer R. Fluid therapy for pediatric patients with diabetic ketoacidosis: current perspectives. Diabetes Metab Syndr Obes. 2019;12:2355–2361. doi:10.2147/DMSO.S194944
4. Tittel SR, Rosenbauer J, Kamrath C, et al. Did the COVID-19 lockdown affect the incidence of pediatric type 1 diabetes in Germany? Diabetes Care. 2020;43(11):e172–e173. doi:10.2337/dc20-1633
5. Ho J, Rosolowsky E, Pacaud D, et al. Diabetic ketoacidosis at type 1 diabetes diagnosis in children during the COVID-19 pandemic. Pediatr Diabetes. 2021;22(4):552–557. doi:10.1111/pedi.13205
6. Kamrath C, Rosenbauer J, Eckert AJ, et al. Incidence of type 1 diabetes in children and adolescents during the COVID-19 pandemic in Germany: results from the DPV Registry. Diabetes Care. 2022;45(8):1762–1771. doi:10.2337/dc21-0969
7. Mameli C, Scaramuzza A, Macedoni M, et al. Type 1 diabetes onset in Lombardy region, Italy, during the COVID-19 pandemic: the double-wave occurrence. EClinicalMedicine. 2021;39:101067. doi:10.1016/j.eclinm.2021.101067
8. Rahmati M, Keshvari M, Mirnasuri S, et al. The global impact of COVID-19 pandemic on the incidence of pediatric new-onset type 1 diabetes and ketoacidosis: a systematic review and meta-analysis. J Med Virol. 2022;94(11):5112–5127. doi:10.1002/jmv.27996
9. Kendall EK, Olaker VR, Kaelber DC, Xu R, Davis PB. Association of SARS-CoV-2 infection with new-onset type 1 diabetes among pediatric patients from 2020 to 2021. JAMA Netw Open. 2022;5(9):e2233014. doi:10.1001/jamanetworkopen.2022.33014
10. Qeadan F, Tingey B, Egbert J, et al. The associations between COVID-19 diagnosis, type 1 diabetes, and the risk of diabetic ketoacidosis: a nationwide cohort from the US using the Cerner Real-World Data. PLoS One. 2022;17(4):e0266809. doi:10.1371/journal.pone.0266809
11. Filippi CM, von Herrath MG. Viral trigger for type 1 diabetes: pros and cons. Diabetes. 2008;57(11):2863–2871. doi:10.2337/db07-1023
12. Müller JA, Groß R, Conzelmann C, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3(2):149–165. doi:10.1038/s42255-021-00347-1
13. Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol. 2020;18(9):2128–2130.e2. doi:10.1016/j.cgh.2020.04.040
14. Kee J, Thudium S, Renner DM, et al. SARS-CoV-2 disrupts host epigenetic regulation via histone mimicry. Nature. 2022;610(7931):381–388. doi:10.1038/s41586-022-05282-z
15. Patterson CC, Harjutsalo V, Rosenbauer J, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: a multicentre prospective registration study. Diabetologia. 2019;62(3):408–417. doi:10.1007/s00125-018-4763-3
16. Salmi H, Heinonen S, Hästbacka J, et al. New-onset type 1 diabetes in Finnish children during the COVID-19 pandemic. Arch Dis Child. 2022;107(2):180–185. doi:10.1136/archdischild-2020-321220
17. Gottesman BL, Yu J, Tanaka C, Longhurst CA, Kim JJ. Incidence of new-onset type 1 diabetes among US children during the COVID-19 global pandemic. JAMA Pediatr. 2022;176(4):414–415. doi:10.1001/jamapediatrics.2021.5801
18. Barrett CE, Koyama AK, Alvarez P, et al. Risk for newly diagnosed diabetes >30 days after SARS-CoV-2 infection among persons aged <18 years - United States, March 1, 2020-June 28, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(2):59–65. doi:10.15585/mmwr.mm7102e2
19. Cherubini V, Grimsmann JM, Åkesson K, et al. Temporal trends in diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes between 2006 and 2016: results from 13 countries in three continents. Diabetologia. 2020;63(8):1530–1541. doi:10.1007/s00125-020-05152-1
20. Birkebaek NH, Kamrath C, Grimsmann JM, et al. Impact of the COVID-19 pandemic on long-term trends in the prevalence of diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes: an international multicentre study based on data from 13 national diabetes registries. Lancet Diabetes Endocrinol. 2022;10(11):786–794. doi:10.1016/S2213-8587(22)00246-7
21. Alfayez OM, Aldmasi KS, Alruwais NH, et al. Incidence of diabetic ketoacidosis among pediatrics with type 1 diabetes prior to and during COVID-19 pandemic: a meta-analysis of observational studies. Front Endocrinol. 2022;13:856958. doi:10.3389/fendo.2022.856958
22. Pillai SS, Cao C, Drees CJ, Chu TC, Mason K, Topor LS. Delays in presentation of new onset diabetes at the start of the COVID-19 pandemic. R I Med J. 2022;105(5):46–50.
23. Leiva-Gea I, Fernández CA, Cardona-Hernandez R, et al. Increased presentation of diabetic ketoacidosis and changes in age and month of type 1 diabetes at onset during the COVID-19 pandemic in Spain. J Clin Med. 2022;11(15):4338. doi:10.3390/jcm11154338
24. Elgenidy A, Awad AK, Saad K, et al. Incidence of diabetic ketoacidosis during COVID-19 pandemic: a meta-analysis of 124,597 children with diabetes. Pediatr Res. 2022:1–12. doi:10.1038/s41390-022-02241-2
25. Alaqeel A, Aljuraibah F, Alsuhaibani M, et al. The impact of COVID-19 pandemic lockdown on the incidence of new-onset type 1 diabetes and ketoacidosis among Saudi children. Front Endocrinol. 2021;12:669302. doi:10.3389/fendo.2021.669302
26. Zubkiewicz-Kucharska A, Seifert M, Stępkowski M, Noczyńska A. Diagnosis of type 1 diabetes during the SARS-CoV-2 pandemic: does lockdown affect the incidence and clinical status of patients? Adv Clin Exp Med. 2021;30(2):127–134. doi:10.17219/acem/130359
27. Jafari K, Koves I, Rutman L, Brown JC. Impact of the COVID-19 pandemic on the severity of diabetic ketoacidosis presentations in a tertiary pediatric emergency department. Pediatr Qual Saf. 2022;7(2):e502. doi:10.1097/pq9.0000000000000502
28. Marks BE, Khilnani A, Meyers A, et al. Increase in the diagnosis and severity of presentation of pediatric type 1 and type 2 diabetes during the COVID-19 pandemic. Horm Res Paediatr. 2021;94(7–8):275–284. doi:10.1159/000519797
29. Mavinkurve M, Jalaludin MY, Chan EWL, et al. Is misdiagnosis of type 1 diabetes mellitus in Malaysian children a common phenomenon? Front Endocrinol. 2021;12:606018. doi:10.3389/fendo.2021.606018
30. Nallasamy K, Jayashree M, Singhi S, Bansal A. Low-dose vs standard-dose insulin in pediatric diabetic ketoacidosis: a randomized clinical trial. JAMA Pediatr. 2014;168(11):999–1005. doi:10.1001/jamapediatrics.2014.1211
31. Williams V, Jayashree M, Nallasamy K, Dayal D, Rawat A. 0.9% saline versus Plasma-Lyte as initial fluid in children with diabetic ketoacidosis (SPinK trial): a double-blind randomized controlled trial. Crit Care. 2020;24(1):1. doi:10.1186/s13054-019-2683-3
32. Chow N, Fleming-Dutra K, Gierke R; CDC COVID-19 Response Team. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382–386. doi:10.15585/mmwr.mm6913e2
33. Kountouri A, Korakas E, Ikonomidis I, et al. Type 1 diabetes mellitus in the SARS-CoV-2 pandemic: oxidative stress as a major pathophysiological mechanism linked to adverse clinical outcomes. Antioxidants. 2021;10(5):752. doi:10.3390/antiox10050752
34. Benoit SR, Zhang Y, Geiss LS, Gregg EW, Albright A. Trends in diabetic ketoacidosis hospitalizations and in-hospital mortality - United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018;67(12):362–365. doi:10.15585/mmwr.mm6712a3
35. Stevens JS, Bogun MM, McMahon DJ, et al. Diabetic ketoacidosis and mortality in COVID-19 infection. Diabetes Metab. 2021;47(6):101267. doi:10.1016/j.diabet.2021.101267
36. Pasquel FJ, Messler J, Booth R, et al. Characteristics of and mortality associated with diabetic ketoacidosis among US patients hospitalized with or without COVID-19. JAMA Netw Open. 2021;4(3):e211091. doi:10.1001/jamanetworkopen.2021.1091
37. Khan F, Paladino L, Sinert R. The impact of COVID-19 on Diabetic Ketoacidosis patients. Diabetes Metab Syndr. 2022;16(1):102389. doi:10.1016/j.dsx.2022.102389
38. Priyambada L, Wolfsdorf JI, Brink SJ, et al. ISPAD clinical practice consensus guideline: diabetic ketoacidosis in the time of COVID-19 and resource-limited settings-role of subcutaneous insulin. Pediatr Diabetes. 2020;21(8):1394–1402. doi:10.1111/pedi.13118
39. Umpierrez GE, Latif K, Stoever J, et al. Efficacy of subcutaneous insulin lispro versus continuous intravenous regular insulin for the treatment of patients with diabetic ketoacidosis. Am J Med. 2004;117(5):291–296. doi:10.1016/j.amjmed.2004.05.010
40. Umpierrez GE, Cuervo R, Karabell A, Latif K, Freire AX, Kitabchi AE. Treatment of diabetic ketoacidosis with subcutaneous insulin aspart. Diabetes Care. 2004;27(8):1873–1878. doi:10.2337/diacare.27.8.1873
41. Ersöz HO, Ukinc K, Köse M, et al. Subcutaneous lispro and intravenous regular insulin treatments are equally effective and safe for the treatment of mild and moderate diabetic ketoacidosis in adult patients. Int J Clin Pract. 2006;60(4):429–433. doi:10.1111/j.1368-5031.2006.00786.x
42. Della Manna T, Steinmetz L, Campos PR, et al. Subcutaneous use of a fast-acting insulin analog: an alternative treatment for pediatric patients with diabetic ketoacidosis. Diabetes Care. 2005;28(8):1856–1861. doi:10.2337/diacare.28.8.1856
43. Vettoretti M, Cappon G, Acciaroli G, Facchinetti A, Sparacino G. Continuous glucose monitoring: current use in diabetes management and possible future applications. J Diabetes Sci Technol. 2018;12(5):1064–1071. doi:10.1177/1932296818774078
44. Unger J. Continuous glucose monitoring overview: features and evidence. Am J Manag Care. 2022;28(4 Suppl):S60–S68. doi:10.37765/ajmc.2022.89206
45. Health Quality Ontario. Continuous monitoring of glucose for type 1 diabetes: a health technology assessment. Ont Health Technol Assess Ser. 2018;18(2):1–160.
46. Hood KK, DiMeglio LA, Riddle MC. Putting continuous glucose monitoring to work for people with type 1 diabetes. Diabetes Care. 2020;43(1):19–21. doi:10.2337/dci19-0054
47. Tauschmann M, Forlenza G, Hood K, et al. ISPAD clinical practice consensus guidelines 2022: diabetes technologies: glucose monitoring. Pediatr Diabetes. 2022;23(8):1390–1405. doi:10.1111/pedi.13451
48. DeSalvo DJ, Miller KM, Hermann JM, et al. Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: international comparison from the T1D exchange and DPV initiative. Pediatr Diabetes. 2018;19(7):1271–1275. doi:10.1111/pedi.12711
49. Korytkowski M, Antinori-Lent K, Drincic A, et al. A pragmatic approach to inpatient diabetes management during the COVID-19 pandemic. J Clin Endocrinol Metab. 2020;105(9):dgaa342. doi:10.1210/clinem/dgaa342
50. Bila R, Varo R, Madrid L, Sitoe A, Bassat Q. Continuous glucose monitoring in resource-constrained settings for hypoglycaemia detection: looking at the problem from the other side of the coin. Biosensors. 2018;8(2):43. doi:10.3390/bios8020043
51. Gherbon A, Frandes M, Timar R, Timar B. The impact of COVID-19 lockdown on glycemic balance in Romanian patients with type 1 diabetes mellitus. Diabetes Metab Syndr Obes. 2022;15:3403–3413. doi:10.2147/DMSO.S386614
52. Scott SN, Fontana FY, Züger T, Laimer M, Stettler C. Use and perception of telemedicine in people with type 1 diabetes during the COVID-19 pandemic-results of a global survey. Endocrinol Diabetes Metab. 2021;4(1):e00180. doi:10.1002/edm2.180
53. Schiller T, Zornitzki T, Ostrovsky V, et al. Following the COVID-19 experience, many patients with type 1 diabetes wish to use telemedicine in a hybrid format. Int J Environ Res Public Health. 2021;18(21):11309. doi:10.3390/ijerph182111309
54. The Lancet Null. Facing up to long COVID. Lancet. 2020;396(10266):1861. doi:10.1016/S0140-6736(20)32662-3
55. Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi:10.1007/s00592-009-0109-4
56. Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi:10.1038/s41591-021-01283-z
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
