Back to Journals » Journal of Pain Research » Volume 17
Patient-Controlled Subcutaneous Analgesia with Hydromorphone versus Oral Oxycontin for Opioid Titration of Cancer Pain: A Prospective Multicenter Randomized Trial
Authors Xiao X, Sun J, Zhang D, Li L, Zhou H, Li Y, Li Q, He Z, Fu Y, Duan Q, Zheng G, Tang Z, Chu Q, Chen Y
Received 11 December 2023
Accepted for publication 4 April 2024
Published 12 April 2024 Volume 2024:17 Pages 1441—1451
DOI https://doi.org/10.2147/JPR.S451698
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amitabh Gulati
Xiaoguang Xiao,1 Jianhai Sun,2 Dongsheng Zhang,3 Linjun Li,4 Haibo Zhou,5 Yongjun Li,6 Quan Li,7 Zhongshi He,8 Yang Fu,9 Qiwen Duan,10 Guping Zheng,11 Ze Tang,12 Qian Chu,1,* Yuan Chen1,*
1Department of Oncology, Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology, Wuhan, Hubei, People’s Republic of China; 2Department of Oncology, Hubei Zhongshan Hospital, Wuhan, Hubei, People’s Republic of China; 3Department of Oncology, Suizhou Hospital, Hubei University of Medicine, Suizhou, Hubei, People’s Republic of China; 4Department of Oncology, Hubei Provincial Hospital of Integrated Chinese and Western Medicine, Wuhan, Hubei, People’s Republic of China; 5Department of Oncology, Yichang Central People’s Hospital, Yichang, Hubei, People’s Republic of China; 6Department of Oncology, Yichang Second People’s Hospital, Yichang, Hubei, People’s Republic of China; 7Department of Oncology, Xiangyang Central Hospital, Xiangyang, Hubei, People’s Republic of China; 8Department of Oncology, Xiangyang No 1 People’s Hospital Affiliated to Hubei University of Medicine, Xiangyang, Hubei, People’s Republic of China; 9Department of Oncology, Xiangyang Hospital of Traditional Chinese Medicine, Xiangyang, Hubei, People’s Republic of China; 10Department of Oncology, Taihe Hospital, Shiyan, Hubei, People’s Republic of China; 11Department of Oncology, Xiaogan Central Hospital, Xiaogan, Hubei, People’s Republic of China; 12Department of Oncology, Huangshi Central Hospital, Huangshi, Hubei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qian Chu; Yuan Chen, Email [email protected]; [email protected]
Background: Studies have shown that oral oxycontin tablets can be used for opioid titration. The European Society for Medical Oncology (ESMO) guidelines for adult cancer pain recommend opioid titration through the parenteral route, usually the intravenous or subcutaneous route. Patient-controlled subcutaneous analgesia (PCSA) with hydromorphone needs further evaluation for opioid titration. This prospective multicenter study was designed to compare the efficacy and safety of hydromorphone PCSA with oral oxycontin tablets for opioid titration of cancer pain.
Patients and Methods: Eligible patients with cancer pain were randomly assigned in a 1:1 ratio to the PCSA group or the oxycontin group for dose titration. Different titration methods were given in both groups depending on whether the patient had an opioid tolerance. The primary endpoint of this study was time to successful titration (TST).
Results: A total of 256 patients completed this study. The PCSA group had a significantly lower TST compared with the oxycontin group (median [95% confidence interval (CI)], 5.5[95% CI:2.5– 11.5] hours vs.16.0 [95% CI:11.5– 22.5] hours; p< 0.001). The frequency (median; interquartile) of breakthrough pain (Btp) over 24 hours was significantly lower in the PCSA group (2.5;2.0– 3.5) than in the oxycontin group.(3.0; 2.5– 4.5) (p=0.04). The pain was evaluated by numeric rating scale (NRS) score at 12 hours after the start of titration. The pain score (median; interquartile) was significantly lower in the PCSA versus the oxycontin group (2.5;1.5– 3.0) vs 4.5;3.0– 6.0) (p=0.02). The equivalent dose of oral morphine (EDOM) for a successful titration was similar in both groups (p=0.29), but there was a significant improvement in quality of life (QoL) in both groups (p=0.03). No between-group difference in the incidence of opioid-related adverse effects was observed (p=0.32).
Conclusion: Compared with oral oxycontin tablet, the use of PCSA with hydromorphone achieved a shorter titration duration for patients with cancer pain (p< 0.001), without significantly increasing adverse events (p=0.32).
Keywords: cancer pain, opioid titration, oxycontin tablets, hydromorphone, patient-controlled subcutaneous analgesia
Introduction
Pain is the most common clinical symptom in patients with cancer.1 Cancer-related pain is the most important factor affecting cancer patients’ quality of life.2 However, effective and fast-onset treatment for cancer pain is still lacking.3–5 Determining the ideal therapeutic opioid dose is the key to successfully controlling cancer pain. The purpose of dose titration is to provide quick and satisfactory pain control, to determine a reasonable therapeutic dose timely, and to avoid side effects due to a high concentration or compromised effects due to a low concentration. According to the European Association for Palliative Care (EAPC) guidelines, the oral immediate-release (IR) and sustained-release (SR) formulations of morphine, oxycontin, and hydromorphone can be used for titration.6 Regarding scheduling and titration, the European Society for Medical Oncology (ESMO) suggested that opioid doses be titrated to take effect as quickly as possible.7
Due to the rapid metabolism of IR morphine and its short efficacy duration, it must be administered repeatedly with evaluation for at least 24 hours when used in patients with moderate to severe cancer pain.8 Thus, that reduces patient compliance and increases the medical staff’s workload.8 Some prospective and retrospective studies have shown that oxycontin tablets can be used for rapid pain titration.9–12In addition, it has been reported that patients treated with hydromorphone have better pain management satisfaction than those treated with morphine.13,14 The ESMO guidelines for adult cancer pain recommend that patients with severe pain should be titrated with opioids for rapid pain relief through a parenteral route, usually an intravenous or subcutaneous route.7
nRotation of opioids, the addition of adjuvant analgesics, and the change of administration route are common methods for treating refractory cancer pain. Patient-controlled analgesia (PCA) technology can be used to titrate hydromorphone.2 Patient-controlled subcutaneous analgesia (PCSA) is used to administer drugs into subcutaneous tissues to achieve the same analgesic effects as intramuscular and intravenous administration. During PCSA, relatively simple monitoring, management, and nursing are needed, and it is associated with few complications, good patient compliance, high safety, and low medical costs.15 Thus, it is very suitable for patients during hospitalization and home application. Other advantages of PCSA over oral and intravenous administration included more comfort and better quality of life for patients.15 However, there is no report on the efficacy and safety of PCSA with hydromorphone for treating moderate to severe cancer pain.
Based on these, we designed this prospective multicenter clinical study to compare the efficacy and safety of PCSA of hydromorphone with oral oxycontin tablets in the titration of cancer pain to provide an important reference for clinical titration treatment of cancer pain and guide personalized drug titration schemes.
Methods and Materials
Study Population
This prospective, multicenter, randomized, controlled trial was approved by the Ethics Committee of TongJi Hospital, TongJi Medical College of HuaZhong University of Science and Technology, China. This study was then registered on the Chinese Clinical Trial Registry with the registration number: ChiCTR2000037845. Between June 2020 and May 2022, patients with moderate to severe cancer pain from eight hospitals in China were enrolled in this study. All patients voluntarily participated in the study and provided their written informed consent. The inclusion criteria included (1) 18–70 years old; (2) patients with cancer pain and numeric rating scale (NRS) score ≥4 in the past 24 hours; (3) being able to cooperate with the medical staff to follow the medication guidance and fill in the investigation form; (4) no history of allergy to narcotic drugs or opioid addiction. The exclusion criteria were as follows: (1) non-cancerous pain or unexplained pain; (2) fasting and unable to take medications orally; (3) having a cognitive impairment assessed by Montreal Cognitive Assessment (MoCA) scale and unable to cooperate with medical staff; (4) having abnormal and clinically significant laboratory results, such as creatinine ≥2 times the upper limit of normal value, ALT or AST≥2.5 times the upper limit of normal value (≥5 times the upper limit of normal value for patients with liver metastasis or primary liver cancer), or Child-Pugh C grade of liver function; or (5) history of allergy to opioids. The patients were 1:1 randomly divided into the PCSA group or the oxycontin group using preset envelopes. The study was conducted in accordance with the principles of the Declaration of Helsinki and good clinical practice.
Study Medications
The hydromorphone hydrochloride injection (2 mL/2 mg, Yichang Humanwell Pharmaceutical Co., Ltd) was used for patients in the PCSA group. The oxycontin tablets (10 mg/tablet, subpackage: Mengti (China) Pharmaceutical Co., Ltd., manufacturer: British BARD PHARMACEUTICAL LIMITED) were used for the oxycontin group.
Administration Plan
The eligible patients with cancer pain were randomly assigned, with a 1:1 ratio, to the PCSA group or the oxycontin group. A trained and experienced nurse implanted the PCSA device for patients in the PCSA group. All research clinicians and nurses were fully trained in the standardized cancer pain program and with traditional opioid titration according to the National Comprehensive Cancer Network (NCCN) guidelines for adult cancer pain.16,17 For patients in both groups, titrations were given depending on whether the patient had opioid tolerance. Opioid tolerance was defined as receiving >60 mg oral morphine daily, >30 mg oral oxycontin daily, or an equianalgesic dose of another opioid for >1 week. The conversion equation of opioid dosage is as follows: oral morphine 60 mg = oral oxycontin 40 mg = fentanyl transdermal patch 4.2 mg = subcutaneous morphine 20 mg = subcutaneous hydromorphone 5 mg. The dose of opioids used by patients before enrollment was converted to the equivalent dose of intravenous morphine or hydromorphone.18
For opioid-naive patients, the initial dose of oxycontin tablets was 10 mg to treat moderate pain (NRS: 4–6) and 20 mg to treat severe pain (NRS: 7–10). For opioid-tolerant patients, the initial dose of oxycontin tablets was based on the conversion of the equivalent dosage of opioids used by the patient in the past 24 hours. The dose of oxycontin tablets was increased by 50–100% if the NRS >3 or the frequency of Btp >3 at 12 hours after the titration. The efficacy and toxicity associated with the treatment were evaluated hourly. Oral morphine IR tablets 10 mg were used to rescue Btp in opioid-naive patients. For opioid-tolerant patients, the rescue dose of oral morphine IR tablets was equivalent to a 10–20% dose of oxycontin tablets. Titration in each patient lasted for a total of 24 hours.
For opioid-naive patients, the initial background dose of hydromorphone in the PCSA was 0.2 mg/h. For opioid-tolerant patients, the converted 2/3 hydromorphone dose of equivalent opioid dosage used by the patient in the past 24 hours was defined as N, and the background dose of hydromorphone in the PCSA was N/24 mg/h. PCSA bolus was the identical dosage of background dose per hour. Lockout time was 15 minutes. The efficacy and toxicity were also evaluated hourly, and the titration lasted 24 hours.
All opioids were managed in strict accordance with the standardized management of narcotic drugs. The PCSA pump and infusion needles were recycled or discarded as required.
Outcome Measures
The primary endpoint of this study was time for successful titration (TST). TST was defined as the time interval from the beginning of titration to achieving stable pain control, indicating that the patient did not have breakthrough pain from the successful titration to the end of the study; or NRS <4 at the end of the study). The secondary endpoints of this study were as follows: (1) The frequency of Btp which is self-reported by the patient during the first 24 hours; (2) the NRS changes which is evaluated hourly during 12 hours by patient-self report, including NRS after 12 hours and ΔNRS which means baseline NRS minus 12h NRS; (3) the equivalent dose of oral morphine (EDOM) up to successful titration; (4) the quality of life (QoL) of patients assessed using the Edmonton Symptom Assessment System (ESAS)19,which needs to be evaluated twice, before enrollment and completion of 24 h titration; (5) adverse effects (AEs) which is evaluated hourly assessed using the Common Terminology Criteria for Adverse Events (CTCAE) 4.0.20
Analysis
According to our preliminary study, the median time to successful titration using PCSA with hydromorphone hydrochloride injection was 6.9 hours, and the median titration success time in the oral rapid titration group of oxycontin tablets was 15.1 hours. Sample size was calculated based on the Log rank test. Assuming as clinically significant a 60% decrease in TST in the PCSA group from an expected 9 hours in the oxycontin group and a power of 80% at a significance level of a 2-sided p<0.05. Considering a dropout rate of 3%, the total number of patients enrolled was 256. Stratified block randomization was conducted centrally by computer with a block size of 6.
Statistical analysis was performed using the SPSS 19.0 software package (IBM, NY, USA). TST between-group differences were analyzed using the Kaplan–Meier method in all patients. Continuous variables were described as medians and interquartile ranges (IQRs). Between-group differences were analyzed using the chi-square test or Fisher exact test. All statistical tests were 2-sided; p<0.05 was considered statistically significant.
Results
Patients
From June 2020 to May 2022, a total of 256 patients were enrolled in this study and randomly assigned to the PCSA and the oxycontin groups (n=128 in each group) from 8 Chinese oncology centers (50 patients for 1 center, 40 each for 3 centers, 26 each for 2 center, and 17 each for 2 centers). There were 50 (57.5%) and 48 (56.5%) opioid-tolerant patients in the PCSA and the oxycontin groups, respectively, and 78 (42.5%) and 80 (43.5%) opioid-naive patients in the PCSA and the oxycontin groups, respectively. The baseline characteristics were well-balanced between the two groups (Table 1). The two groups were matched in terms of gender, age, tolerance status, primary and metastatic site, pain type, baseline ESAS, baseline NRS before the intervention (all p>0.05). The study followed the consolidated standards of reporting trials (CONSORT) guidelines for reporting research (Figure 1).
 |
Table 1 The Baseline Characteristic of Two Groups |
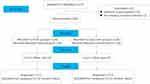 |
Figure 1 CONSORT flow diagram. |
Tst
Of the 256 subjects, 250 patients completed the opioid titration for pain control within 24 hours. Titration failed in one patient in the PCSA group and five patients in the oxycontin group within 24 hours, all of whom were opioid-tolerant.
For all patients, the median TST of pain control in the PCSA group was 5.5 (95% CI: 2.5–11.5) hours, which was significantly lower than that in the oxycontin group (16.0 [95% CI: 11.5–22.5] hours) (p<0.001). For opioid-tolerant patients, the median TST of pain control in the PCSA group was 8.0 (95% CI: 3.5–13.5) hours, which was significantly lower compared with the oxycontin group (18.0 [95% CI: 12.0–24.0] hours) (p<0.001). Among opioid-naive patients, the PCSA group also had a significantly lower TST of pain (4.5 [95% CI: 1.5–8.0] hours) than the oxycontin group (12.0 [95% CI: 11.0–20.5] hours) (p<0.001) (Figure 2).
Frequency of Btp in 24 Hours
Btp could occur for patients in both groups during the opioid titration. For the oxycontin group, rescue analgesia to treat Btp was provided by oral morphine IR tablets. For the PCSA group, rescue analgesia to treat Btp was provided by PCSA hydromorphone bolus. The frequency (median; IQRs) of Btp over 24 hours was significantly lower in the PCSA group (2.5;2.0–3.5) than in the oxycontin group (3.0; 2.5–4.5). (p=0.04).
NRS and ΔNRS Assessment of Pain at 12 Hours After Titration
The pain was evaluated by NRS at 12 hours after the start of titration. NRS is a patient self-assessment. ΔNRS which means baseline NRS minus 12h NRS. The median average pain score [IQRs] was significantly lower in the PCSA versus the oxycontin group (2.5 [1.5–3.0]) vs 4.5 [3.0–6.0]) (Figure 3) (p=0.02). For opioid-naive patients, the median NRS scores at 12 hours of titration were significantly lower in the PCSA group (2.5 [2.0–4.0]) compared with the oxycontin group (4.0[3.0–5.0]) (p=0.03). For opioid-tolerant patients, the PCSA group had significantly lower median NRS scores at 12 hours after titration (2.0 [1.0–3.0]) compared with the oxycontin group (5.0 [4.0–6.0]) (p=0.02).
ΔNRS was also assessed at 12 hours after the start of titration. The median ΔNRS [IQRs] of all patients in the PCSA and oxycontin groups was 5.0 [3.0–6.0] and 4.0 [3.0–5.0], respectively (p=0.04). The median ΔNRS [IQRs] for the opioid-naive patients was 4.0 [3.5–4.0] and 4.0 [3.0–5.0] in the PCSA and the oxycontin groups, respectively (p=0.89). The medianΔNRS[IQRs] for opioid-tolerant patients was significantly higher in the PCSA group (6.0 [3.5–7.0]) than that in the oxycontin group (4.0 [2.0–5.0]) (p=0.03).
EDOM for a Successful Titration
For all patients, the median EDOM[IQRs] was 68.73mg [39.58mg-91.26mg] in the PCSA group and 71.39mg [42.46mg-114mg] in the oxycontin group (p=0.29) (Figure 4). For the opioid-tolerant patients, the median EDOM was 86.82mg [68.75mg-94.58mg] and 91.79mg [69.34mg-115.52mg] in the PCSA and the oxycontin groups, respectively (p=0.31). For the opioid-naive patients, the median EDOM was 55.77mg [30.49mg-79.65mg] and 59.81mg [41.11mg-84.21mg] in the PCSA and the oxycontin group, respectively (p=0.59).
Evaluation of QoL
ESAS scores at hour 24 were not significantly different between the PCA and oxycontin groups (p=0.18), and they were significantly decreased in PCSA and oxycontin group compared with baseline (p=0.03,p=0.04 respectively) (Supplementary Table S1).
Safety
AEs were comparable between the two groups (Supplementary Table S2). There was no catheter-related AEs (such as infection, edema, etc.). The most common AEs in both groups was constipation.
Discussion
The classical drug used for pain titration is morphine IR tablets. Its dosage must be adjusted at a 24-hour interval to complete the titration. Thus, it is impossible to achieve rapid and effective pain relief, and the clinical practice is also cumbersome. As far as we know, this is the first prospective study to compare the PCSA of hydromorphone with oxycontin in rapid opioid titration to treat cancer pain. Furthermore, this is also a single-arm prospective study for oxycontin tablets used in rapid opioid titration and dose adjustment performed at 12-hour intervals.
Oxycontin tablets have stable pharmacokinetics in vivo and exert analgesic effects for 12 hours after rapid onset. An appropriate amount of oxycontin tablets can achieve effective analgesia for 12 hours without the tedious titration process of morphine IR tablets.21 According to the EAPC guidelines, the IR and SR dosage forms of morphine, oxycontin, and hydromorphone can be used for dose titration to treat cancer pain. Clinical practice has shown that hydromorphone is not inferior to morphine in rapid titration and pain control in cancer pain treatment. Due to the pharmacokinetic characteristics of rapid onset, short half-life, and mild drug accumulation, hydromorphone may be more suitable for rapid titration than morphine. Hydromorphone has high water solubility and can be prepared into high concentration solutions. This high concentration and low infusion volume drug solution are particularly suitable for implantable drug infusion systems in the body, which can reduce the number of injections and extend the service life of the equipment. Through the PSCA model, patients can fully achieve “on-demand administration”, which is convenient for clinical promotion and practice. In our study, only five patients in the oxycontin group failed the titration within 24 hours. The median TST in the oxycontin group was 16.0 hours, and 95% of patients completed the titration successfully within 24 hours. In a meta-analysis, Zhou et al22 found that the pain control rate was 86% and 82.98% when oxycontin and morphine were used for titration, respectively. Liang et al23 also reported that oxycontin and morphine led to a pain control rate of 98.0% and 94.5%, respectively). These are similar to our results, suggesting good analgesic efficacy and fast onset of the 12-hour rapid-dose titration algorithm.
The hydromorphone PCA approach can be used for titration in treating cancer pain. Lin et al5 reported that intravenous hydromorphone titration for severe cancer pain was achieved more effectively with PCA than with non-PCA administration. Continuous subcutaneous analgesia is mainly used in treating intractable cancer pain and breakthrough pain, as well as opioid-dose titration and rapid adjustment. The characteristics of PCSA technology highlighted the unique advantages of subcutaneous administration of medicine.24 In our study, 99% of patients had successful opioid-dose titration within 24 hours using the PCSA. The PCSA group had a significantly lower TST compared with the oxycontin group (median [95% CI)], 5.5[2.5–11.5] hours vs.16.0 [11.5–22.5] hours; p<0.001). The frequency (median; IQRs) of Btp over 24 hours was significantly lower in the PCSA group (2.5;2.0–3.5) than in the oxycontin group (3.0; 2.5–4.5) (p=0.04). The pain was evaluated by NRS score at 12 hours after the start of titration. The pain score (median; IQRs) was significantly lower in the PCSA versus the oxycontin group (2.5;1.5–3.0) vs 4.5;3.0–6.0) (p=0.02). The EDOM for a successful titration was similar in both groups (p=0.29), but there was a significant improvement in QoL in both groups (p=0.03). No between-group difference in the incidence of opioid-related adverse effects was observed (p=0.32). In our study, the use of PCSA resulted in faster pain titration than oxycontin. The main explanation for this result is the background dose of PCSA, which maintains a relatively stable blood concentration and reduces the number of administration times of rescue dose.24 The setting of background dose was based on the type of patients: (1) For opioid-tolerant patients, a 2/3 dose was used as the background dose according to the 24-hour subcutaneous dose converted from the equivalent opioids dose, and the background dose per hour is calculated by dividing by 24. (2) For opioid-naive patients, the maintenance dose was 0.2 mg/h morphine or other opioids with an equivalent dose. Nonetheless, the PCSA bolus dosage in opioid-naive patients is still controversial.24,25 The adult cancer pain guideline from MD Anderson Cancer Center recommends that PCSA devices should be set to 0.2 mg (range, 0.1–0.5mg) intravenous boluses of hydromorphone with a lockout interval of 10 to 30 minutes for opioid-naive patients.26 Therefore, according to this guideline, we set 0.2 mg as the bolus dosage in our study. Lin et al5 investigated PCA vs non-PCA hydromorphone titration for severe cancer pain, with the PCA bolus set to 0.5 mg of hydromorphone (equal to 3.3 mg of morphine) for opioid-naïve patients. They found that the mean TSTs were 1.99 hours in opioid-naïve patients and 2.3 hours in opioid-tolerant patients. The shorter TST in their study may be attributable to a larger bolus dose than that used in our study. In subgroup analysis, a significant difference in TST between the PCSA and oxycontin group was both seen in opioid-tolerant patients and opioid-naïve patients. However, the ΔNRS for the opioid-naive patients in the PCSA and the oxycontin groups was not found significant difference. We presumed that both groups can provide excellent pain relief within 12 hours for opioid-naive patients, which might explain the significant difference in TST among opioid-tolerant and opioid-naive patients.
Regarding the assessment of QoL, the ESAS scores at 24 hours were comparable between the PCSA and oxycontin groups, and both groups showed improved QoL compared with baseline, especially due to the pain (Supplementary Table S1). No difference in the incidence of opioid-related AEs was observed. No patient in either group attempted suicide, and there were no catheter-related AEs. The most common AEs in both groups was constipation. The utilization of PCSA led to relatively few complications and is relatively simple in monitoring, management, and nursing.27
Our trial also had some limitations. First, although PCSA is relatively simple and safe, it is an invasive procedure that may not be applied to every patient, especially for patients with unconsciousness, systemic edema, and peripheral and subcutaneous circulation disorders.28 Second, Hydromorphone is administered subcutaneously, while oxycontin is administered orally. The different administration methods of the two drugs lead to differences in pharmacokinetic characteristics. Different administration approaches of the two drugs may confound the current research results. Third, a patient’s pain experience is generally subjective and based on sociocultural, psychologic, cognitive, and emotion variables that may not be captured by pain scores. Because the trial was designed to compare PCSA and oxycontin group methods, double blinding was not possible. However, the open-label design may have provided a feeling of security, increasing satisfaction and, in turn, improving their pain experience. Furthermore, the cost-effectiveness of PCSA should be explored in future studies.29
In conclusion, compared with oral oxycontin tablet, the use of PCSA with hydromorphone achieved a shorter titration duration for patients with cancer pain without increasing adverse events.30
Data Sharing Statement
The original data is saved by excel spreadsheet and shared at the Chinese Clinical Trial Registration Center. The authors are willing to share these data and the data can be downloaded at https://www.chictr.org.cn/showproj.html?proj=60437 permanently.
Acknowledgments
We appreciate Medjaden Bioscience Limited for polishing the language of the manuscript. The abstract of this paper was presented at the 20203 ASCO Conference with
Patient-controlled subcutaneous analgesia with hydromorphone versus oral oxycodone for opioid titration of cancer pain: A prospective multicenter randomized trial
as a poster presentation (Abstract:404398;Poster:492). The poster’s abstract was published in ”Poster Abstracts” in Journal of Clinical Oncology. https://ascopubs.org/doi/10.1200/JCO.2023.41.16_suppl.12124.
Disclosure
The authors report no conflicts of interest in this work.
References
1. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn JKessels AG et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437–1449. doi:10.1093/annonc/mdm056
2. Lin R, Zhu J, Luo Y, et al. Intravenous patient-controlled analgesia versus oral opioid to maintain analgesia for severe cancer pain: a randomized phase II trial. J Natl Compr Canc Netw. 2022;20(9):1013–1021. doi:10.6004/jnccn.2022.7034
3. Mercadante S. Opioid titration in cancer pain: a critical review. Eur J Pain. 2007;11(8):823–830. doi:10.1016/j.ejpain.2007.01.003
4. Syrjala KL, Jensen MP, Mendoza ME, et al. Psychological and behavioral approaches to cancer pain management. J Clin Oncol. 2014;32(16):1703–1711. doi:10.1200/JCO.2013.54.4825
5. Lin R, Lin S, Feng S, et al. Comparing patient-controlled analgesia versus non-pca hydromorphone titration for severe cancer pain: a randomized phase III trial. J Natl Compr Canc Netw. 2021;19(10):1148–1155. doi:10.6004/jnccn.2020.7699
6. Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13(2):e58–68. doi:10.1016/S1470-2045(12)70040-2
7. Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol. 2018;29:166–191. doi:10.1093/annonc/mdy152
8. Sun Y, Wei H, Yu M, et al. Rapid titration with oral sustained-release morphine plus subcutaneous morphine in a multi-center, randomized control study of cancer patients with moderate to severe cancer pain. Jpn J Clin Oncol. 2022;52(11):1303–1310. doi:10.1093/jjco/hyac128
9. Bruera E, Belzile M, Pituskin E, et al. Randomized, double-blind, cross-over trial comparing safety and efficacy of oral controlled-release oxycodone with controlled-release morphine in patients with cancer pain. J Clin Oncol. 1998;16(10):3222–3229。. doi:10.1200/JCO.1998.16.10.3222
10. Jun G, Jing L, Bo L, et al. Effect of hydrochloride oxycodone controlled-release tablets with dose titration for moderate or severe cancer pain. Chin J Cancer Prev Treat. 2017;15:1319–1322.
11. Pan H, Shen P, Shu Q, et al. Efficacy and safety of sustained-release oxycodone compared with immediate-release morphine for pain titration in cancer patients: a multicenter, open-label, randomized controlled trial (SOCIAL). Medicine. 2019;98:e15505. doi:10.1097/MD.0000000000015505
12. Feng W, Wang Y, Ran F. Ran F et al.The effectiveness and safety of the rapid titration strategy of background controlled-release oxycodone hydrochloride for patients with moderate-to-severe cancer pain: a retrospective cohort study. Front Med. 2022;9:918468. doi:10.3389/fmed.2022.918468
13. Song EK, Shim H, Han H-S. A prospective multicentre study to evaluate the efficacy and tolerability of osmotic release oral system (Oros ®) hydromorphone in opioid-naive cancer patients: results of the Korean south west oncology group study. Pain Res Manag. 2015;20(6):293–299. doi:10.1155/2015/458389
14. Oldenmenger WH, Lieverse PJ, Janssen PJ, Taal W, van der Rijt CC, Jager A. Efficacy of opioid rotation to continuous parenteral hydromorphone in advanced cancer patients failing on other opioids. Support Care Cancer. 2012;20(8):1639–1647. doi:10.1007/s00520-011-1254-1.
15. Bartz L, Klein C, Seifert A, et al. Subcutaneous administration of drugs in palliative care: results of a systematic observational study. J Pain Symptom Manag. 2014;48(4):540–547. doi:10.1016/j.jpainsymman.2013.10.018
16. Johannessen LEF. The commensuration of pain: how nurses transform subjective experience into objective numbers. Soc Sci Med. 2019;233:38–46. doi:10.1016/j.socscimed.2019.05.042
17. Yu SY, Wang JJ, Huang YG, et al. Managing pain in patients with cancer: the Chinese good pain management experience. J Glob Oncol. 2016;3(5):583–595. doi:10.1200/JGO.2016.005686
18. Swarm RA, Youngwerth JM, Agne JL, et al. NCCN clinical practice guidelines in oncology: adult cancer pain. Version 1.2022. Accessed March 1, 2022. To view the most recent version, visit NCCN.org.
19. Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: past, present, and future developments. J Pain Symptom Manage. 2017;53(3):630–643. doi:10.1016/j.jpainsymman.2016.10.370
20. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017 https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
21. Guo KK, Deng CQ, Lu GJ, et al. Comparison of analgesic effect of oxycodone and morphine on patients with moderate and advanced cancer pain: a meta-analysis. BMC Anesthesiol. 2018;18(1):132. doi:10.1186/s12871-018-0583-8
22. Zhou J, Wang Y, Jiang G. Gang Jiang.Oxycodone versus morphine for cancer pain titration: a systematic review and pharmacoeconomic evaluation. PLoS One. 2020;15(4):e0231763. doi:10.1371/journal.pone.0231763
23. Liang J, Chen L, Yang S, et al. A 12-hour rapid titration method for cancer pain: a randomized, controlled, open-label study. Ann Palliat Med. 2021;10(1):88–96. doi:10.21037/apm-20-2336
24. Wan C-F, Meng Q-Z, Wang Y-W. Yan-Wei Wang et al.Patient-controlled subcutaneous analgesia using sufentainil or morphine in home care treatment in patients with stage III-IV cancer: a multicenter randomized controlled clinical trial. Cancer Med. 2020;9(15):5345–5352. doi:10.1002/cam4.3194
25. Sousa AM, de Santana Neto J, Guimaraes GMN, et al. Safety profile of intravenous patient-controlled analgesia for breakthrough pain in cancer patients: a case series study. Support Care Cancer. 2014;22(3):795–801. doi:10.1007/s00520-013-2036-8
26. University of Texas MD Anderson Cancer Center Cancer Pain – Adult,version 12; 2020.
27. Gonul S, Ayse B, Yanik F. Fazli Y.The comparison of two different techniques of remifentanil administration during implantable vascular access device procedures. J Perianesth Nurs. 2021;36(6):664–671. doi:10.1016/j.jopan.2021.02.007
28. Gibney M, Xue Z, Swinney M, et al. Reduced silent occlusions with a novel catheter infusion set (BD FlowSmart): results from two open-label comparative studies. Diabetes Technol Ther. 2016;18(3):136–143. doi:10.1089/dia.2015.0342
29. Oosten AW, Oldenmenger WH, Mathijssen RH, et al. A systematic review of prospective studies reporting adverse events of commonly used opioids for cancer-related pain: a call for the use of standardized outcome measures. J Pain. 2015;16(10):935–946. doi:10.1016/j.jpain.2015.05.006
30. Xiao X, Chu Q, Chen Y. Patient-controlled subcutaneous analgesia with hydromorphone versus oral oxycodone for opioid titration of cancer pain: a prospective multicenter randomized trial. J Clin Oncol. 2023;41(16):12124. doi:10.1200/JCO.2023.41.16_suppl.12124
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



