Back to Journals » International Journal of General Medicine » Volume 17
Patients with Hemophagocytic Lymphohistiocytosis Who Need Intensive Care Can Be Successfully Rescued by Timely Using Etoposide-Based HLH Regimens
Authors Lv K, Cheng X, Zhou Y, Yu M, Wang S, Shen H, Li F
Received 7 November 2023
Accepted for publication 28 January 2024
Published 3 February 2024 Volume 2024:17 Pages 431—446
DOI https://doi.org/10.2147/IJGM.S443774
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Woon-Man Kung
Kebing Lv,1,* Xiaoye Cheng,1– 3,* Yulan Zhou,1– 3 Min Yu,1– 3 Shixuan Wang,1– 3 Huimin Shen,1 Fei Li1– 3
1Center of Hematology, The First Affiliated Hospital of Nanchang University, Nanchang, People’s Republic of China; 2Jiangxi Clinical Research Center for Hematologic Disease, Nanchang, People’s Republic of China; 3Institute of Lymphoma and Myeloma, Nanchang University, Nanchang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fei Li, Email [email protected]
Background: Hemophagocytic lymphohistiocytosis (HLH) patients who need intensive care usually have multiple organ failure and poor prognosis. However, the clinical characteristics, therapeutic efficacy and outcome in these critically ill HLH patients have remained unclear.
Methods: We performed a retrospective study of 50 critically ill HLH patients from September 2013 to October 2022. Patients’ information was collected, and the overall survival rate was estimated.
Results: Fifty HLH patients need intensive care, and the median sequential organ failure assessment (SOFA) score was 8. 66.00% patients had septic shock, 60.00% had disseminated intravascular coagulation (DIC) and 56.00% had acute respiratory distress syndrome (ARDS). 64.00% patients needed vasoactive drugs, 60.00% needed invasive or non-invasive positive pressure mechanical ventilation, and 12.00% needed continuous renal replacement therapy (CRRT). Among 18 patients received the etoposide-based regimens, the median time for 17 patients to remove ECG monitoring was 13 days (4– 30 days); the median time to remove respiratory support in 10 patients was 8.5 days (4– 21 days); the median time for 5 patient to convert from dominant DIC to non-dominant DIC was 4 days (1– 14 days) and the median time for 6 patients to stop using vasoactive drugs was 10 days (2– 14 days). After 4 weeks of treatment, 7 patients were evaluated as NR, 6 achieved PR, and 5 could not be evaluated. The ORR was 55.56%. Up to the last follow-up, the OS rate of patients receiving etoposide-based regimens was 66.67%. In contrast, all 32 HLH patients in other groups died. Univariate analysis showed that PCT > 0.5 ug/L, PT prolonged > 6 s, TBil > 25umol/L, respiratory failure, renal failure, liver failure and did not receive etoposide- based regimens were the negative factors affecting survival (P = 0.001, 0.017, 0.043, 0.001, 0.000, 0.029, 0.000).
Conclusion: HLH patients who need intensive care timely used etoposide-based HLH regimens might rescue critically ill patients successfully.
Keywords: hemophagocytic lymphohistiocytosis, etoposide-based treatment, critically ill patients, prognosis
Introduction
As a cytokine release syndrome caused by uncontrolled immune activation, hemophagocytic lymphohistiocytosis (HLH) can quickly lead to organ infiltration, systemic disease, and poor prognosis.1 Genetic defects in primary HLH affect cytotoxic activity, which has been widely studied in pediatric patients.2 Secondary HLH is often caused by infection, malignancy, and autoimmune diseases and can be seen in patients of all age groups.3 Adult HLH patients lack comprehensive data, and treatment measures depend on expert opinions and the HLH-94/2004 protocol verified by pediatric patients.4,5 Based on the rapid and dangerous disease process of HLH, some patients have multiple organ involvement at diagnosis, including respiratory failure, coma, shock, acute liver and kidney failure, and so on. Mortality in intensive care units (ICU) is 50–80%.6,7 Therefore, in addition to standard organ support, thorough etiological screening and emergency treatment of HLH triggers are necessary for HLH treatment.8 “Specific” HLH therapies (corticosteroids, multivalent immunoglobulins, etoposide, anti-cytokine therapy, and so on) are usually used for the most severe cases of HLH in the ICU.6,9,10 The death-related risk factors are poorly understood among critically ill HLH patients who need intensive care. The efficacy of the etoposide-based regimens has also not been intensively identified and reported.
In our retrospective cohort, we analyzed triggering factors, clinical characteristics, treatment, and survival prognosis in critically ill HLH patients with intensive care, hoping to provide more detailed insights and clinical guidance information.
Methods
Patients and Selection Criteria
From September 2013 to October 2022, 372 patients diagnosed with HLH were enrolled in the Department of Hematology of the First Affiliated Hospital of Nanchang University, and 50 of them needed life support and intensive care. The inclusion criteria were as follows.11 1. All patients were diagnosed according to the revised diagnostic criteria guidelines within the definitive HLH-2004 protocol. 2. The patients had life-threatening complications at diagnosis, such as shock, sepsis, acute respiratory distress syndrome (ARDS),12 disseminated intravascular coagulation (DIC),13 acute heart failure, serious arrhythmia, liver dysfunction, liver failure, renal failure, gastrointestinal bleeding, coma, delirium, severe water-electrolyte disorder, acid–base imbalance, multiple organ dysfunction syndrome (MODS),14 and so on. These complications led to instability of vital signs (temperature ≥37.8°C, heart rate ≥100 beats per minute, respiratory rate > 24 breaths per minute, systolic blood pressure ≤90 mmHg, or oxygen saturation <90%,15 requiring respiratory support, circulatory support, plasma purification, and other life support. Exclusion criteria were as follows. 1. Patients did not meet the above inclusion criteria. 2. Patients were lost during follow-up. 3. Lack of clinical data.
Treatment Regimens
HLH-1994/2004 regimen, dexamethasone at 10 mg/m2/day during weeks 1 and 2, 5 mg/m2/day during weeks 3 and 4, 2.5 mg/m2/day during weeks 5 and 6, and 1.25 mg/m2/day during weeks 7 and 8; etoposide at 100 mg/m2/day two times weekly during weeks 1 and 2 and 100 mg/m2/day once each week during weeks 3–8. CSA (6 mg/m2/day) and VP-16 were used simultaneously.
Efficacy Assessment
According to the efficacy evaluation standard formulated by the Midwest cooperation group of the United States,16 the following six indexes were used as the reference basis for clinical efficacy evaluation after 4 weeks and 8 weeks of treatment: (1) soluble CD25 (sCD25); (2) serum ferritin; (3) blood cell count; (4) triacylglycerol; (5) hemophagocytosis; and (6) level of consciousness, such as HLH patients with central nervous system (CNS) symptoms. CR: All the above indicators returned to normal. PR: More than two symptoms or laboratory makers were improved by at least 25%, and individual indicators also needed to meet the following criteria: (1) sCD25 level was decreased by at least 1/3; (2) the levels of ferritin (FER) and triglyceride (TG) were decreased by more than 25%; (3) in case of transfusion: increase of neutrophil count by at least 100% to >0.5×109/L in the absence of blood transfusion for patients with an initial neutrophil count <0.5×109/L or increase of neutrophil count by at least 100% to >2.0×109/L for patients with an initial neutrophil count of 0.5 to 2.0×109/L; and (4) patients with alanine aminotransferase (ALT) >400 U/L should be reduced by more than 50%. ORR: CR+PR.
Statistical Analysis
All statistical analyses were performed using IBM SPSS version 24 and GraphPad prism 8.0. The paired test was used for data before and after treatment, the t-test was used for data with normal distribution, and the rank sum test was used for data with non-normal distribution. Overall survival (OS) was defined as the time from the date of diagnosis to the date of death of any triggering event or the last follow-up. Patients without available follow-up data were excluded from further analysis. Kaplan–Meier method was used to analyze the prognosis of patients, and COX proportional hazards model was used for multivariate analysis. In all analyses, P < 0.05 was considered statistically significant.
Results
Clinical and Laboratory Examination Characteristics
A total of 50 HLH patients (27 males and 23 females) were included in this study. The median age at diagnosis was 51.5 years (range, 9–86 years). Five patients (10.00%) were under 18 years of age. None of the patients fulfilled the diagnostic criteria for primary HLH. The clinical manifestations of HLH included various symptom combinations, while persistent fever, systemic edema, and serous effusion were the most common symptoms (98.00%, 80.00%, and 84.00% of patients, respectively). In addition, hemophagocytosis in bone marrow samples was detected in 29 patients (69.05%). Moreover, 50 patients (100.00%) needed transfusions, 30 patients (60.00%) needed invasive or non-invasive positive pressure mechanical ventilation, 32 patients (64.00%) needed vasoactive drugs, and 6 patients (12.00%) needed continuous renal replacement therapy (CRRT). These clinical features and their distribution among various etiologies are shown in Tables 1 and 2 lists the laboratory characteristics. HLH patients had a higher proportion of impaired liver function, thrombocytopenia, hemoglobin reduction, ALB reduction, and abnormal inflammatory indexes, evidenced by elevated procalcitonin (PCT).
 |
Table 1 Clinical Manifestations in 50 Critically Ill Patients with HLH |
 |
Table 2 Laboratory Data in 50 Critically Ill Patients with HLH |
Triggering Diseases of HLH
The most frequent triggering diseases of HLH in our cohort were infections (36/50, 72.00%), followed by neoplastic factors (6/50, 12.00%) and autoimmune factors (2/50, 4.00%), and 12.00% of patients had unknown inducement. Except for 4 patients with severe pulmonary infection, but the pathogen was not identified, bacterial infections (17/36, 47.22%) were most prevalent in infection-associated HLH. 5 patients were infected with Gram-positive bacteria, including 1 case of vancomycin-resistant Enterococcus, 1 case of cephalosporin Staphylococcus, and 3 unknown cases. Gram-negative bacteria infected 10 patients, including 4 cases of Klebsiella pneumoniae, 1 case of Stenotrophomonas maltophilia, 1 case of Pseudomonas stephensi, 3 cases of Acinetobacter baumannii, and 1 case of Xanthomonas campestris. In addition, 2 patient suffered from Kikuchi disease. Fungal infections occurred in two patients (2/36, 5.56%), and both were Candida infections. Besides, 13 patients (13/36, 36.11%) were infected with a virus, including 11 with EBV, 1 with adenovirus, and 1 with the respiratory syncytial virus. Moreover, 6 patients with neoplastic factors were caused by lymphoma. Figure 1 illustrates various etiological classifications.
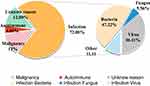 |
Figure 1 The proportion of etiological classification in 50 critically ill HLH patients. |
Mods
All 50 patients had organ or system involvements at diagnosis, including cardiovascular, coagulation function, lung, liver, kidney, gastrointestinal tract, and CNS (Figure 2).14 The median sequential organ failure assessment (SOFA) score was 8 (4–18). Cardiovascular, coagulation, and respiratory system involvement were the most common clinical manifestations. There were 33 cases of septic shock (66.00%), 10 cases of heart failure (20.00%), 30 cases of DIC (60.00%), and 28 cases of ARDS (55.60%).
 |
Figure 2 Organ or system involvements in 50 critically ill HLH patients. |
Treatment and Response
A total of 18 patients received the etoposide-based regimens (including HLH-1994/2004 regimen). Underlying diseases of 18 patients were infections, including 14 cases of septic shock, 1 case of non-shock sepsis, 3 cases of heart failure, 11 cases of DIC, 5 cases of respiratory failure, 9 cases of serous exudation, and 2 cases of gastrointestinal bleeding. Among 18 patients treated with the etoposide-based regimens, the median time for 17 patients to remove ECG monitoring was 13 days (4–30 days). The median time to remove respiratory support in 10 patients was 8.5 days (4–21 days). The median time for 5 patients to convert from dominant DIC to non-dominant DIC was 4 days (1–14 days) and the median time for 6 patients to stop using vasoactive drugs was 10 days (2–14 days). Two patients with acute heart failure did not deteriorate after treatment. One patient with gastrointestinal bleeding improved significantly after 3 days. After 4 weeks of treatment, 7 patients were evaluated as NR (4 achieved PR in the follow-up), 6 patients achieved PR (2 achieved CR in the follow-up), and 5 patients could not be evaluated. The ORR after 4 weeks was 55.56% (Table 3).
 |
Table 3 Treatment and Outcome of 18 Severe HLH Patients |
Among 18 critically ill patients treated with HLH regimens, 13 were evaluated after 4 weeks. White blood cell (WBC), absolute neutrophil count (ANC), HB, platelet (PLT), D-D dimer, lactate dehydrogenase (LDH), aspartate aminotransferase (AST), ALT, sCD25, FER, total bilirubin (TBIL), TG, albumin (ALB), FBG, and other laboratory indexes were compared and analyzed. After 4 weeks of HLH treatment, the concentrations of PLT and ALB were increased significantly (P = 0.000 and P = 0.005, respectively). The concentrations of D-D dimer, LDH, AST, sCD25, and FER were decreased significantly (P < 0.05). No significant difference was found in other indexes (P > 0.05) (Figure 3).
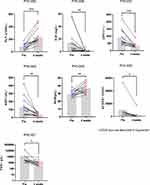 |
Figure 3 Changes of some evaluation indexes before and after treatment with etoposide-based regimen in 13 critically ill HLH patients (*P ≤0.05; **P ≤0.01; ***P ≤0.001). |
Moreover, 32 critically ill patients did not receive the etoposide-based HLH regimen. Among them, 6 patients were willing to receive etoposide, but ultimately were unable to continue infusion (less than one day) and withdrew due to objective reasons. Including 5 patients who were unable to maintain liver, kidney, and respiratory functions due to infusion, and 1 patient experiencing serious side effects such as abdominal pain and diarrhea; 26 patient families are concerned about drug toxicity and refuse to accept the use of etoposide. Out of 32 patients, 10 were treated with corticosteroids alone. All patients received one or more symptomatic treatments, such as anti-infection, antipyretic, sedation, analgesia, ALB infusion, and rehydration. Unfortunately, all died before discharge. The specific information of these patients are presented in Table S1.
Comparison of Characteristics of Clinical Parameter Between Patients in the Etoposide-Based Regimens and Other Treatment Groups
The related indicators of HLH and multiple organ failure of patients in the etoposide-based regimens and other treatment groups are presented in Table 4. With respect to laboratory-test results, HB, PLT, APTT, Creatinine (CRE), PCT, hemophagocytosis in two groups showed significant differences. With respect to clinical characteristics, respiratory failure showed significant differences.
 |
Table 4 The Characteristics of Clinical Parameters Between the Etoposide-Based Regimen and Other Treatment Groups |
Survival Analysis
For the 50 patients with survival data available, the median OS of the entire cohort was 0.55 months (range: 0–25 months). The 1-year survival rate was 24.0%, and the median follow-up time from diagnosis was 15 months (Figure 4A). The 30-day and 90-day mortality rates were 60.0% and 70.0%, respectively. We divided patients into etoposide-based treatment group, corticosteroid treatment group and symptomatic treatment group based on the therapy they received. In the etoposide-based treatment group, the survival rate of patients as of the last follow-up was 66.67%. All 32 patients in other groups died before discharge. The OS of etoposide-based treatment group was significantly better than that of corticosteroid treatment group and symptomatic treatment group (not reached versus 0.45 months, not reached versus 0.20 months; P = 0.000). There was no significant difference in OS between the corticosteroid treatment group and the symptomatic treatment group (P = 0.1585) (Figure 4B). In addition, 157 of the 363 patients in our center received etoposide-based HLH regimens. Except for the 9 patients who were lost during follow-up, the 152 patients were divided into severe (n = 18) and general patient groups (n = 134) according to whether their vital signs were stable.There was no significant difference in survival between the severe group and the general group (P = 0.094) (Figure 4C).
Univariate analyse was conducted to identify possible prognostic factors for overall mortality. Patients with liver failure (median OS, 0.267 vs 0.920 months, P = 0.029, Figure 5A), respiratory failure (median OS, 0.259 vs 2.500 months, P = 0.001, Figure 5B), renal failure (median OS, 0.200 vs 1.000 months, P = 0.000, Figure 5C), PCT > 0.5 µg/L (median OS, 0.450 vs 13.000 months, P = 0.024, Figure 5D), PT prolonged > 6 s (median OS, 0.185 vs 0.684 months, P = 0.017, Figure 5E), TBil > 25umol/L (median OS, 0.415 vs 0.960 months, P = 0.043, Figure 5F) and patients who did not receive etoposide-based regimens (median OS, 0.240 vs not reached months, P = 0.000) were associated with a poor outcome (Table 5).
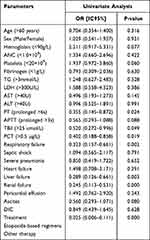 |
Table 5 Univariate and Multivariate Cox Regression Analysis of Prognostic Factors in Severe HLH Patients |
Discussion
HLH is an acute disease that may lead to organ failure and premature death. Numerous retrospective observational studies have described HLH patients admitted to the ICU.17–20 However, some critically ill patients with HLH fail to enter the ICU due to economic and other factors. Therefore, our cohort included patients who needed supportive life care, such as mechanical ventilation, CRRT, and vasoactive drug application, to avoid the possible data loss caused by the inclusion of patients only from the ICU.
In previous retrospective studies, the proportion of HLH caused by infection is about 30%, while it is increased significantly to 40–50% in patients with severe HLH.21–23 Of the 50 patients we included, up to 72% were infected. This is related to the large bias caused by the limited number of cases in our single center. But combined with previous reports, there is indeed an increased proportion of infectious causes in severe patients. Under the stimulation of pathogens, monocytes/macrophages and neutrophils are continuously activated and secrete many pro-inflammatory factors, such as TNF-α, IL-6, IFN-γand IL-β, which further promote the activation of macrophages and prolong the survival time.24,25 The above process predisposes patients to hemodynamic instability due to endothelial dysfunction. In our present study, 86.11% (31/36) induced by infection were complicated with septic shock. Microcirculation disturbance leads to fibrin deposition and PLT aggregation in microvessels, which, together with the primary cause, leads to DIC.26–28 Thrombosis and hemorrhage in the course of DIC aggravate the shock. In short, DIC and shock are mutually causal, which form a vicious circle, further destroying the blood supply to organs and promoting the development of MODS. Therefore, HLH patients with infection are more prone to rapid deterioration.
In terms of clinical manifestations, in addition to HLH-related symptoms, the liver function damage is the most prominent in our group of critically ill patients. The biochemical results showed a high proportion of abnormal liver enzymes in patients (68.00% ALT, 88.00% AST, and 40.00% TBil), and 26.00% (13/50) of patients had liver failure. The mechanism underlying the HLH-induced liver injury is unclear. It is generally believed to be caused by infiltration of activated hemophagocytic tissue cells or excessive production of cytokines,29 resulting in anemia, elevated LDH, hyperbilirubinemia, hypoalbuminemia, further cytokine increase, and even inflammatory factor storm.30–33 Liver failure is rare in HLH.34 In the existing reports of severe patients, 1–29% of patients are diagnosed with acute liver failure (ALF) during hospitalization,17,35 which is similar to our results. Our data also showed that ALF is a risk factor associated with mortality, suggesting that timely intervention in controlling liver diseases and avoiding the end-stage of the related organ were highly significant.
Judging from previous reports, critically ill patients with HLH could benefit from etoposide-based regimens. Etoposide and corticosteroids can effectively inhibit the proliferation of cytotoxic T lymphocytes and macrophage activity to control the inflammatory response, which is related to reduced lactate levels and improved organ failure.34,35 Aoyagi et al have improved respiratory status and inflammatory marker levels in ARDS patients with etoposide and corticosteroids.36 In addition, Vigneron et al have observed a reduction in non-PLT SOFA (npSOFA) in HLH patients treated with etoposide.37 Therefore, we chose the etoposide-based regimen for critically ill patients.
However, in the face of HLH patients in a critical state, there is no international consensus on whether to treat HLH quickly and timely while providing life support or waiting for the vital signs to stabilize before therapy. In our present study, 18 HLH patients started etoposide-based treatment under critical conditions and withdrew from ECG monitoring (median time: 13 days), significantly improving respiratory and circulatory systems. After 4 weeks of treatment, 7 indicators, including sCD25 and FER concentrations, in 13 evaluable patients were significantly improved. Six patients achieved PR, and the 30-day survival rate was 100%. As of the last follow-up, 66.67% of patients were alive. Of the patients receiving symptomatic treatment only, 10 patients received corticosteroids, and no survival benefit was observed, which was consistent with previous studies.38 All patients in this group died before discharge, and the longest survival time was 1.7 months. The experience of these patients showed that when critically ill patients were receiving life support technology, early initiation of HLH treatment could rapidly improve and reverse the patient’s multisystem failure, thereby saving lives and curing diseases. However, symptomatic treatment and life support technology alone could not stabilize vital signs and change the outcome of rapid death.
We further compared the survival prognosis of 134 general patients who received an etoposide-based HLH regimens with 18 severe patients in our center and found that there was no significant difference in survival between the severe group and the general group (P = 0.094). It was worth noting that the proportion of underlying diseases with poor prognoses (EBV infection and malignancy-associated HLH) in the severe group was much lower than that in the general group (38.89% vs 70.89%). It also illustrated that even if the patients with non-EBV infection and non-malignancy-associated HLH were critically ill, they could still be cured after receiving etoposide therapy timely and achieve long-term survival compared with ordinary patients. It is necessary to expand the sample size for further comparison and validation.
Conclusions
Collectively, our data showed that the prognosis of critically ill HLH patients was poor. Patients with PCT > 0.5 µg/L, TBil > 25umol/L, PT prolonged > 6 s, respiratory failure, renal failure, liver failure and did not receive an etoposide-based regimen were at higher risk of death. These severe HLH patients required increased awareness and prompt early intervention of HLH treatment. For these patients, the etoposide-based regimens might be an effective treatment to reverse worsening clinical progression and gain a chance of survival.
Data Sharing Statement
Please contact the author for data requests.
Statement of Ethics
Our work was approved by the Medical Research Ethics Review Committee of the First Affiliated Hospital of Nanchang University (IIT2023297).
We confirm that the informed consent of the study participants has been obtained and a parent or legal guardian of patients under 18 years of age provided informed consent. The guidelines outlined in the Declaration of Helsinki have been complied with.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
We thank all the patients and their families for participating in this research. In addition, we acknowledge the support of the grants from the National Natural Science Foundation of China (81960043, 82160043), the Natural Science Foundation of Jiangxi Province (20192ACB20030), and the Science and Technology Innovation Base Construction Project of Jiangxi Province (20212BCG74001 and 20211ZDG02006).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Knaak C, Schuster FS, Nyvlt P, et al. Treatment and mortality of hemophagocytic lymphohistiocytosis in adult critically ill patients: a systematic review with pooled analysis. Crit Care Med. 2020;48(11):e1137–e1146. PMID: 32947471. doi:10.1097/CCM.0000000000004581
2. Henter JI, Elinder G, Söder O, Hansson M, Andersson B, Andersson U. Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood. 1991;78(11):2918–2922. PMID: 1954380.
3. Yildiz H, Van Den Neste E, Defour JP, Danse E, Yombi JC. Adult haemophagocytic lymphohistiocytosis: a Review. QJM. 2020:hcaa011. PMID: 31943120. doi:10.1093/qjmed/hcaa011
4. Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. PMID: 16937360. doi:10.1002/pbc.21039
5. La Rosée P. Treatment of hemophagocytic lymphohistiocytosis in adults. Hematology Am Soc Hematol Educ Program. 2015;2015:190–196.
6. Kapoor S, Morgan CK, Siddique MA, Guntupalli KK. Intensive care unit complications and outcomes of adult patients with hemophagocytic lymphohistiocytosis: a retrospective study of 16 cases. World J Crit Care Med. 2018;7(6):73–83. PMID: 30596029; PMCID: PMC6305525. doi:10.5492/wjccm.v7.i6.73
7. Buyse S, Teixeira L, Galicier L, et al. Critical care management of patients with hemophagocytic lymphohistiocytosis. Intensive Care Med. 2010;36(10):1695–1702. PMID: 20532477. doi:10.1007/s00134-010-1936-z
8. Bichon A, Bourenne J, Allardet-Servent J, et al. High Mortality of HLH in ICU Regardless Etiology or Treatment. Front Med. 2021;8:735796. PMID: 34692727; PMCID: PMC8526960. doi:10.3389/fmed.2021.735796
9. Trottestam H, Horne A, Aricò M, et al.; Histiocyte Society. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011;118(17):4577–4584. PMID: 21900192; PMCID: PMC3208276. doi:10.1182/blood-2011-06-356261
10. Bergsten E, Horne A, Aricó M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130(25):2728–2738. PMID: 28935695; PMCID: PMC5785801. doi:10.1182/blood-2017-06-788349
11. Zhang JR. HLH-2004 protocol: diagnostic and therapeutic guidelines for childhood hemophagocytic lymphohistiocytosis. Zhongguo Dang Dai Er Ke Za Zhi. 2013;15(8):686–688.
12. Bellani G, Laffey JG, Pham T, et al.; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016; 315(8):788–800. Erratum in: JAMA. 2016 Jul 19;316(3):350. Erratum in: JAMA. 2016 Jul 19;316(3):350. PMID: 26903337. doi:10.1001/jama.2016.0291
13. Taylor FB, Toh CH, Hoots WK, Wada H, Levi M. Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330. PMID: 11816725.
14. Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638–1652. PMID: 7587228. doi:10.1097/00003246-199510000-00007
15. Akram AR, Chalmers JD, Taylor JK, Rutherford J, Singanayagam A, Hill AT. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect. 2013;19(12):1174–1180. PMID: 23438068. doi:10.1111/1469-0691.12173
16. Marsh RA, Allen CE, McClain KL, et al. Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab. Pediatr Blood Cancer. 2013;60(1):101–109. PMID: 22522603; PMCID: PMC3410971. doi:10.1002/pbc.24188
17. Knaak C, Schuster FS, Spies C, et al. Hemophagocytic Lymphohistiocytosis in Critically Ill Patients. Shock. 2020;53(6):701–709. PMID: 31626037. doi:10.1097/SHK.0000000000001454
18. Valade S, Azoulay E, Galicier L, et al. Coagulation disorders and bleedings in critically ill patients with hemophagocytic lymphohistiocytosis. Medicine. 2015;94(40):e1692. PMID: 26448017; PMCID: PMC4616770. doi:10.1097/MD.0000000000001692
19. Han AR, Lee HR, Park BB, et al. Lymphoma-associated hemophagocytic syndrome: clinical features and treatment outcome. Ann Hematol. 2007;86(7):493–498. PMID: 17347847. doi:10.1007/s00277-007-0278-6
20. Eichenauer DA, Lachmann G, La Rosée P. Hemophagocytic lymphohistiocytosis in critically ill patients. Med Klin Intensivmed Notfmed. 2021;116(2):129–134.
21. Birndt S, Schenk T, Heinevetter B, et al. Hemophagocytic lymphohistiocytosis in adults: collaborative analysis of 137 cases of a nationwide German registry. J Cancer Res Clin Oncol. 2020;146(4):1065–1077. PMID: 32076823; PMCID: PMC7085479. doi:10.1007/s00432-020-03139-4
22. Herpich F, Rincon F. Management of Acute Ischemic Stroke. Crit Care Med. 2020;48(11):1654–1663. PMID: 32947473; PMCID: PMC7540624. doi:10.1097/CCM.0000000000004597
23. Kaya Z, Bay A, Albayrak M, Kocak U, Yenicesu I, Gursel T. Prognostic factors and long-term outcome in 52 Turkish children with hemophagocytic lymphohistiocytosis. Pediatr Crit Care Med. 2015;16(6):e165–e173. PMID: 25901543. doi:10.1097/PCC.0000000000000449
24. Delano MJ, Ward PA. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Invest. 2016;126(1):23–31. PMID: 26727230; PMCID: PMC4701539. doi:10.1172/JCI82224
25. Delano MJ, Ward PA. The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunol Rev. 2016;274(1):330–353. PMID: 27782333; PMCID: PMC5111634. doi:10.1111/imr.12499
26. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. PMID: 26903338; PMCID: PMC4968574. doi:10.1001/jama.2016.0287
27. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. PMID: 12700374. doi:10.1056/NEJMoa022139
28. Murata A, Okamoto K, Mayumi T, Muramatsu K, Matsuda S. The recent time trend of outcomes of disseminated intravascular coagulation in Japan: an observational study based on a national administrative database. J Thromb Thrombolysis. 2014;38(3):364–371. PMID: 24823684. doi:10.1007/s11239-014-1068-3
29. Chen JH, Fleming MD, Pinkus GS, et al. Pathology of the liver in familial hemophagocytic lymphohistiocytosis. Am J Surg Pathol. 2010;34(6):852–867. PMID: 20442642. doi:10.1097/PAS.0b013e3181dbbb17
30. Fukaya S, Yasuda S, Hashimoto T, et al. Clinical features of haemophagocytic syndrome in patients with systemic autoimmune diseases: analysis of 30 cases. Rheumatology. 2008;47(11):1686–1691. PMID: 18782855. doi:10.1093/rheumatology/ken342
31. Palazzi DL, McClain KL, Kaplan SL. Hemophagocytic syndrome in children: an important diagnostic consideration in fever of unknown origin. Clin Infect Dis. 2003;36(3):306–312. PMID: 12539072. doi:10.1086/345903
32. Ost A, Nilsson-Ardnor S, Henter JI. Autopsy findings in 27 children with haemophagocytic lymphohistiocytosis. Histopathology. 1998;32(4):310–316. PMID: 9602326. doi:10.1046/j.1365-2559.1998.00377.x
33. Lin M, Park S, Hayden A, et al. Clinical utility of soluble interleukin-2 receptor in hemophagocytic syndromes: a systematic scoping review. Ann Hematol. 2017;96(8):1241–1251. PMID: 28497365. doi:10.1007/s00277-017-2993-y
34. Li F, Yang Y, Jin F, et al. Clinical characteristics and prognostic factors of adult hemophagocytic syndrome patients: a retrospective study of increasing awareness of a disease from a single-center in China. Orphanet J Rare Dis. 2015;10:20. PMID: 25757854; PMCID: PMC4355377. doi:10.1186/s13023-015-0224-y
35. Al Nasrallah N, Al-Hader A, Samala N, Sears CR. Hemophagocytic lymphohistiocytosis in the medical ICU: a single-institution cohort study on acute liver failure and mortality. Crit Care Explor. 2021;3(1):e0318. PMID: 33458685; PMCID: PMC7803668. doi:10.1097/CCE.0000000000000318
36. Aoyagi T, Sato Y, Baba H, et al. Case report: successful treatment of five critically ill Coronavirus Disease 2019 patients using combination therapy with etoposide and corticosteroids. Front Med. 2021;8:718641. PMID: 34631741; PMCID: PMC8496678. doi:10.3389/fmed.2021.718641
37. Vigneron C, Le Stang V, Decroocq J, et al. Etoposide-containing regimens for the treatment of critically ill patients with hematological malignancy-related hemophagocytic lymphohistiocytosis. Acta Oncol. 2022;61(5):608–610. PMID: 35243961. doi:10.1080/0284186X.2022.2044517
38. Brito-Zerón P, Kostov B, Moral-Moral P, et al.; REGHEM-GEAS-SEMI Study Group. Prognostic factors of death in 151 adults with hemophagocytic syndrome: etiopathogenically driven analysis. Mayo Clin Proc Innov Qual Outcomes. 2018;2(3):267–276. PMID: 30225460; PMCID: PMC6132215. doi:10.1016/j.mayocpiqo.2018.06.006
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


