Back to Journals » Patient Preference and Adherence » Volume 16
Patient Preference Studies for Advanced Prostate Cancer Treatment Along the Medical Product Life Cycle: Systematic Literature Review
Authors Menges D , Piatti MC, Cerny T, Puhan MA
Received 17 February 2022
Accepted for publication 16 June 2022
Published 28 June 2022 Volume 2022:16 Pages 1539—1557
DOI https://doi.org/10.2147/PPA.S362802
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Johnny Chen
Dominik Menges,1 Michela C Piatti,1 Thomas Cerny,2,3 Milo A Puhan1
1Epidemiology, Biostatistics and Prevention Institute (EBPI), University of Zurich (UZH), Zurich, Switzerland; 2Foundation Board, Cancer Research Switzerland (Krebsforschung Schweiz KFS), Bern, Switzerland; 3Human Medicines Expert Committee (HMEC), Swissmedic, Bern, Switzerland
Correspondence: Dominik Menges, Epidemiology, Biostatistics and Prevention Institute (EBPI), University of Zurich (UZH), Hirschengraben 84, Zurich, CH-8001, Switzerland, Tel +41 44 634 46 15, Email [email protected]
Background: Patient preference studies can inform decision-making across all stages of the medical product life cycle (MPLC). The treatment landscape for advanced prostate cancer (APC) treatment has substantially changed in recent years. However, the most patient-relevant aspects of APC treatment remain unclear. This systematic review of patient preference studies in APC aimed to summarize the evidence on patient preferences and patient-relevant aspects of APC treatments, and to evaluate the potential contribution of existing studies to decision-making within the respective stages of the MPLC.
Methods: We searched MEDLINE and EMBASE for studies evaluating patient preferences related to APC treatment up to October 2020. Two reviewers independently performed screening, data extraction and quality assessment in duplicate. We descriptively summarized the findings and analyzed the studies regarding their contribution within the MPLC using an analytical framework.
Results: Seven quantitative preference studies were included. One study each was conducted in the marketing approval and the health technology assessment (HTA) and reimbursement stage, and five were conducted in the post-marketing stage of the MPLC. While almost all stated to inform clinical practice, the specific contributions to clinical decision-making remained unclear for almost all studies. Evaluated attributes related to benefits, harms, and other treatment-related aspects and their relative importance varied relevantly between studies. All studies were judged of high quality overall, but some methodological issues regarding sample selection and the definition of patient-relevant treatment attributes were identified.
Conclusion: The most patient-relevant aspects regarding the benefits and harms of APC treatment are not yet established, and it remains unclear which APC treatments are preferred by patients. Findings from this study highlight the importance of transparent reporting and discussion of study findings according to their aims and with respect to their stage within the MPLC. Future research may benefit from using the MPLC framework for analyzing or determining the aims and design of patient preference studies.
Keywords: patient preferences, medical product life cycle, preference research, benefit-harm assessment, patient-centered care, prostate cancer, systematic review
Introduction
Patient preferences are an essential component of patient-centered care1 and are rapidly gaining importance in the development and evaluation of novel medical products.2–8 The United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have both integrated the evaluation of the values and perspectives of patients in their approval processes.2–5 Large public–private partnerships such as the Medical Device Innovation Consortium (MDIC) and the Innovative Medicines Initiative Patient Preferences in Benefit Risk Assessments During the Drug Life Cycle (IMI PREFER) initiative are conducting methodological research on how patient preferences can be incorporated in the medical product life cycle (MPLC).6–8
Previous research has shown potential benefits of patient preference information not just for decision-making in clinical practice, but throughout all MPLC stages.3,6,9–12 In the discovery stage, patient preferences may aid the assessment of unmet medical needs3,6,9,10,12 and the design and selection of novel product prototypes.3,6,10–12 During pre-clinical and clinical development, preference information may be used in the design of clinical trials by defining patient-relevant outcomes and study populations,3,6,9–12 understanding important benefit-risk trade-offs,3,6,10 and exploring preference heterogeneity between patients.6,12 Patient preferences may support marketing authorization, health technology assessment (HTA) and reimbursement by complementing benefit-risk assessment6,9,10,12 and economic evaluation,10,12 as well as by informing value propositions and marketing strategies for industry.6,9,12 In the post-marketing stage, preference information may guide safety monitoring and post-authorization benefit-risk assessment,6,9–12 inform industry regarding market opportunities and further product development,6,9–12 and enhance clinical practice by informing practice guidelines and enabling more patient-centered decision-making.3,6,11 Various methods for eliciting patient preferences exist, which need to be carefully selected depending on the study aims and information required at the respective stage along the MPLC.6,13,14 To date, no study has explicitly investigated the design and the stated aims of existing preference studies to evaluate the extent to which they were suited to inform decision-making along the MPLC.
Patient preferences play an important role in clinical decision-making in advanced prostate cancer (APC).15–18 In recent years, this field has been significantly transformed by the development and approval of various novel treatments. To date, optimal treatment strategies have not been established and the balance of benefits and harms needs to be evaluated for each patient individually.15–18 Thus, there is a need for a better understanding about which aspects of treatment are most relevant for patients and warrant consideration when eliciting preferences regarding APC treatment. Given the latest developments in preference research and the recent market approval of several novel treatments, APC provides an ideal example to evaluate potential contributions of patient preference studies along the MPLC.
With this systematic literature review, we pursued two aims. First, we aimed to describe the design and findings of previously conducted patient preference studies in APC, focusing on the selection and definition of patient-relevant aspects of treatment. Second, we aimed to assess the potential contribution of these studies according to their stage along the MPLC and identify potential gaps for future research.
Methods
This systematic literature review is reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.19 The study was part of the first stage of the development of a preference study, which aims to elicit patient preferences for the later conduct of a benefit-harm assessment to inform clinical practice in the context of APC. A protocol for the full project including this systematic review was published on the Open Science Framework platform.20 The evaluation of the identified preference studies with respect to the MPLC was added during the conduct of the review, since we considered it an important emerging aspect that added substantially to the interpretation of the findings.
Eligibility Criteria
Eligible target decision contexts for APC were metastatic hormone-sensitive prostate cancer and non-metastatic or metastatic castration-resistant prostate cancer. We deemed studies eligible if they involved individuals from the general population, patients with localized prostate cancer, or APC patients. The rationale for including populations at risk of developing APC (ie, general population and prostate cancer patients with localized disease) was that it is currently unclear whether it is more appropriate to elicit preferences from patients with disease experience or populations that have not yet faced the relevant decision and its consequences.6,21,22 We considered studies eligible if they elicited patient preferences related to treatment outcomes in APC. Studies investigating patient preferences unrelated to treatment outcomes (eg, decision-making preferences), studies related to the treatment of localized prostate cancer, and studies exclusively involving clinical experts or other stakeholders were not considered eligible. Studies were eligible if they used methods allowing to elicit the relative importance and trade-offs between benefits, harms and other aspects of treatment made by patients (eg, discrete choice experiments, best-worst scaling exercises, time trade-off, visual analogue scales, and other approaches).
Information Sources and Search Strategy
We systematically searched MEDLINE (accessed via PubMed) and EMBASE (accessed via Elsevier) up to 5 October 2020 for relevant records using terms and medical subject headings (MeSH) related to patient preferences, benefit and risk assessment, and APC. The full search strategies are provided in Supplementary Tables S1 and S2. We restricted our search to the time since 1 January 2000, since we deemed records before this period to be likely of limited relevance given the more recent establishment of patient preference studies and elicitation approaches,6,13 as well as recent advances in the treatment of APC.15,16,23 We further restricted the search to records in English and German. We complemented the systematic search by screening included study reports and relevant related publications for additional records.
Screening and Data Extraction
We screened the de-duplicated records for eligibility based on their titles, abstracts and full text. For included studies, we extracted data regarding the target decision context (ie, disease stage or treatment context of interest), study population characteristics, preference elicitation methodology, supportive research conducted to inform the study design, evaluated aspects of treatment (ie, treatment attributes and attribute levels), main study findings, and study funding. Screening and data extraction for all studies was performed independently and in duplicate by two reviewers, with disagreements resolved by consensus.
Quality Assessment
We assessed the quality of included studies based on the International Society of Preference and Outcome Research (ISPOR) checklist.24 While this checklist has been developed as a guide for good research practices in conducting conjoint analyses, it has also previously been applied for the evaluation of patient preference studies.25–27 Other available tools28–30 did not cover all methodological items that were of interest for our study. We individually rated each of the 30 checklist items and separately report items that could not be assessed due to inconclusive or missing information. Two reviewers conducted quality assessment independently for all studies and resolved disagreements by consensus.
Analysis and Synthesis
We summarized the extracted data and descriptively analyzed the studies for differences and similarities in their characteristics, methodology, and quality. Furthermore, we assessed the selection and definition of treatment attributes and their relative importance within and across studies. Treatment attributes in preference studies may describe expected benefits, harms, mode of administration, costs, or other patient-relevant aspects of treatment.24,26,31 The selection of well-defined and patient-relevant attributes and attribute levels is an essential component in conducting quantitative preference studies.6,24,32 We categorized the identified attributes into benefit outcomes, harm outcomes and other aspects of treatment for analysis.
To evaluate the potential contributions of the identified preference studies to decision-making with respect to the MPLC stage during which they were conducted, we used an analytical framework based on previous work by the FDA,3 the MDIC,6,7 as well as the IMI PREFER initiative.9–12 We extracted key information on the potential uses of patient preference studies from the published reports and condensed the information according to the different stages of the MPLC. We additionally considered further potential contributions of patient preference studies to clinical practice, such as exploring preference sensitivity and heterogeneity,6 informing the creation of patient decision aids,3,6,33 informing guideline development,1 and conducting highly stratified benefit-harm assessment.34 We categorized the potential contributions along the MPLC into three categories: informing industry processes and product marketing, informing regulatory assessment and reimbursement, and informing clinical practice and patient-centered decision-making. The resulting analytical framework is presented in Figure 1.
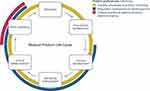 |
Figure 1 Stages of the medical product life cycle and processes that can be informed by patient preference information. Abbreviation: HTA, Health Technology Assessment. |
We then mapped the studies to their respective stage along the MPLC. Mapping was performed based on the studies’ stated aims, the context provided in the introduction and discussion, Phase III trials and clinical practice guidelines cited within the study reports, the attributes and attribute levels used in the studies, study dates, and study funders. We used this information to determine the specific APC treatment or treatment comparisons for which patient preferences were evaluated by the individual studies. Subsequently, we determined the most likely MPLC stage based on the contextual information. Finally, we sought to compare the different studies by identifying similarities and differences between preference studies conducted at similar stages or with similar aims by assessing their potential contributions according to the analytical framework.
Results
Study Selection and Characteristics
We identified 1140 records through database searches and 9 records through manual searches of reference lists (Figure 2). We screened 807 records for eligibility and included 7 studies with data from total 1357 participants in our analysis35–41 (a list of excluded records is provided in Supplementary Table S3). The characteristics of the included patient preference studies are summarized in Table 1. Two studies each were conducted in multiple European countries,35,36 the United States,38,40 and Japan,39,41 and one in the United Kingdom.37 The target decision context was metastatic hormone-sensitive prostate cancer (mHSPC)35,37,38 and metastatic castration-resistant prostate cancer (mCRPC)36,39,41 in three studies each, and non-metastatic castration-resistant prostate cancer (nmCRPC)40 in one study. The study populations covered a wide range, including APC patients in the respective target decision context,35,36,39–41 APC patients in different APC disease stages,35,36 patients with localized or locally advanced prostate cancer,35,38,39 men from the general public,37 caregivers (ie, partners, relatives or friends),40 and physicians.39 Sample sizes (median 200, range 65 to 292) and average age of participants (range 35 to 75 years) varied considerably between studies.
 |
Table 1 Study Characteristics and Results from Quality Assessment |
 |
Figure 2 Flow chart of the systematic literature search, screening and inclusion of patient preference studies in the systematic review. |
The design and primary findings of the included studies are shown in Table 2. All but one study applied a discrete choice experiment (DCE) to elicit patient preferences,35,36,38–41 while the other used a time trade-off approach in combination with a visual analogue scale.37 Exploratory research conducted to define patient-relevant attributes or health states included literature reviews,35–41 patient35,37,40 and expert35–37,40,41 interviews, focus groups,39 expert input,38 as well as additional information from product labels,36 regulatory reports,40 or clinical trials.41
 |  |  |
Table 2 Study Design, Attributes (Ranked by Relative Importance) and Attribute Levels of the Included Preference Studies |
Quality of Studies
Overall, we judged the quality of the studies as high. Studies fulfilled between 23 and 27 (median 25) out of 30 items of the ISPOR checklist (Table 1). Some information necessary to evaluate all checklist items was missing in all studies (number of non-reported items ranging from 2 to 4). All studies stated a well-defined rationale and provided adequate information on the decision-making context. The studies used an appropriate elicitation format, and their experimental design was generally well reported. The selection of attributes was described in adequate detail and supported by evidence in all studies. In contrast, the methods and rationale used to select and define attribute levels were less well described and considered insufficient in three studies.35,36,39 Three studies involved patients in exploratory research guiding the study design35,37,39 (Table 2). Furthermore, the specification or justification of the sample size and sampling strategy was insufficient in five studies,35,37–39,41 an examination or testing of respondent characteristics and subgroups was lacking in four studies,35,36,38,39 and an assessment of the quality of responses (eg, internal validity) was missing in five studies.35–37,39 Meanwhile, all studies presented and discussed their results and limitations appropriately with respect to the existing literature. Details of the quality assessment are provided in Supplementary Table S4.
Relation of Studies to Medical Product Life Cycle
The findings on study aims and contextual information of importance for evaluating the studies’ potential contributions along the MPLC are demonstrated in Table 3. The assessment of the temporal relation between study conduct and its stage in the MPLC was complicated due to missing information on study timeframes in all but one study.40 All studies were funded by a pharmaceutical company and six were co-authored by industry representatives.35–37,39–41 Furthermore, all studies discussed or cited clinical phase III trial results or clinical practice guidelines corresponding to a drug marketed by the study funder in the respective context. Based on study reports and contextual information, we categorized one study to have been conducted in the marketing authorization stage,40 one in the HTA and reimbursement stage,37 and five in the post-marketing stage of the MPLC.35,36,38,39,41
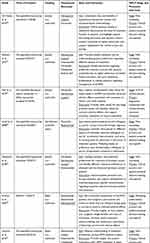 |
Table 3 Study Aims, Evaluated Treatments and Potential Contributions to Decision-Making Along the Medical Product Life Cycle (MPLC) Stages |
Various potential contributions along the MPLC were discussed in the aims or discussion of the studies. The study conducted in the HTA and reimbursement stage explicitly stated the aim of informing regulatory assessment and reimbursement through deriving dis-/utility values for economic evaluation.37 Meanwhile, the other six studies stated to aim at informing clinical practice and patient-centered decision-making, for example by enabling a discussion of preferences between patients and physicians,35,36,38,39,41 by examining differences in preferences between patients and physicians39 or caregivers,40 or by quantifying the value placed by patients on specific treatment attributes.35,38,40 One study additionally mentioned potential uses of patient preference information during marketing authorization and reimbursement negotiations,35 and another calculated the willingness to pay for treatments based on patients’ preferences.38 However, none of the studies aiming to inform clinical practice explicitly evaluated preference heterogeneity within its decision context, and we deemed it insufficiently clear based on the stated aims as to how the six studies intended to influence clinical practice in reality. Therefore, we categorized the contribution of these six studies to clinical practice as non-specific.
Study Attributes and Their Relative Importance
Among the six studies using a DCE methodology, four defined the attributes describing the benefits of treatment using directly patient-relevant outcomes,42 such as overall survival38,40,41 and health-related quality of life39 (Table 2, Figure 3). Three studies used pain control,35,36,41 and a majority of five studies used surrogate endpoints for defining benefits of treatment, such as progression-free survival (defined as time to disease progression or effect on keeping disease stable),35,39 time to chemotherapy,36 time to symptomatic skeletal event,41 or time to pain progression.40 Surrogate outcomes and pain control ranked among the three most important attributes most frequently (three studies each), followed by overall survival (two out of three studies) and quality of life (one study). In all studies including patients with localized prostate cancer, a survival or progression-free survival outcome ranked highest of all treatment attributes,35,38,39 while harms and pain control were rated as most important in the other DCE studies.36,40,41
For defining potential harms of treatment, the DCE studies used a wide range of different attributes depending on the decision context and specific treatment of interest. Most frequently used harm attributes were fatigue,35,36,40,41 nausea, vomiting or diarrhea,35,38 and cognitive disorder.36,40 One study used a more generic “side effects” attribute.39 Of note, all but one DCE study defined the harm attributes by using different levels of risk of experiencing a certain harm outcome (eg, 5% or 10% risk of fatigue).35,36,38,39,41 One study additionally used severity levels for defining harm outcomes (eg, mild or moderate fatigue).40 Cognitive or memory disorder ranked among the three most important attributes most frequently (two studies), with fatigue, nausea/vomiting or diarrhea, hematuria, fractures, and falls each ranking among the top three in one study. No harm outcome with the exception of (generic) “side effects” ranked last in any of the DCE studies.
All DCE studies included further attributes unrelated to treatment outcomes, such as mode of administration,35,38,39,41 need of co-medication,36 drug interactions,36 food restrictions,36 lost work days,41 or out-of-pocket costs.38 Among these, mode of administration ranked among the three most important attributes in two studies, but also ranked last in one study. Similarly, out-of-pocket costs, lost work days, and food restrictions ranked last in the respective studies. Attributes regarding mode of administration and need of co-medication commonly reflected the treatments targeted by the studies (eg, intravenous administration of docetaxel, cabazitaxel and Radium-223 compared with oral administration of abiraterone acetate, darolutamide or bicalutamide; Table 3).
The study which applied a combination of a time trade-off approach and a visual analogue scale used three defined base health states corresponding to newly diagnosed patients, patients receiving chemotherapy, and patients post-chemotherapy.37 In addition, five combinations of the health state of patients receiving chemotherapy with different adverse effect experiences (fatigue, nausea and vomiting, diarrhea, fluid retention, susceptibility to infection, and alopecia) were evaluated. In this study, the base health state of patients receiving chemotherapy was less preferred by participants than the base health state of newly diagnosed patients and patients post-chemotherapy. Among the adverse effects, nausea and vomiting, diarrhea, and susceptibility to infection were rated as the most important (ie, having the highest disutility), while alopecia ranked last in importance (ie, had the smallest disutility).
The two studies conducted in the marketing authorization and in the HTA and reimbursement stage differed from the ones conducted in the post-marketing stage of the MPLC. The study conducted in the marketing authorization stage was the only one in which a benefit attribute ranked last and which found all four harm attributes to be more important than the two benefit outcomes.40 This study was also the only one in the target decision context of non-metastatic castration-resistant prostate cancer and defined benefits as time to pain progression and months of additional survival beyond 4 years. Meanwhile, the study conducted in the HTA and reimbursement stage took a health state valuation approach and was the only one using a general population sample, which was in line with its aim of deriving dis-/utility values for economic evaluation.37
Discussion
In this systematic review of seven patient preference studies related to the treatment of APC, we found substantial variation in the definition and the relative importance of patient-relevant benefits, harms, and other aspects of APC treatment across studies. The identified studies explored patient preferences in all relevant decision-contexts of APC treatment. We considered five of the included preference studies to be located in the post-marketing stage of the MPLC, and one study each in the marketing approval and the HTA and reimbursement stage. All but one study were conducted in the past five years, reflecting the recent advances in APC treatment.
Study Contributions Along the Medical Product Life Cycle
One study located in the HTA and reimbursement stage explicitly aimed to inform economic evaluation, while all other studies stated to aim at informing clinical practice and patient-centered decision-making. Among these studies, none provided further detail about how it had intended to inform clinical decision-making. In the post-marketing setting, patient preference studies may influence clinical practice in various ways, such as by examining the preference sensitivity of a specific context,6 exploring preference heterogeneity between patients,6 informing shared decision-making tools such as patient decision aids,3,6,33 informing guideline development,1 or enabling highly stratified patient-centered benefit-harm assessment.34 We found none of the studies to be specifically designed to inform these processes. Studies frequently stated the aims of highlighting attributes of importance to patients and facilitating discussions regarding preferred treatments between patients and physicians. Meanwhile, none of the studies provided information on preference heterogeneity.
For information from patient preference studies to be useful in clinical practice, an exploration of preference heterogeneity between patients is warranted.6 While the most important aspects of treatment (ie, the most or least preferred attributes) are important to discuss in clinical practice, attributes for which there is the largest between-patient variation may be most relevant for patient-centered decision-making. Having information on the relative importance of benefits and harms may help to determine the benefit-harm balance of different treatments. Meanwhile, personalized discussions and treatment decisions – as opposed to making generalized recommendations for the whole population – may be most useful when based on aspects that are valued differently by individual patients. Furthermore, attributes that are most distinctive between different treatment options may matter more in clinical practice than those that are similar. For example, if all treatment options cause fatigue as an adverse effect and to a similar extent (ie, similar risk or severity), other adverse effects may be more relevant for determining patient preferences for the different treatment options. Last, most studies used risk levels for defining harm attributes, with only one using severity levels for certain harms.40 In the design of DCEs, risk levels combine expectations about both the severity and risk of a harm outcome.43 However, since harm risks are usually largely known in the post-marketing stage, preferences for different levels of severity may be more informative for assessing the benefit-harm balance for individual patients.
All identified studies were funded by the pharmaceutical industry. Patient preference studies may have an important role in informing marketing strategies and information material, extension of product labels or indications, or future product development.3,6,10–12 Yet, potential applications of patient preference studies to inform industry processes and product marketing were not mentioned in almost all studies. Only one study stated that preference studies may support submission of application dossiers and negotiations with health authorities during regulatory approval.35 Since the conduct of patient preference studies is costly and time-consuming, funding for such research may be difficult to obtain.10 It appears that to date, the strongest interest in conducting preference studies in APC came from the pharmaceutical industry. Potential conflicts of interest arising from this role highlight the need for transparent reporting of the exploratory research conducted, the justifications for choosing attributes, attribute levels and sampling strategies, as well as the aims regarding which processes along the MPLC should be informed by the study.
Quality of Studies and Attribute Selection
While we judged the quality of studies to be high overall, we also identified some shortcomings. One observed issue was related to the justification of the chosen sampling strategy and sample sizes, which were not well specified in several studies. The choice of the study population is considered a key factor in the design of patient preference studies and may influence the interpretability and transferability of study findings.6,9,10 It is thus important to assess the representativeness of the study population with respect to the target population and to evaluate potential differences between population subgroups (eg, patients at different disease stages), as well as between responders and non-responders.6,10,26,28 Several studies lacked an examination of respondent characteristics and subgroups, and an evaluation of characteristics of responders and non-responders was not possible based on the presented data. In combination, these issues may impair the interpretation of the respective studies. This is especially relevant when translating the study findings into clinical practice, as substantial heterogeneity in preferences between patients at different disease stages or even individual patients may have to be expected.6
We found a relevant variation in the treatment attributes used in the studies. All studies selected attributes based on exploratory research and provided an adequate rationale for their selection. However, only little or no detail was provided regarding the selection of attribute levels. In addition, only three studies (43%) involved patients in the attribute selection process,35,37,39 which we identified as a potentially important methodological issue in the quality assessment. This shortcoming was also identified in other studies.26 The translation of the exploratory research into the definition of attributes and attribute levels is an integral part of the design of quantitative preference studies and always bears some degree of subjectivity of the involved researchers.10,24,32 The choice and framing of such attributes and attribute levels may relevantly influence the findings of a study, which has implications for their applicability and translation into the relevant decision-making context.6,10,44 It is thus crucial that all aspects of attribute and attribute level selection are transparently reported24 and related to the stated aims of the study within the corresponding stage along the MPLC.
While some differences regarding the selection of attributes between studies were expected depending on the studies’ aims, decision contexts, or treatments of interest, only few attributes were used relatively consistently. To capture preferences regarding treatment benefit, DCE studies most frequently used surrogate outcomes and pain control, with survival and health-related quality of life being used less frequently. Surrogate outcomes and pain control also most frequently ranked among the three most important attributes in the respective studies. This is especially interesting since survival and quality of life are commonly considered the most patient-relevant outcomes in advanced cancer settings.45,46 Meanwhile, pain due to bone metastases is the most frequent symptom of APC.47 This may explain the relative importance of pain reduction in the DCE studies evaluating this attribute. Definitions of surrogate outcomes were highly heterogenous across studies, and none was used in more than one study.
Regarding treatment harms, fatigue was the only outcome used in more than two DCE studies. It is, however, expected that harms would differ more strongly across contexts due to differences in adverse effect profiles of the respective treatments and target populations (eg, regarding age and (co-)morbidity). We found that mode of administration was a frequently used and important attribute, most especially in contexts where there were substantial differences in the administration of the discussed treatments.35,38,39,41 Meanwhile, treatment cost (defined as monthly out-of-pocket costs to the patient regardless of insurance coverage in a United States health-care setting) was used as an attribute in only one study among patients with localized prostate cancer, ranking last in importance.38 The latter is surprising, as out-of-pocket costs may be relevant given the price of novel APC treatments, depending on insurance coverage in the countries of study conduct.26
Our findings are similar to those of another recent systematic review of patient preference studies in metastatic prostate cancer,48 which found treatment benefits – expressed as treatment effectiveness and bone pain control – and fatigue to be the most frequently used and most important attributes. In comparison, the inclusion of additional quantitative preference studies in our review revealed more substantial heterogeneity in the definitions and relative importance of benefit and harm attributes related to APC treatment. Thus, we currently consider the evidence to be insufficient to allow judgements about what the most patient-relevant aspects or the preferred treatments are in APC.
We identified differences in the primary study findings between studies conducted in the post-marketing stage compared to the study conducted in the marketing authorization stage, in which treatment benefits were found to be less important than the harm outcomes.40 However, these differences may also arise due to the different target decision-context or study population. Pain progression (eg, due to bone metastases) may not have been considered relevant by participants in the non-metastatic setting, and expected survival in this setting is longer than in later disease stages (which is also reflected in the definition of the survival attributes). Meanwhile, pain reduction was commonly rated as highly important in studies investigating metastatic prostate cancer.35,36,41 Hence, it remains unclear to what extent these different factors influenced the design and primary findings of this study.
Directions for Future Research
Based on our systematic review, we identified several gaps to be addressed by future research. First, it is currently unclear which treatment attributes are most appropriate to be used in patient preference studies in APC and whether there is substantial preference heterogeneity between individual patients in this context. Future research should further explore key attributes that are both important for patients and relevant for decision-making in clinical practice. Second, the included preference studies allowed only a limited exploration of potential contributions of such studies along the MPLC. Further insights from studies conducted at different stages of the MPLC, with different perspectives or aims, and targeting other disease contexts should be gathered in future studies. Third, using the MPLC as a framework may be helpful for clarifying the research questions and aims of future preference studies. Preference researchers may use the MPLC framework to plan studies aiming to inform clinical practice and patient-centered decision-making, industry processes and product marketing, or regulatory assessment and reimbursement.
Limitations
The systematic review was focused on APC as an example for an innovative field and is thus limited in its scope. While we aimed at exploring the potential contribution of preference studies along the MPLC, most studies were conducted in the post-marketing stage and stated to inform clinical practice and patient-centered decision-making. By widening the topic to other treatment contexts or disease areas, we may have identified further preference studies conducted in other MPLC stages or with different aims. Considering further databases or different search strategies may have yielded additional studies providing further insights.49 However, based on other systematic reviews of preference studies in advanced cancer settings,26,50 we deem our study to provide a representative example of studies in this context.
The quality assessment in this review was based on the ISPOR checklist. While this allowed a comprehensive evaluation of the studies, the checklist was not originally intended for such assessments. Thus, it may miss important aspects of study design and does not include a thorough evaluation of the potential risk of bias of studies. Other tools are available to assess the quality,26,28 risk of bias,29 and certainty of evidence30 in preference studies. However, a standard for assessment has not yet been established and there is a relevant overlap between available checklists. We thus chose a methodology that is most comparable to existing research.25–27 Since we were not interested in specific estimates or the certainty of the available evidence, we deemed the checklist to sufficiently cover all dimensions of relevance to this study. More research is needed to establish a standardized assessment covering all relevant dimensions of methodological and reporting quality, as well as the risk of bias of preference studies.
To assess the stages and potential contributions of studies along the MPLC, we conducted a synthesis of existing research which we used as a basic framework for analysis.3,6,9–12 However, the incorporation of patient preference information along the MPLC is a recent development that requires more methodological research and experiences. Thus, we consider the applied framework to be a starting point for discussion which warrants further development and more detailed examination. Meanwhile, we found it useful for categorizing the studies and enabling a discussion about what would constitute useful evidence in the post-marketing setting. We hope that other authors are encouraged by our work to assess preference studies in light of their stage along the MPLC to determine their potential contributions and value for industry, regulatory or clinical decision-making.
Conclusion
In this systematic review of patient preference studies in APC, we found that studies used a wide variety of different attributes for defining benefits and harms of treatment. While the quality of studies was high overall, we identified issues with respect to sample selection and definition of attribute levels. All studies were industry-funded, and most were conducted in the post-marketing stage of the MPLC. All but one study stated the aim to inform patient-centered decision-making, but the specific contributions to clinical practice remained unclear. Hence, no judgements regarding the most patient-relevant aspects of APC treatment or preferred APC treatments are currently possible and further research aimed at informing clinical practice in this context is warranted. As this review is one of the first to apply the MPLC framework in the analysis of preference studies in a specific decision-making context, future research may further explore and refine this framework as an analytical tool in other contexts. In addition, an explicit consideration of the MPLC may also help to determine the aims and design of future preference studies.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article and its Supplementary Information Files.
Ethics Approval
As a systematic literature review relying on aggregate information from published studies, this research project did not require ethical approval under the Swiss Human Research Act.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the Swiss Cancer Research Foundation (Stiftung Krebsforschung Schweiz, Bern, Switzerland; Health Services Research Grant No. HSR-4950-11-2019) and the Swiss Cancer Foundation (Zug, Switzerland). The salary of Dominik Menges was covered by a fellowship by the Béatrice Ederer-Weber Foundation (Zurich, Switzerland). The study funders had no role in the study design, data collection, analysis, interpretation, decision to publish, or writing of this report.
Disclosure
The authors declare that they have no conflicts of interest related to this work. The article consists of an extended analysis based on the research conducted as part of the doctoral thesis of Michela C Piatti.
References
1. Montori VM, Brito JP, Murad MH. The optimal practice of evidence-based medicine: incorporating patient preferences in practice guidelines. JAMA. 2013;310(23):2503–2504. doi:10.1001/jama.2013.281422
2. US Food & Drug Administration (FDA). Patient-focused drug development guidance series for enhancing the incorporation of the patient’s voice in medical product development and regulatory decision making; 2020. Available from: https://www.fda.gov/media/139088/download.
3. US Food & Drug Administration (FDA). Patient preference information - voluntary submission, review in premarket approval applications, humanitarian device exemption applications, and de novo requests, and inclusion in decision summaries and device labeling: guidance for industry, food and drug administration staff, and other stakeholders; 2016. Available from: https://www.fda.gov/media/92593/download.
4. European Medicines Agency (EMA). The patient’s voice in the evaluation of medicines; 2013. Available from: https://www.ema.europa.eu/en/documents/report/report-workshop-patients-voice-evaluation-medicines_en.pdf.
5. Mühlbacher AC, Juhnke C, Beyer AR, Garner S. Patient-focused benefit-risk analysis to inform regulatory decisions: the European Union perspective. Value Health. 2016;19(6):734–740. doi:10.1016/j.jval.2016.04.006
6. Medical Device Innovation Consortium (MDIC). Patient centered benefit-risk project report: a framework for incorporating information on patient preferences regarding benefit and risk into regulatory assessments of new medical technology; 2015. Available from: https://www.fda.gov/media/95591/download.
7. Ho M, Saha A, McCleary KK, et al. A framework for incorporating patient preferences regarding benefits and risks into regulatory assessment of medical technologies. Value Health. 2016;19(6):746–750. doi:10.1016/j.jval.2016.02.019
8. de Bekker-Grob EW, Berlin C, Levitan B, et al. Giving patients’ preferences a voice in medical treatment life cycle: the PREFER public–private project. Patient. 2017;10(3):263–266. doi:10.1007/s40271-017-0222-3
9. van Overbeeke E, Janssens R, Whichello C, et al. Design, conduct, and use of patient preference studies in the medical product life cycle: a multi-method study. Front Pharmacol. 2019;10:10. doi:10.3389/fphar.2019.01395
10. van Overbeeke E, Whichello C, Janssens R, et al. Factors and situations influencing the value of patient preference studies along the medical product lifecycle: a literature review. Drug Discov Today. 2019;24(1):57–68. doi:10.1016/j.drudis.2018.09.015
11. Whichello C, Bywall KS, Mauer J, et al. An overview of critical decision-points in the medical product lifecycle: where to include patient preference information in the decision-making process? Health Policy. 2020;124(12):1325–1332. doi:10.1016/j.healthpol.2020.07.007
12. Janssens R, Huys I, van Overbeeke E, et al. Opportunities and challenges for the inclusion of patient preferences in the medical product life cycle: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):189. doi:10.1186/s12911-019-0875-z
13. Soekhai V, Whichello C, Levitan B, et al. Methods for exploring and eliciting patient preferences in the medical product lifecycle: a literature review. Drug Discov Today. 2019;24(7):1324–1331. doi:10.1016/j.drudis.2019.05.001
14. Whichello C, Levitan B, Juhaeri J, et al. Appraising patient preference methods for decision-making in the medical product lifecycle: an empirical comparison. BMC Med Inform Decis Mak. 2020;20(1):114. doi:10.1186/s12911-020-01142-w
15. Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: report of the Advanced Prostate Cancer Consensus Conference (APCCC) 2019. Eur Urol. 2020;77(4):508–547. doi:10.1016/j.eururo.2020.01.012
16. Parker C, Castro E, Fizazi K, et al. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(9):1119–1134. doi:10.1016/j.annonc.2020.06.011
17. Lowrance WT, Breau Rodney H, Roger C, et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART I. J Urol. 2021;205(1):14–21. doi:10.1097/JU.0000000000001375
18. Lowrance WT, Breau Rodney H, Roger C, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline PART II. J Urol. 2021;205(1):22–29. doi:10.1097/JU.0000000000001376
19. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
20. Menges D, Braun J, Piatti MC, Puhan M. Patient preferences regarding benefits and harms of advanced prostate cancer treatments in Switzerland. OSF Res Protocol. 2021. doi:10.17605/OSF.IO/UN682
21. Najafzadeh M, Schneeweiss S, Choudhry NK, Avorn J, Gagne JJ. General population vs. patient preferences in anticoagulant therapy: a discrete choice experiment. Patient. 2019;12(2):235–246. doi:10.1007/s40271-018-0329-1
22. Goodwin E, Green C, Hawton A. What difference does it make? A comparison of health state preferences elicited from the general population and from people with multiple sclerosis. Value Health. 2019. doi:10.1016/j.jval.2019.08.009
23. Weiner AB, Nettey OS, Morgans AK. Management of Metastatic Hormone-Sensitive Prostate Cancer (mHSPC): an evolving treatment paradigm. Curr Treat Options Oncol. 2019;20(9):69. doi:10.1007/s11864-019-0668-8
24. Bridges JFP, Hauber AB, Marshall D, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–413. doi:10.1016/j.jval.2010.11.013
25. Wortley S, Wong G, Kieu A, Howard K. Assessing stated preferences for colorectal cancer screening: a critical systematic review of discrete choice experiments. Patient. 2014;7(3):271–282. doi:10.1007/s40271-014-0054-3
26. Bien DR, Danner M, Vennedey V, Civello D, Evers SM, Hiligsmann M. Patients’ preferences for outcome, process and cost attributes in cancer treatment: a systematic review of discrete choice experiments. Patient. 2017;10(5):553–565. doi:10.1007/s40271-017-0235-y
27. Webb EJD, Meads D, Eskyte I, et al. A systematic review of discrete-choice experiments and conjoint analysis studies in people with multiple sclerosis. Patient. 2018;11(4):391–402. doi:10.1007/s40271-017-0296-y
28. Joy SM, Little E, Maruthur NM, Purnell TS, Bridges JFP. Patient preferences for the treatment of type 2 diabetes: a scoping review. PharmacoEconomics. 2013;31(10):877–892. doi:10.1007/s40273-013-0089-7
29. Yepes-Nuñez JJ, Zhang Y, Xie F, et al. Forty-two systematic reviews generated 23 items for assessing the risk of bias in values and preferences’ studies. J Clin Epidemiol. 2017;85:21–31. doi:10.1016/j.jclinepi.2017.04.019
30. Zhang Y, Alonso-Coello P, Guyatt GH, et al. GRADE guidelines: 19. Assessing the certainty of evidence in the importance of outcomes or values and preferences - risk of bias and indirectness. J Clin Epidemiol. 2019;111:94–104. doi:10.1016/j.jclinepi.2018.01.013
31. Clark MD, Determann D, Petrou S, Moro D, de Bekker-Grob EW. Discrete choice experiments in health economics: a review of the literature. PharmacoEconomics. 2014;32(9):883–902. doi:10.1007/s40273-014-0170-x
32. Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making - a user’s guide. PharmacoEconomics. 2008;26(8):661–677. doi:10.2165/00019053-200826080-00004
33. O’Connor AM, Légaré F, Stacey D. Risk communication in practice: the contribution of decision aids. BMJ. 2003;327(7417):736–740. doi:10.1136/bmj.327.7417.736
34. Aschmann HE, Boyd CM, Robbins CW, et al. Informing patient-centered care through stakeholder engagement and highly stratified quantitative benefit-harm assessments. Value Health. 2020;23(5):616–624. doi:10.1016/j.jval.2019.11.007
35. de Freitas HM, Ito T, Hadi M, et al. Patient preferences for metastatic hormone-sensitive prostate cancer treatments: a discrete choice experiment among men in Three European Countries. Adv Ther. 2019;36(2):318–332. doi:10.1007/s12325-018-0861-3
36. Eliasson L, de Freitas HM, Dearden L, Calimlim B, Lloyd AJ. Patients’ preferences for the treatment of metastatic castrate-resistant prostate cancer: a discrete choice experiment. Clin Ther. 2017;39(4):723–737. doi:10.1016/j.clinthera.2017.02.009
37. Hall F, de Freitas HM, Kerr C, et al. Estimating utilities/disutilities for high-risk metastatic hormone-sensitive prostate cancer (mHSPC) and treatment-related adverse events. Qual Life Res. 2019;28(5):1191–1199. doi:10.1007/s11136-019-02117-9
38. Lloyd A, Penson D, Dewilde S, Kleinman L. Eliciting patient preferences for hormonal therapy options in the treatment of metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2008;11(2):153–159. doi:10.1038/sj.pcan.4500992
39. Nakayama M, Kobayashi H, Okazaki M, Imanaka K, Yoshizawa K, Mahlich J. Patient preferences and urologist judgments on prostate cancer therapy in Japan. Am J Mens Health. 2018;12(4):1094–1101. doi:10.1177/1557988318776123
40. Srinivas S, Mohamed AF, Appukkuttan S, et al. Patient and caregiver benefit‐risk preferences for nonmetastatic castration‐resistant prostate cancer treatment. Cancer Med. 2020;9(18):6586–6596. doi:10.1002/cam4.3321
41. Uemura H, Matsubara N, Kimura G, et al. Patient preferences for treatment of castration-resistant prostate cancer in Japan: a discrete-choice experiment. BMC Urol. 2016;16(1):63. doi:10.1186/s12894-016-0182-2
42. Booth CM, Eisenhauer EA. Progression-free survival: meaningful or simply measurable? J Clin Oncol. 2012;30(10):1030–1033. doi:10.1200/JCO.2011.38.7571
43. Mühlbacher AC, Bethge S, Sadler A. Compound attributes for side effect in discrete choice experiments: risk or severity - what is more important to Hepatitis C patients? Value Health. 2015;18(7):A629–A630. doi:10.1016/j.jval.2015.09.2223
44. Veldwijk J, Essers BAB, Lambooij MS, Dirksen CD, Smit HA, de Wit GA. Survival or mortality: does risk attribute framing influence decision-making behavior in a discrete choice experiment? Value Health. 2016;19(2):202–209. doi:10.1016/j.jval.2015.11.004
45. European Medicines Agency (EMA). Evaluation of anticancer medicinal products in man - revision 5; 2017. Available from: https://www.ema.europa.eu/en/evaluation-anticancer-medicinal-products-man.
46. Prasad V, Kim C, Burotto M, Vandross A. The strength of association between surrogate end points and survival in oncology: a systematic review of trial-level meta-analyses. JAMA Intern Med. 2015;175(8):1389–1398. doi:10.1001/jamainternmed.2015.2829
47. Smith JA, Soloway MS, Young MJ. Complications of advanced prostate cancer. Urology. 1999;54(6, Supplement 1):8–14. doi:10.1016/S0090-4295(99)00448-3
48. Connor MJ, Genie MG, Burns D, et al. A systematic review of patients’ values, preferences, and expectations for the treatment of metastatic prostate cancer. Eur Urol Open Sci. 2021;36:9–18. doi:10.1016/j.euros.2021.10.003
49. Yu T, Enkh-Amgalan N, Zorigt G. Methods to perform systematic reviews of patient preferences: a literature survey. BMC Med Res Methodol. 2017;17(1):166. doi:10.1186/s12874-017-0448-8
50. Blinman P, Alam M, Duric V, McLachlan SA, Stockler MR. Patients’ preferences for chemotherapy in non-small-cell lung cancer: a systematic review. Lung Cancer. 2010;69(2):141–147. doi:10.1016/j.lungcan.2010.05.001
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

