Back to Journals » Veterinary Medicine: Research and Reports » Volume 12
Pathogenicity of Field Marek’s Disease Virus Serotype-1 and Vaccine Efficacy Test in Chicken in Eastern Shewa Ethiopia
Authors Yimer YM , Asfaw Ali D , Getachew Ayalew B, Bitew Asires M , Gelaye E
Received 10 August 2021
Accepted for publication 2 December 2021
Published 22 December 2021 Volume 2021:12 Pages 347—357
DOI https://doi.org/10.2147/VMRR.S332737
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Yasin Mohammed Yimer,1 Destaw Asfaw Ali,2 Belayneh Getachew Ayalew,3 Molalegne Bitew Asires,4 Esayas Gelaye3
1College of Veterinary Medicine, Samara University, Samara, Ethiopia; 2College of Veterinary Medicine and Animal Sciences, University of Gondar, Gondar, Ethiopia; 3National Veterinary Institute, Bishoftu, Ethiopia; 4Ethiopian Biotechnology Institute, Addis Ababa, Ethiopia
Correspondence: Destaw Asfaw Ali
College of Veterinary Medicine and Animal Science, University of Gondar, P.O. Box 196, Gondar, Ethiopia
Email [email protected]
Background: Marek’s disease is a chicken lymphoproliferative viral illness. As new viruses emerge, vaccination immunity is being broken and hence pathogenecity assessment and vaccine evaluation related to the pathogen is critical for developing vaccine immunity in the field.
Methods: An experimental investigation was conducted to determine the pathogenicity of field isolates against Marek’s disease in antibody-free chicks and to assess the protective efficacy of the Marek’s disease vaccination. The viral isolates in question were discovered during an outbreak investigation for a previous study. The pathogenicity and effectiveness trial used a complete random design.
Results: In the pathogenicity trial, chickens inoculated with Bishoftu and Mojo field isolate had lower body weight 77.7± 3.757 and 78.15± 1.95 g at 10 dpi, respectively, when compared to un-inoculated controls, 89.85± 3.838 g at 10 dpi. Incidence of early mortality syndrome (35% and 25%), lymphoma (53.8% and 40%), and overall mortality (50% and 45%) between Bishoftu and Mojo isolates, respectively, was discovered. Vaccinations with Herpes virus of turkey challenged chickens were provided complete protection against Marek’s disease.
Conclusion: Based on the findings in pathogenecity assessment experimental trials, Bishoftu and Mojo isolates were designated as virulent Marek’s disease viruses. Regular vaccinations with Herpes virus of turkey vaccine and supported by biosecurity measures in poultry farms are important to prevent the disease.
Keywords: chicken, Marek’s disease virus, pathogenicity, vaccine efficacy, Ethiopia
Introduction
Poultry farming is a significant agricultural activity in most rural communities around the world. It provides a consistent source of petty income as well as scarce animal protein in the form of meat and eggs to rural households.1 For a long time, home chicken farming has been an essential component of the rural economy, with small and large-scale poultry farms rapidly expanding throughout Ethiopia.2 However, many developing countries’ poultry industries are facing major challenges as a result of the growing threat of infectious diseases in general, and viral diseases in particular, which makes poultry production difficult.3 Marek’s disease (MD) is one of the main viral diseases that cause significant economic losses.4 The disease is a highly contagious and economically important oncogenic and paralytic viral illness of chicken.5
Marek’s disease (MD) is caused by an enveloped DNAAlpha-herpes virus that infects bird species (Gallus gallus domesticus) and causes chronic infection.6 This virus, which belongs to the Herpesviridae family, was previously categorized as a Gammaherpesvirus because of its lymphotropic nature. MDV is now classified as a family of Herpesviridae, subfamily Alphaherpesvirinae, genus Mardivirus, and species Gallid Alphaherpesvirus 2 (GaHV-2) based on genomic organization and repetitive structures validated by virus sequencing research.7,8 The MDV group of viruses is divided into three serotypes: 1, 2, and 3, on the basis of their antigenic resemblance and they showed major differences in genome and biological features. Serotype-1 MDV has also been divided into four pathotypes: moderate (m), virulent (v), very virulent (vv), and very virulent plus (vv+), based on several parameters such as lesions, death rate, and vaccine strain protection.9,10
The source of infectious virus is thought to be the epithelium of the feather follicle, which sheds infectious virions.11 Inhalation of infectious virions from shed feather dander is the first step in MDV infection. The virus is present in the spleen, thymus, and bursa of the host bird within 24 hours of contact in the lungs by infected macrophages,12,13 which transfer the virus to B lymphocytes. Early cytolytic infection, latent infection, late cytolytic infection with immune suppression, and transformation phase are the four stages of pathogenesis. Marek’s disease is characterized by paralysis of the legs, wings, and neck, as well as weight loss, abnormal pupil, and eyesight impairment. The vaccination provides lifelong protection and, more particularly, induces an anti-tumor immune response to later infection with field strains which reduces mortality rates. It does not guard against virus infection, reproduction, or spread.14,15 MDV vaccine strains such as serotype 1 (GaHV-2) attenuated live vaccine (strain CVI988/Rispens), serotype 2 (GaHV-3) strains SB-1, and serotype 3 (MeHV-1) Herpesvirus of Turkey (HVT) are commercially available. Both serotypes 2 and 3 are reflected non-virulent and generate an immune response which is protective against some serotype 1 field strains.16
Marek’s disease issues in the field based on the advent of the first MD vaccinations, primarily the serotype 3 herpesvirus of turkeys (HVT) vaccine, the disease appeared to be under control.17 However, due to the presence of a constant virus reservoir, the vaccine was unable to induce immune responses that protected against infection, and the number of vaccinated birds decreased in these flocks. This constantly circulating virus population served as a foundation for the development of new MD virus strains. These isolates were discovered to be able to break the protection induced by the first-generation HVT vaccinations in the late 1970s. New pathotypes have emerged, showing that MDV is continuing to evolve towards increased virulence.11 The lack of a global pathotyping method that can be used in many laboratories throughout the world is a big stumbling block to MD research development. Currently, researchers are working to encourage pathotyping technologies to become more standardized.10
Because Marek’s disease virus is highly cell-associated, suspensions must contain viable cells or living cells to assure MDV survival.18 Adopting the ficoll-paque method, lymphocytes from heparinized blood, lymphoma cells, and spleen cells must be isolated and preserved in liquid nitrogen using dimethyle sulfoxide (DMSO).19 Marek’s disease virus has become a priority problem in commercial and backyard poultry production systems, despite vaccination practices using HVT vaccine, and improved biosecurity measures in Ethiopia are limited. In addition to this difficulty, Ethiopia still lacks information on which pathotypes are in circulation. However, a few researchers have used MDV isolation and genetic characterization to determine serotypes;5 no research has been done to determine which pathotypes are circulating in the country.
As the basis of this study, outbreak field Marek’s disease virus strain was molecularly identified and alignment with phylogenetic analysis with a reference pathotype strain using the meq gene nucleotide sequence was clustered (M Yasin, email [or personal] communication, August, 2021). The Marek’s disease virus was isolated from commercial chickens of all ages and breeds reared under intensive management systems that had experienced Marek’s disease outbreaks. The chicken was often vaccinated bythe provider company at day one, which could suggest the field outbreaks could be from vaccine failure or management issues. However, the experimental pathotyping trial methodologies used by different laboratories have not been consistent and particular pathotyping and vaccine efficacy evaluation is needed. The best fit approach employing commercially available SPF hens could produce pathotype rankings that are comparable to the traditional ADOL method.10 In laboratories where prototype MDV reference strains are not available, a modified best fit pathotyping approach is useful.20 Therefore, the lack of specific pathotyping identification against the circulating virus serotype makes it difficult to control and prevent Marek’s disease in Ethiopia. On the basis of this particular study, nucleotide sequences were compared with vMDV, vvMDV, and vv+MDV sequences available on the Gene Bank from a previous study (Bishoftu_2019_MDV_Isolate_Meq_gene_partial cds and Mojo_2019_MDV_Isolate_Meq_gene_partial cds). As a result, this study was conducted to identify the pathogenicity of Marek’s Disease Virus serotype-1 in antibody-free chicks and to assess the protective efficacy of the MD vaccination against the virus circulating in chicken in Eastern Shewa poultry farms of Ethiopia.
Materials and Methods
Study Area
The study was carried out based on outbreak field Marek’s disease virus serotype-1 from poultry farms found in Bishoftu and Mojo, East Shewa zone of Oromia regional state, Ethiopia. These areas are well known as the main belt for poultry production in Ethiopia. The laboratory investigation of pathogenicity and vaccine efficacy study were conducted in the National Veterinary Institute (NVI) located at Bishoftu.
Bishoftu town is located 47 km south east of Addis Ababa at 9°N and 40°E, at an altitude of 1,850 meters above sea level (m.a.s.l) in the central high land of Ethiopia. It has a short rainy season from March to April and a long rainy season from June to September. The average annual rainfall is 1,150 mm, while the maximum and minimum temperatures are 28.6°C and 12.9°C, respectively. Mojo town is located at about 88 km south east of Addis Ababa. Its mean monthly temperature of the area ranges from 22°C to 34°C.21
Study Animal and Study Designs
A controlled experimental study was implemented to determine the pathogenicity of field isolates and vaccine efficacy using day-old white leghorn breed chicks which were hatched at the NVI. A completely random design (CRD) method was employed to assign the chicks. In total, 120 white leghorn day-old layer chicks were assigned randomly for the pathogenecity trial. For the pathogenecity assessment trials there were two experimental groups consisting of three and four pens: (1) testing the pathogenicity of two different field isolates from Bishoftu and Mojoby, with criteria like: decrease in body weight, incidence of early mortality syndrome (EMS), MD lymphoma, and overall mortality to compare with reference strain; (2) to assess the protective ability of monovalent (HVT) vaccine (NVI product) against challenge with two different field isolates; to evaluate the capacity of field isolate breaking vaccine immunity. Observed data such as the percentage EMS, lymphoma incidence, and overall mortality between pre-vaccinated challenged and unvaccinated challenged control were recorded.
The chickens were randomly assigned either to the three pens for testing pathogenicity (two treatment groups and one uninoculated control) or to the four pens to assess the protective ability of monovalent vaccine (two pre-vaccinated by two different field isolate groups and two unvaccinated by two different field isolates groups). Experimental chickens were followed for a periods of 7-weeks post-challenge for the first trial in pathogenicity and 7-weeks post-challenge for the second trial in the vaccine efficacy test. At the termination of the experiment, all remaining chickens were necropsied. Experimental pathotyping trial by comparing its pathogenicity and vaccine efficacy with reference strain that most closely look like, especially in the most critical parameters by best fit method. All of the tests on chickens were carried out humanely, according to ethical guidelines.
Challenge Virus Titration
Duck embryonic fibroblast (DEF) cell lines were commercially purchased and used for virus titration of Marek’s disease virus serotype-1. The tissue culture suspension to be titrated was diluted in sterile tubes and 10-fold virus dilutions were prepared from 10−1 to 10−5 (0.5 mL viral suspension in 4.5 mL of GMEM without serum) of field isolate. The 100 μL viral dilutions of 10−5 were dispensed into each of 10 wells of column 1 to 10 in Row E and continue for every dilution step towards Row A (dilution 10−1) micro plate wells containing 100 μL DEF cells. Column 11 was left empty and column 12 was inoculated only cell for controls. Finally, the plate wells were sealed by micro plate sealer and incubated at 38°C in the presence of a 5% CO2 incubator for up to 10 days. The inoculated plates were observed under an inverted microscope daily starting from inoculation date up to day ten. The titers for each virus isolates were determined according to the following formula (titer/mL).22
The Spearman-Karber formula:
Log10=((xo−(d/2)+d(∑ri/ni)))
where xo=Log 10 of the reciprocal of the lowest dilution at which all set monolayer’s are positive;
d=Log 10 of the dilution factor that is the difference between the log dilution intervals;
ri=number of positive test monolayer’s out of ni; and
∑(ri/ni)=∑(P) sum proportion of the tests beginning at the lowest dilution showing a 100% positive result and the summation was started at dilution Xo.
Using the Spearman Karber method, the challenge virus titer was determined by the Tissue Culture Infective Dose (TCID 50) assay and the result was converted to Plaque Forming Units (PFU) [assuming 1 TCID50 = 0.69 PFU].23 The field isolates selected for the challenge study were prepared in 1,000 PFU/dose as per the sample titer result.20
Pathotyping Trial
Pathotyping of the field isolates was achieved by best fit pathotyping trial by comparing its pathogenicity and evaluating the protective efficacy of vaccines with appropriate reference strains described by earlier experimental works.10,24 The modified best fit pathotyping method is useful to laboratories where prototype reference strains of MDV are not accessible.20 Firstly, non-vaccinated chickens were challenged and the pathogenicity test identified points such as early mortality syndrome, lymphoma incidence, and overall mortality. In the second trial, monovalent HVT vaccinated chickens challenged along with non-vaccinated challenged control for vaccine efficacy test.
Experimental Animal and Managements
Specific pathogen free (SPF) eggs were imported from Germany and stored in sterile conditions, then entered into a cleaned and disinfected digital egg incubator at the NVI hatchery rooms. For the first 18 days, eggs were placed in a yellow egg tray by way of adjusting the humidity and temperature. On the morning of day 18, the eggs were moved from the yellow egg tray to black hatching crates at the bottom of the incubator and the chicks started hatching on day 21. Then SPF day-old White Leghorn chicks obtained after hatched were used for the MDV serotype 1 pathotyping trial. All groups were housed in separate classes with a local wooden made partition. The experimental house ceiling, walls, and floor were disinfected using 1% formalin. Clean disinfected deep litter was spread over the floor for bedding. Equipment including a drinker and feeder were cleaned, disinfected, and introduced to the houses and the house was kept closed and warmed before the chicks were introduced. The 120 experimental chicks were reared in a pen with infrared bulbs for heating and deeplitter for bedding. The chicks were fed on purchased starter commercial ration for 20 days and fed a rearing commercial ration until the end of the experiment and clean water was given. Mortality was recorded daily. All experiments were performed in animal facilities according to Ethical Guidelines.
Pathogenicity Test
To evaluate the pathotypes of field isolates, we used a pathogenicity test on the chicks. Sixty of 1-day-old SPF unvaccinated layer chicks were randomly divided into two treatment groups; A and B each containing 20 chicks for both Bishoftu and Mojo isolates, respectively. The remaining 20 chickens were kept as control. This was used to compare pathogenicity of field isolate with reference strain, especially in the most critical parameters, such as decrease in body weight, early mortality syndrome (EMS) (between 8 and 18 dpi), lymphoma incidence, and overall mortality. Pathotypes were identified by the comparison with appropriate reference strains described by earlier works. The birds were inoculated with 1,000 PFU of challenge virus of individual isolate via intra-peritonial route except for the negative controls at day 1 with the use of identification wing tags. The chickens were carefully kept in separate rooms to prevent transmission between groups and for easy observation of changes. Bodyweights were measured 10 dpi.20 The presence of challenge virus in experimental birds was confirmed by PCR with DNA extracted from feather tips from challenged chickens at 20 dpi. The incidence of lymphoma and overall mortality were assessed until 7 weeks from dead birds and necropsy at the termination of experiment from the remaining birds.25
Vaccine Efficacy Test
To evaluate the protective efficacy of monovalent vaccine, the isolate used in the first trial was used for this efficacy test. Sixty 1-day-old SPF layer chicks were first randomly divided into two groups; A and B, each containing 40 vaccinated chicks and 20 unvaccinated chicks for control. Forty chicks were vaccinated with monovalent HVT subcutaneously at day 1 with the use of identification wing tags. The remaining groups of 20 chicks were maintained as unvaccinated control.
The serum samples were collected at 7 days post-vaccination and screened by agar gel immunodiffusion (AGID) test.26 The tests were conducted using a petri dish by making a 1% agarose in 8% sodium chloride mixture and the agar was poured to a thickness of 2–3 mm. Holes were cut in the agar using an agar cutter with a center well and four wells were spaced at equal distance around the center well. The antigen was placed in the center well and the standard antiserum and test serum samples were placed in alternate exterior wells. The antigen used in this test was MDV-infected tissue culture cells. Plates were examined after 24 h of incubation. The appearance of one or more clearly definable precipitation lines after 24 h constituted a positive test result. Absence of any precipitation lines was recorded as a negative test result.18
Ten days later, the vaccinated and unvaccinated groups were randomly divided into four groups; two vaccinated and two unvaccinated control. Two vaccinated groups, each with 20 chicks per pen, and two unvaccinated control groups, each with 10 chicks per pen. All chicks from each group, including the unvaccinated controls, were challenged with approximately 1,000 PFU/chick of both Mojo and Bishoftu isolates. This indicates the ability of isolates to be protected by specific vaccine. The outcome data (vaccine efficacy) are generally expressed as a proportionate reduction in disease attack rate (AR) between the unvaccinated (ARU) and vaccinated (ARV) studies.27
Where: VE=vaccine efficacy,
ARU=Attack rate (both MD lesions and mortality rate) of unvaccinated chickens, and
ARV=Attack rate (both MD lesions and mortality rate) of vaccinated chickens.
New growth feathers from each chicken were collected 20 days post challenge (dpc), and identification of DNA by PCR for MDV serotype 1 was done. Chickens were observed for 7 weeks after the challenge.25 For vaccine efficacy the percentage of EMS, lymphoma incidence (examined for gross lesions from chickens died during experimental period and from chickens killed at the end of the experiment) and overall mortality in experimental birds were recorded up to 7 weeks post challenge (dpc).
Data Analysis
The data collected from the experimental trial were carefully entered into Microsoft excel. The entire data was transferred to the STATA (version 14) software program for analysis. Descriptive statistics were done to present data using tables frequency was used to summarize data of EMS, lymphoma development and overall mortality percentages.One- way ANOVA was applied to check the difference between groups. Multiple comparison tests byBonferroni tests were used to know which group of mean is the difference significant. A P-value of <0.05 was used to declare statistical significance. The mean value and standard error (SE) of decreased body weight between groups were determined according to treatment groups.
Results
The isolation of the Marek’s Disease Virus serotype-1 and the basic data was from a previous study. The meq gene sequence data was presented in the previous study with the result of genetic distance between Bishoftu and Mojo isolates at 0.000 (100% similar, not different). Comparison of the Meq gene nucleotide sequence of these isolates with that of 20 reference strains showed a high similarity with virulent BC-1 strain of USA (genetic distance of 0.004 or 99.996%) and virulent MPF57 and 04CRE strains of Australia (0.007 and 0.009 genetic distance, respectively). Nucleotide sequences were also compared with vMDV, vvMDV, and vv+MDV sequences available on the Gene Bank from a previous study (Bishoftu_2019_MDV_Isolate_Meq_gene_partial cds and Mojo_2019_MDV_Isolate_Meq_gene_partial cds).
Infective Dose of the Marek’s Disease Virus Serotype-1
The titration for infectivity of Bishoftu and Mojo isolates to Duck embryonic fibroblast (DEF) continuous cell was determined by calculating the 50% end point. The total infectious titers of DEF by Bishoftu and Mojo isolates per ml were log103.6 TCID50/mL (103.6=3,981 TCID50/mL, 1TCID50=0.69 PFU, then 3,981 TCID50=2,747 PFU) and log103.5 TCID50/mL (103.5=3,162 TCID50/mL=2,182 PFU), respectively. The challenging dose in PFU was 1,000 PFU (0.4 mL) and 1,000 PFU (0.5 mL) in Bishoftu and Mojo isolates, respectively.
Result of Pathogenicity Test
One-way ANOVA was used to see the mean difference between groups. Mean difference between groups was statistically significant (P<0.05). Both isolates were pathogenic in challenged chickens: 35% and 25% of chickens were displayed EMS; 53.8% and 40% lymphoma, and 50% and 45% overall mortality, respectively. The body weight in the two isolates was lower when compared with uninoculated controls at 10 dpi (Table 1). The conventional PCR test was performed for identification of MDV serotype 1 from new growing feather tips at 20 days post inoculation and the result was indicated as positive.
 |
Table 1 Pathogenicity Test Result Between Bishoftu and Mojo Challenge Virus Isolates |
Multiple comparison tests showed that the mean differences between treatment and control groups were statistically significant (P<0.05) but the mean difference between the Mojo and Bishoftu isolates challenged groups was non-significant (P>0.05) (Table 2). Gross pathological changes induced in experimental birds by field isolates are shown in Figure 1.
 |
Table 2 Multiple Comparison Test of Body Weight Between Groups by Bonferroni |
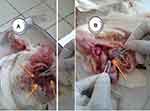 |
Figure 1 Post mortem lesions: Liver tumour (A) and Spleen tumour (B). |
Efficacy Test Results
Agar gel immunodiffusion (AGID) test result showed all collected serum samples from pre-vaccinated experimental chickens were positive. The results were read over a lamp in a darkened room and the appearance of the precipitation line was observed after a 24 h incubation period (Figure 2).
 |
Figure 2 AGID test result showed precipitation line. |
Both Bishoftu and Mojo challenge virus isolates were pathogenic in unvaccinated challenged chickens: 30% and 20% chickens displayed EMS; 42.8% and 37.5% lymphoma, and 50% and 40% overall mortality, respectively. However, chicken groups vaccinated with HVT vaccine were safe with the absence of any MD lesions and mortality. The PCR test for identification of MDV serotype 1 was positive from new growing feather tips at 20 days post inoculation. Vaccinations with HVT challenged groups were provided complete protection against the MD lesions. Both Bishoftu and Mojo isolates were unable to break vaccinal immunity, as indicated in Table 3 and Figure 3.
 |
Table 3 Vaccine Efficacy Test Result Between the Pre-Vaccinated and Unvaccinated Challenged Groups |
 |
Figure 3 Efficacy test result between pre-vaccinated (A) and unvaccinated challenged group (B). |
Discussion
Marek’s disease virus (MDV) isolation and molecular detection were obtained in previously conducted research of this research team. This study was conducted to determine the pathotypes of the virus and monitor the vaccine efficacy against the virus from outbreak MDV sick chicken in the study areas isolated so far. Marek’s disease vaccines could not prevent the evolution of MDV due to the inability of the vaccines to convene sterile immunity and hence new emergent viruses have been a capacity of breaking vaccine immunity.10 Pathotype designations reflect important biological properties that correlate with the breakthrough of vaccinal immunity in the field. Evaluation of MDV isolates for the pathotyping gives information about isolatable virus pathotype to find a suitable vaccine candidate.
In this study, a pathogenicity trial was conducted by best fit method, and comparisons with appropriate reference strains described by earlier works were investigated. This involved two groups of chickens; non-vaccinated and FC126-vaccinated (HVT). The pathotyping system such as the Avian Disease and Oncology Laboratory or best fit systems still becomes ian mportant method to determine pathogenicity level of MDV-1.10 Although the evolution into more pathogenic pathotypes has been recognized, the phylogenetical clustered finding of a previous follow-up study offered that isolates obtained from the Bishoftu and Mojo groups in the current study belong to the lower end, with virulent MDV strains in the USA and Australia. This might be due to identical use of vaccines like HVT vaccines because MD vaccines are not capable of inducing immune responses that protect against infection.
The pathogenicity test was done to determine the pathogenicity of field (Bishoftu and Mojo) isolate in vivo. Based on this pathogenicity trial, the present study indicated both Bishoftu and Mojo isolates were pathogenic in unvaccinated chickens, inducing MD lesions in 53.8% and 40%, respectively. This finding is in line with reports which stated that the incidence of lymphoma in unvaccinated chickens was 50–66%, designated a virulent pathotype.20 In addition, the incidence of very virulent and virulent lymphoma was 93% and 62%, respectively, as reported in Korea.27 HVT vaccination provides good protection against all of these effects.25 This study also showed that early mortality syndrome (EMS) in Bishoftu and Mojo isolates was 35% and 25%, respectively. This finding is in agreement with the other findings which reported 33–40% EMS within a few weeks of inoculations.20 The percentages of overall mortality in the present study were 50 and 45% in Bishoftu and Mojo isolates in challenged non-vaccinated chickens, respectively. The virulent strain was responsible for 53–66% of overall mortality in non-vaccinated chickens. This variation might be due to variations in the number of experimental chickens per pens, differences in virus dose, age of inoculation, or chicken breed used.
The mean difference between groups was statistically significant but it does not tell us which group of the mean is the difference significant. Therefore, multiple Bonferroni comparison tests were used to indicate in which group of the mean the difference is significant. It showed that the mean difference between treatment and control groups was statistically significant (P<0.05) but the mean difference between Mojo and Bishoftu isolates challenged groups were non-significant (P>0.05). The present isolates were less body weight depressive than the study conducted that showed that chickens inoculated with very virulent and virulent strains were depress in body weight 53.70±1.01 and 63.38±0.93 at 10 dpi, respectively.20 This might be due to variation in feed ration, feeding time, and amount of feed given to the chicks, and due to differences in viral dose during challenge. Based on these pathogenicity comparisons the isolates from Bishoftu and Mojo were designated as virulent pathotypes.
The challenge test was also done to evaluate the efficacy of HVT (FC-126) vaccine. To confirm the pathogenicity comparison result, each pathotype appears to be associated with the ability to be protected by specific MD vaccines. The efficacy of MD vaccines used was determined post-vaccination in vitro by measuring the antibody level and in vivo by challenge with field MDV. In this study, the productions of antibodies in vaccinated chicks were tested by AGID method at 9 days post-vaccination due to inaccessibility of the ELISA kit. As described in the in standard,18 the agar gel immunodiffusion (AGID) test was employed most commonly to detect antibodies. In virulent MDV pathotype, response in HVT-vaccinated chickens did not differ from reference virulent pathotype. The control groups responded to the challenge virus by EMS, and lymphoma incidence and overall mortality rate were recorded. There were MD lesions on visceral organs after post-mortem examination in control unvaccinated challenged groups. However, there were no MD lesions found on visceral organs or death occurred in pre-vaccinated challenged chickens. The chickens vaccinated with HVT (FC-126) vaccine were fully protected against the effect of the challenge virus that could be due to absence of variation from virulent pathotypes. Therefore, HVT (FC-126) is efficacious to protect the vMDV circulating in Bishoftu and Mojo isolates. This finding is in agreement with the stated vaccination that HVT provided complete protection against MD lesions in virulent pathotypes in Australia.25
The result of these pathogenicity and efficacy tests confirmed that the circulation of field viruses of MDV-1 were virulent pathotypes in both Bishoftu and Mojo poultry farms. The vaccination program or utilization of the MDV vaccine based on HVT as a monovalent product is still available in many countries. The vaccine is inexpensive, available as cell-free and effective when the field exposure is not a severe or virulent pathotype (vMDV).28–30 This report was in agreement with the present study, circulation of oncogenic field viruses of MDV-1 were virulent pathotype and can be protected well by HVT vaccine. Although the HVT vaccine has been successful in controlling major losses from the disease, there were occurrences of MD outbreaks. This might be due to the challenge with virulent viruses before the development of vaccinal immunity and improper use of the vaccine.
Conclusions
The study shows that isolates originated from the study areas were pathogenic in unvaccinated challenged chickens. Vaccinations with HVT challenged chickens provided complete protection against the MD lesions and the isolates were unable to breaks vaccinal immunity. The present study was challenged with a lack of ELISA kit for quantifying produced antibody after immunization. Regular vaccinations with HVT (FC-126) vaccine and supported by biosecurity measures in poultry farms is needed to prevent introduction of the Marek’s Disease Vaccine and timely flock immunity level after immunization should be quantified to assess and improve the preventive measure in the sector.
Abbreviations
ADOL, Avian Disease Oncology Laboratory; AGID, Agar Gel Immunodiffusion; DNA, Deoxyribonucleic Acid; DMSO, Dimethyle Sulfoxide; EMS, Early Mortality Syndrome; GaHV-2, Gallid alpha Herpesvirus Type 2; GMEM, Glasgow Minimum Essential Medium; HVT, Herpes Virus of Turkey; ISO, International Standardazation Organization; MDV, Marek’s Disease Virus; NVI, National Veterinary Institute; OIE, “World Organization for Animal Health”; vv+MDV, very virulent plus Marek’s Disease Virus.
Data Sharing Statement
The original data to validate this experiment was obtained from the molecularly confirmed field isolate of the virus maintained in a virology laboratory by the same authors. Additional data will be available from the first and corresponding authors on reasonable request.
Ethics Approval and Consent to Participate
The incorporated work was ethically cleared by the Institutional Review Board of the National Veterinary Institute, NVI/ERC/01/2018. All methods were carried out in accordance with relevant guidelines and regulations set by ISO and OIE, on involving humans and birds in the study. No fees were requested.
Acknowledgments
The work incorporated in this research was undertaken using the research grant allocated by the National Veterinary Institute. We are very grateful to the institute. It is with great appreciation that we express our great thanks and hearty appreciation to technician staff of the National Veterinary Institute for their cooperativeness and unreserved technical support in all the laboratory works.
Author Contributions
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tadelle D, Million T, Alemu Y, Peters K. Village chicken production systems in Ethiopia: 2. Use patterns and performance valuation and chicken products and socio-economic functions of chicken. Livest Res Rural Dev. 2003;15:1.
2. Halima H, Nesser F, Van Marle-koster E, De Kock A. Village based indigenous chicken production system in north-west Ethiopia. Trop Anim Health Prod. 2007;39:189–197. doi:10.1007/s11250-007-9004-6
3. Dessie T, Ogle B. Village poultry production systems in the central highlands of Ethiopia. Tro Ani Health Prod. 2001;33:521–537. doi:10.1023/A:1012740832558
4. Zeleke A, Sori T, Gelaye E, et al. Newcastle disease in village chickens in the southern and rift valley districts in Ethiopia. Inter J Poul Sci. 2005;7:508–510. doi:10.3923/ijps.2005.507.510
5. Berhan D. Isolation and Molecular Characterization of Marek’s Disease Virus Circulating in Ethiopian Chickens. [MVSc thesis]. Debre Zeit, Ethiopia: Addis Ababa University, College of Veterinary Medicine and Agriculture; 2014:25–46. Available from: http://etd.aau.edu.et/handle/123456789/4222
6. Boodhoo N, Gurung A, Sharif S, Behboudi S. Marek’s disease in chickens: a review with focus on immunology. Vet Res. 2016;47:119. doi:10.1186/s13567-016-0404-3
7. Tulman E, Afonso C, Lu Z, Zsak L, Rock D, Kutish G. The genome of a very virulent Marek’s disease virus. J Virol. 2000;74:7980–7988. doi:10.1128/JVI.74.17.7980-7988.2000
8. Tischer B, Schumacher D, Chabanne D, Zelnik V, Vautherot J, Osterrie N. High-level expression of Marek’s disease virus glycoprotein C is detrimental to virus growth in vitro. J Virol. 2005;79:5889–5899. doi:10.1128/JVI.79.10.5889-5899.2005
9. Davison F, Nair V. Use of Marek’s disease vaccines: could they be driving the virus to increasing virulence. Expert Rev Vaccines. 2005;4(1):77–88. doi:10.1586/14760584.4.1.77
10. Witter R, Calnek B, Buscaglia C, Gimeno I, Schat K. Classification of Marek’s disease viruses according to pathotype: philosophy and methodology. Avian Pathol. 2005;34:75–90. doi:10.1080/03079450500059255
11. Venugopal K, Jones C, Gough R. Herpes viridae. In: Jordan F, Pattison M, Alexander D, Faragher T, editors. Poultry Disease.
12. Calnek B. Pathogenesis of Marek’s disease. In: Hirai K, editor. Marek’s Disease. Berlin, Germany: Springer; 2001:25–55.
13. Barrow A, Burgess C, Baigent J, Howes K, Nair K. Infection of macrophages by a lymphotropic herpesvirus: a new tropism for Marek’s disease virus. J Gen Virol. 2003;84:2635–2645. doi:10.1099/vir.0.19206-0
14. Baigent S, Davison F. Marek’s disease virus: biology and life cycle. In: Davison F, Nair V, editors. Marek’s Disease: An Evolving Problem. London, UK: Elsevier Academic Press; 2004:62–77. Available from: https://www.elsevier.com/books/mareks-disease/davison/978-0-12-088379-0
15. Gimeno I. Marek’s disease vaccines: a solution for today but a worry for tomorrow. Vaccine. 2008;18:26. doi:10.1016/j.vaccine.2008.04.009
16. Baigent S, Smith P, Petherbridge J, Nair K. Defferential quantification of clonedCVI988 vaccine strain and virulent RB-1B strain of Marek’s disease virus in chicken tissue, using real time PCR. Res Vet Sci. 2011;91:167–174. doi:10.1016/j.rvsc.2010.08.002
17. Osterrieder N, Kamil J, Schumacher D, Tischer B, Trapp S. Marek’s disease virus from miasma to model. Nat Rev Micro. 2006;4:753–761. doi:10.1038/nrmicro1382
18. OIE. Office International des Epizootics, chapter 2. 3.13: Marek’s disease, Manual of diagnostic test and vaccines for terrestrial animals. 2017:1–13. Available from: https://www.oie.int/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access/
19. Jianming T, Julie C, Nadeene C, Gregory A, Tannoc K. Optimization of methods for the isolation of Marek’s disease viruses in primary chicken cell cultures. J Virol Methodol. 2008;147:312–318. doi:10.1016/j.jviromet.2007.09.011
20. Suresh P, Johnson Rajeswar J, Sukumar K, Harikrishnan T, Srinivasan P. Pathotyping of recent Indian field isolates of Marek’s disease virus serotype 1. Acta Viro. 2015;59:156–165. doi:10.4149/av_2015_02_156
21. NMSA. Rainfall and Temperature Data of Debre Zeit. Addis Ababa, Ethiopia: National Meteorological Service Agency; 2010.
22. Spearman C. The method of ‘right and wrong cases (constant stimuli) without Gauss’s formulae. Brit J Psychol. 1908;2:227–242. doi:10.1111/j.2044-8295.1908.tb00176.x
23. Luria S, Darnell J, Baltimore D, Campbell A. General Virology.
24. Dudnikova E, Norkina S, Vlasov A, Slobodchuk A, Le L, Witter L. Evaluation of Marek’s disease field isolates by the “best fit” pathotyping assay. Avian Pathol. 2007;36:135–143. doi:10.1080/03079450701209857
25. Katrin G, Julie C, Nadeene C, et al. Pathotyping of Australian isolates of Marek's disease virus and association of pathogenicity with meq gene polymorphism. Avian Pathol. 2012; 41(2):161-76. doi:10.1080/03079457.2012.656077
26. Witter R. Protective efficacy of Marek’s disease vaccines. Curr Top Micro Immunol. 2001;255:57–90. doi:10.1007/978-3-642-56863-3_3
27. Orenstein W, Bernier R, Dondero T, Hinman A, Marks J, Bart K. Field evaluation of vaccine efficacy. Bull World Health Organ. 1985;63(6):1055–1068. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2536484/
28. Sung H. Recent increase of Marek’s disease in Korea related to the virulence increase of the virus. Avian Dis. 2002;46:517–524. doi:10.1637/0005-2086(2002)046[0517:RIOMSD]2.0.CO;2
29. Payne L, Venugopal N. Neoplastic diseases: Marek’s disease, avian leukosis and reticuloendotheliosis. Revue Sci tech. 2000;19(2):544–564. doi:10.20506/rst.19.2.1226
30. Nicole G. Attempts to Visualize Lymphocytes Latently Infected with Marek’s Disease Virus in situ. [Master of Science thesis]. Canada: Simon Fraser University, Faculty of Health Sciences. 2015:1–21.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
