Back to Journals » International Journal of General Medicine » Volume 13
Park Algorithm as Predictor of Premature Ventricular Contraction Origin in Three‐Dimensional Mapping Electrophysiological Studies
Authors Amir M, Mappangara I, Kabo P, Hasanuddin Z, Setiadji R, Zam SM
Received 25 August 2020
Accepted for publication 6 October 2020
Published 11 November 2020 Volume 2020:13 Pages 1083—1092
DOI https://doi.org/10.2147/IJGM.S275188
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Video abstract presented by Muzakkir Amir.
Views: 685
Muzakkir Amir,1 Idar Mappangara,1 Peter Kabo,1 Zulkifli Hasanuddin,1 Robertus Setiadji,2 Sitti Multa Zam1
1Department of Cardiology and Vascular Medicine, Faculty of Medicine, Hasanuddin University, Makassar, South Sulawesi, Indonesia; 2Department of Pharmacology, Faculty of Medicine, Hasanuddin University, Makassar, South Sulawesi, Indonesia
Correspondence: Sitti Multa Zam
Department of Cardiology and Vascular Medicine, Faculty of Medicine, Hasanuddin University, Perintis Kemerdekaan St, Makassar, South Sulawesi 90241, Indonesia
Tel +6281342265956
Email [email protected]
Purpose: In the past few years, premature ventricular contraction (PVC) has attracted immense attention, both in patients with or without structural heart disease. Despite the technological advancement, no guiding tools are currently available to assist in the prediction of origin of PVC using a 12‐lead electrocardiogram (ECG) before electrophysiology and ablation procedures. Park and co‐workers compiled the existing algorithms for the morphology of ECG from the literature and generated a single algorithm based on specific features of ECG for the prediction of PVC origin. The Park algorithm is limited to idiopathic PVC and has not been evaluated clinically. In the present study, the Park algorithm was used to predict PVC origin in patients with or without structural heart disease and compared with the gold standard examination based on three-dimensional electrophysiological mapping studies.
Patients and Methods: A cross‐sectional study employing ECG data and electrophysiology study (EPS) reports from patients’ medical records at Integrated Heart Center Wahidin Sudirohusodo Hospital, Makassar, Indonesia was conducted. The study was performed from April 2018 to June 2019 with a total of 31 samples; however, four samples were excluded during the EPS.
Results: In the present study, the incidence of structural heart disease was 45.2%. The suitability of the Park algorithm for electrophysiological evaluation was 85.2%, both in the case of PVC with and/or without structural heart disease. The prediction of the origin of PVC in the right or left heart using the Park algorithm showed a sensitivity of 95%, specificity of 100%, positive predictive value of 100%, negative predictive value of 87.5%, and accuracy of 96%.
Conclusion: The findings of the study suggest significant accuracy of the Park algorithm in the prediction of location of origin of PVC. High sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the Park algorithm highlight its suitability to be used for determining the location of PVC origin in the right or left heart.
Keywords: premature ventricular contraction, ECG, structural heart disease, ablation therapy, arrhythmia
Introduction
Premature ventricular contraction (PVC) is one of the most commonly occurring arrhythmia arising due to early depolarization of ventricular myocardium. In the general population, PVC shows a prevalence of 14% when assessed on a standard 12‐lead ECG, which increases to 40–75% when a 24‐ or 48‐hour Holter monitoring is used. PVC is often seen to be associated with structural heart diseases and represents an increased risk of cardiovascular diseases and sudden death. However, PVC can also occur in individuals without any underlying heart disease. Although PVC is known to be associated with 2-times higher risk of cardiovascular complication like stroke and death, it was considered benign in the absence of any structural heart disease. However, studies in the last decade suggest that PVC induces cardiomyopathy even in subjects without any structural heart disease. PVC‐induced cardiomyopathy is an interesting topic and a significant amount of evidence is rapidly emerging in support of this subject.1
Among the various therapies available for PVC management, antiarrhythmic therapy was the preferred choice of treatment in the past and radiofrequency ablation therapy was only considered in patients with recurrent ventricular tachycardia (VT) despite antiarrhythmic therapy. The management of patients with PVC is often dictated by symptoms, precipitating factors, and most importantly the presence or absence of structural heart disease. In addition to this, the use of antiarrhythmic therapy is often limited owing to its efficacy and associated side-effects.2 So far, radiofrequency ablation therapy has been reported to be more beneficial as compared to antiarrhythmic drugs in the management of patients with PVC. According to the 2015 European Society of Cardiology (ESC) guidelines, radiofrequency ablation is recommended in patients with symptomatic and frequent PVC. The recent past has witnessed an increase in the management options using an ablation catheter. Several previous studies have established the superiority of three‐dimensional electroanatomic mapping in EPS over the conventional methods. The use of three‐dimensional mapping aids in the reduction of duration of fluoroscopy and radiation dose as compared to the conventional mapping strategies.3 In order to define the target of ablation, it is important to determine the origin of arrhythmia. Earlier, only EPS were used to determine the location of PVC origin. Recent developments in the field of ECG, particularly for PVC, can be used in the prediction of the origin of PVC. This will further assist in the pre‐procedural planning and improvement of outcomes of ablation therapy.4 In addition to this, prediction of PVC origin using ECG measurement will be helpful in planning ablation strategies, and all this information will enable better patient education regarding the length of procedure and risks associated with vascular access, mapping, and ablation.5
Many studies have previously evaluated the efficacy of ECG as an early predictor before EPS procedure; however, no fixed algorithm is available for the prediction of origin of PVC using a standard 12‐lead ECG before the electrophysiological procedure. In 2012, Park et al6 developed a single algorithm by compilation of various algorithms used in earlier studies for the prediction of origin of PVC based on ECG morphology in patients without structural heart disease. The characteristics of ECG used for PVC origin prediction by Park et al involved various electrophysiological diagnostic tools. The present study aimed to evaluate and establish the congruity of the Park algorithm and EPS using three‐dimensional mapping in the prediction of origin of PVC in patients with or without structural heart disease.
Materials and Methods
Study Design and Data Collection
A cross‐sectional study was conducted from April 2018 to June 2019 using ECG data and EPS reports obtained from the medical records of patients registered at Integrated Heart Center Wahidin Sudirohusodo Hospital, Makassar, Indonesia. The sample in the present study included PVC patients undergoing three‐dimensional EPS. The location of the origin of PVC obtained from EP studies was compared with the one predicted using the Park algorithm performed on a 12‐lead ECG data set that were acquired prior to electrophysiology procedures.
Basic Principles of ECG Used in the Prediction of the Origin of PVC
For the prediction of the origin of PVC, it is important to assess the morphology of PVC obtained from 12‐lead ECG. The QRS complex pattern for PVC was made according to the distance of its origin. This further spreads depending upon the direction of myocardial activation (moving towards or away from the recording electrode). Therefore, the general principles established for the geometry of ventricles and the direction of their activation will play a pivotal role in the determination of the PVC pattern. Anatomical variation plays a pivotal role in the assessment of the direction of electrical current towards the body surface during the prediction of PVC origin. Variations are recorded whenever there is a shift of the heart towards the wall of the chest.6
The following general principles were used for the prediction of location of PVC origin:
QRS Axis
A PVC originating from the top of the heart will spread from top to bottom. The electrical axis for PVC will be directed inferiorly. Therefore, PVC will appear positive in inferior leads, namely, lead II, III, and aVF. In comparison to this, if PVC originates from the bottom (inferior) of the heart, it will spread from bottom to top and the electrical axis will be directed superiorly. A right superior QRS axis indicates the presence of origin of PVC in apical septal or lateral apical site. This is frequently observed as QS wave in leads I, II, and III and QS or rS wave in leads V5 and V6. A right inferior axis indicates a high basal location of origin (high lateral left ventricle or high lateral LV). Generally, a left inferior axis shows PVC originating from the top of the LV septum. However, in certain cases the QRS axis does not match with the PVC/VT exit site, as reported in the case of myocardial infarction (MI) in the apical region. These differences can arise due to abnormalities in the exit conduction of the reentry circuit towards the myocardium.7,8
In electrophysiology, the scar area is defined as an area that is electrically inactive and does not show any electromagnetic pattern. This area is considered non‐conductive. During VT, an isthmus is defined as a conductive myocardial tissue that is delineated or surrounded by non‐conductive tissue (scar).9
Bundle Branch Block
The activation of left ventricle prior to right ventricle in the case of PVC arising from LV is suggestive of morphological resemblance with the right bundle branch block (RBBB). However, PVC arising from the septum will provide morphology similar to the left bundle branch block (LBBB) if the exit is from the septum towards the right ventricle. PVC arising from the right side of the heart will activate the right ventricle first followed by the left ventricle. Thus, in accordance with the sequence of activation of the ventricles it will show a pattern similar to LBBB on ECG. The morphology of PVC or VT is described as “LBBB Pattern” and “RBBB Pattern” in reference/accordance to the lead V1. A negative (or negative dominant) PVC in V1 indicates a LBBB pattern, while positive PVC in V1 is suggestive of a RBBB pattern.7 Most of the ventricular arrhythmias with RBBB pattern have been found to be associated with inferior and/or extensive anterior MI.8
Concordance
VTs with positive concordance in all precordial leads (V1–V6) arise/originate only at the base of the heart, where ventricular activation occurs towards the anterior and apical direction, such as left ventricle outflow tract (LVOT), along the mitral or aortic valves, or in the basal septum. Conversely, negative concordance is observed only in VTs originating near the apical septum, especially in anteroseptal MI, where the direction of activation is away from the chest wall. Here, concordance refers to the scenario where QRS complex for PVC from V1–V6 are in the same direction (positive or negative).8
QS Complex
The presence of a QS complex in any lead is indicative of the propagation of the wave away from its location. Thus, QS complex in inferior leads suggests that the activation is originating from the inferior wall, whereas QS complex in the precordial is indicative of the activation moving away from the anterior wall. QS complexes in V2–V4, V3–V5, and V5 and V6 suggest the location of origin in the anterior wall, apical region, and lateral wall, respectively.8
Outflow Tract
PVC arising from the outflow tract (OT) is characterized by an inferior frontal axis along with an LBBB or RBBB pattern in lead V1. In case PVC is located more towards the left of the heart, precordial transitions will occur earlier (PVC patterns will become more positive in precordial leads), and thus the origin of PVC that is located more towards the left will show a RBBB pattern.7
Park Algorithm for Predicting PVC Origin
In 2012, Park et al6 compiled the characteristics of 12‐lead ECG obtained for various idiopathic PVC locations based on earlier studies. This data was simplified and used to generate a single algorithm to assist in the prediction of PVC location based on ECG morphology.
Statistical Analysis
The acquired data was analyzed using the Statistical Package for Social Science (SPSS) program, version 21. Analysis of Park algorithm as a predictor for PVC location based on EPS was performed using ANOVA Test. Park algorithm as a predictor of PVC location from the left and right heart based on EPS was analyzed using cross‐tabulation and Fischer’s exact test.
Results
The present study included 31 patients, the majority of which were females in the age group 19–73 years, with average age of 43.35±12.77 years). Most of the participants were adults (45.2%), with no structural heart disease (54.8%), and showed normal left ventricular systolic function (LV ejection fraction >50%) (83.9%). The most common type of PVC that was observed was bigeminy (32.3%), followed by frequent PVC (29%), ventricular tachycardia (VT) (16.1%), trigeminy PVC (12.9%), and quadrigeminy PVC (9.7%). The findings of the 12‐lead surface ECG suggested that most of the subjects were characterized by single PVC focus, ie, unifocal (90.3%). All these characteristics for the acquired data are summarized in Table 1.
 |
Table 1 Characteristics of Samples Undergoing Electrophysiology Study |
According to EPS data, the origin of PVC was located mostly in the right heart (64.5%). PVC arising from the left heart was observed only in 22.6% cases. The PVC arising from the right heart was found to be mostly of right ventricle outflow tract (RVOT) origin (58%), whereas the PVC location in the left heart showed mostly LV origin. The PVC arising from the left heart was contributed by anterolateral (papillary muscle anterior, 9.7%), posteromedial (papillary muscle posterior, 6.5%), and mitral annulus (6.5%) location (Table 2).
 |
Table 2 Characteristics of PVC Origin in EPS |
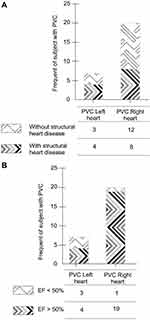
PVC origin predicted from 12‐lead ECG data using the Park algorithm and EPS were found to be congruent in 23 samples (85.2%), whereas four samples were non‐congruent (14.8%) (Figure 1). The results of the ANOVA test indicated no significant correlation in the congruity of PVC locations obtained from ECG using the Park algorithm and EPS (P=0.79) (Figure 2). The cross‐tabulation and chi‐square analysis of the acquired data showed no significant correlation between PVC with or without structural heart disease and the suitability of the Park algorithm (P=0.39) (Figure 3).
 |
Figure 1 Congruity of PVC based on Park Algorithm and EPS. |
 |
Figure 2 Correlation in Congruity of PVC locations based on ECG using the Park Algorithm and EPS. |
 |
Figure 3 Correlations between PVC with or without structural heart disease. |
In general, PVC in patients without structural heart disease has been found to originate from the right heart. However, the chi-square analysis in the present study indicated the absence of any significant correlation between the location of origin of PVC (left or right heart) and the absence or presence of structural heart disease (P=0.43). PVC with an ejection fraction of >50% were found to be frequently associated with the right heart, whereas PVC with a fraction ejection < 50% originated frequently from the left heart. This showed a significant correlation of P=0.01, as shown in Figure 4.
The results of the cross‐tabulation analysis indicated that the use of the Park algorithm in the prediction of location of the right or left heart PVC was characterized by 95% sensitivity, 100% specificity, positive predictive value of 100%, negative predictive value of 87.5%, and 96% accuracy (P<0.001) (Table 3).
 |
Table 3 Correlation of PVC Origin Predicted by Park Algorithm and PVC Origin Obtained from Electrophysiological Studies |
Discussion
Baseline Characteristic of PVC Origin for Subjects Undergoing Electrophysiology Study
Sample Size and Age Group
In 2019, Amir et al10 evaluated a total of 8,847 ECGs, collected from the general population residing at Makassar city of Indonesia. Among these, PVC was observed only in 98 ECGs (1.1%). The findings of this study were consistent with the previous study by Ahn,1 which stated that the prevalence of PVC in the general population was 1%, as studied using standard methods. Most of these people with PVC belonged to the group of healthy people (40–75%). The present study included 31 patients with PVC that underwent electrophysiology study (EPS) at Integrated Heart Center Wahidin Sudirohusodo Hospital, Makassar, Indonesia. Several previous studies have suggested a positive correlation between the prevalence of PVC and age, irrespective of the presence of structural heart disease. However, no information is available on the underlying mechanism responsible for this linear relationship between age and the occurrence of PVC.11 In fact, there are reports that suggest that the incidence and frequency of PVC increase with age. One possible explanation could be the effect of coronary heart disease and increase in age that result in an increase in the incidence and frequency of PVC. Another study conducted by Kostis et al12 reported an increase in the incidence and frequency of PVC with age, even in the absence of coronary heart disease which was excluded by coronary angiography. The report suggested that the degenerative changes increase with age.
Amir et al10 reported the highest prevalence of PVC in the age group 45–65 years, which differs from the findings of the present study where most of the PVC patients belonged to the adult age group (26–45 years). This difference could be attributed to less use of long‐term antiarrhythmic drugs in younger age groups, who generally prefer ablation therapy. This is in accordance with the findings of Matthew et al,13 which stated that the patients aged <65 years prefer ablation therapy as compared to antiarrhythmic drugs, whereas patients aged >65 years generally choose antiarrhythmic drugs over ablation (P<0.001).
PVC Prevalence in Patients with Structural Heart Disease
In the present study, echocardiography was used as a tool to identify PVC patients with or without structural heart disease. PVC with structural heart disease was determined on the basis of echocardiography examination with one or more of the following: dilated cardiac chambers, valvular regurgitation or stenosis, left kinetic or hypokinetic segments, and left ventricular hypertrophy. Structural heart disease was reported in 45.2% of the PVC samples. The occurrence of PVC in patients with a structurally normal heart is called “idiopathic PVC”, which is devoid of any scar‐related mechanism. According to the echocardiography parameters, most of the samples belonged to the category of PVC without structural heart disease (54.8%).
The prognosis of patients with PVC is generally determined on the basis of coexistence of structural heart disease. Nevertheless, the presence of PVC in patients with normal heart has been found to be associated with sudden death. In 2017, Lin et al14 conducted a cohort study on 5,778 subjects with normal heart structures and followed up to 10 years. It was observed that PVC>12 beats was an independent predictor for all the deaths (hazard ratio, HR=1.4), cardiovascular hospitalization (HR=1.1), all‐cause hospitalization (HR=1.1), and new onset of heart failure (HR=1.41).
Types of PVC
The majority of the PVC cases reported in the present study were repetitive PVC (bigeminy, quadrigeminy, and trigeminy), with bigeminy being most prevalent. Generally, the repetitive type of PVC, especially bigeminy, occurs via a mechanism of re-entry (micro‐re-entry, branch to branch, and/or between fibrotic tissue).15 Clinically, repetitive PVC results in an increase in the heart rate and appears as an irregular rhythm or pounding heart. This is followed by the appearance of disturbing symptoms in patient that require treatment. In the last decade, the findings of several studies suggested that PVC can induce cardiomyopathy.16 Niwano et al17 reported the occurrence of progressive deterioration of LV function in patients with frequent PVC (>1,000 beats/day). In comparison to this, Amir et al10 reported higher incidences (64%) of non‐repetitive or occasional type PVC, regardless of the clinical conditions, in the study conducted on the sample population residing in Makassar.
Focus of PVC Origin
In the present study, most of the participants showed unifocal PVC (n=28; 90.3%), whereas only a small number of subjects displayed evidences of PVC with multiple ectopic foci (n=3; 9.7%). These findings are consistent with those of Amir et al,10 where higher incidences of unifocal PVC were reported (91.8%) as compared to multifocal PVC (8.2%). In 1971, Lown and Wolf18 devised a grading system, Lown grading system, for ventricular arrhythmia where PVC was classified according to its complexity (unifocal, frequency, multifocal, consecutive, phenomenon “R on T”). This study reported the absence of any correlation between the level of complexity of PVC and patient’s prognosis, except in coronary heart disease.
Origin of PVC
According to the EPS reports, a large number of samples showed PVC arising from the right heart, particularly from RVOT (58.1%), whereas no PVC origin was found to originate from tricuspid valve and LVOT. This could be attributed to the rare prevalence of tricuspid and LVOT origin of PVC as reported in a study conducted by Tada et al,19 where only 8% of 454 idiopathic PVC patients showed PVC originating from the tricuspid annulus. Electrophysiology studies sometimes do not always provide satisfactory results in PVC patients, especially in the cases arising from RVOT. This is particularly seen in certain cases, where arrhythmias cannot be induced in patients by programmed stimulation or administration of isoproterenol. This is one of the major limitations during catheter ablation handling. In addition to this, patients with non‐induced PVC require a longer time for catheter procedures and radiation exposure.20 The present study included 12.9% cases where PVC was not induced during electrophysiological. According to the predictions of the Park algorithm, three out of four PVC showed an origin from OT. The procedure for EPS is usually performed under mild sedation using fentanyl as sedative, which can increase the vagal tone. Since PVC is very sensitive to sedation, focus of arrhythmia is easily suppressed upon sedation, especially in the cases that arise from OT.21 The underlying mechanism responsible for origin of PVC from the OT is a re-entry mechanism that is induced either by a triggering activity or delay after depolarization. Mutations in G protein that stimulate an increase in intracellular levels of cAMP have been found to be associated with arrhythmogenic focus of myocardial OT.22
Congruity Between the Prediction of the Park Algorithm and Results of Electrophysiology Studies for Patients with or without Structural Heart Disease
Most of the PVC locations (85.2%) predicted using the Park algorithm were consistent with the findings of the EPS. However, no significant difference (P=0.79) was reported in the ANOVA test for the branching group of the Park algorithm. This suggests that the discrepancies in the predictive ability of Park algorithm for PVC location based on EPS are not contributed by some error in one of the branches of the algorithm. This cannot be addressed using statistical tools due to the presence of empty columns. Further studies with larger sample size will be helpful in addressing this issue.
Many characteristics of PVC have been explored in the past based on ECG data. With reference to the cardiac anatomy, Park et al complied the characteristic of a typical ECG for each PVC location based on bundle branch block (BBB) type, frontal axis, precordial transition, and PVC morphology into V1, V6, and I leads. A single algorithm was generated to predict PVC location that showed significant congruity with the gold standard three-dimensional mapping used in the present study (for further details see Supplementary Table 1). Although the Park algorithm is limited by the complexity of the existing anatomy, its congruity is significant enough for the prediction of location of PVC on the basis of differences in the main characteristics. Currently, no algorithm is available to determine the location of PVC origin which can be used as a fixed procedure before EPS. Although the Park algorithm has not been proven statistically owing to limited sample size, it can predict PVC location with 85% similarity to the results of EPS, and thus can be employed in clinical practice.
The Park algorithm is designed on the basis of the typical characteristics of ECG reported in previous studies conducted for idiopathic PVC. The present study included PVC with structural heart disease. No significant differences were observed between PVC with or without structural heart disease as predicted using this algorithm (P=0.39). Thus, it can be concluded that the presence or absence of structural heart disease in the case of PVC has no effect on the characteristics of PVC location obtained using 12‐lead ECG, and this algorithm can be used to determine the location of PVC origin in patients with or without structural heart disease.
Several previous studies have established the superiority of three‐dimensional electrophysiology studies over conventional ones. In particular, the application of three‐dimensional mapping results in the reduction of fluoroscopy duration and radiation dose as compared to the conventional mapping strategies. In addition to this, three‐dimensional mapping reduces the duration of procedure and improves the success rate, especially in the cases of complex arrhythmia, such as VT and atrial fibrillation (AF), that require ablation.3,23 Park et al6 generated a single algorithm by compiling the findings of various previous studies that utilized different tools, some used three‐dimensional mapping, while others used conventional mapping strategies. In the present study, all PVCs used three‐dimensional mapping and the results for most of them were compatible with the findings of the Park algorithm. Most of the PVCs arising from the right side of the heart showed a preserved ejection function as compared to the PVCs arising from the left heart. This difference was statistically significant, with P=0.01. In the present study, most PVCs originating from the right heart were localized to the RVOT, where the mechanism is not related to scarring. The underlying pathomechanisms responsible for the occurrence of the left and right heart PVC are quite similar. Even in the present case, PVC with and without structural heart disease showed no significant correlation with the location, left or right heart PVC (P=0.43). However, the presence of structural heart disease was found to be more associated with a decrease in ejection fraction (P=0.07). PVC mechanism of arrhythmias in structural heart disease is associated with fibrotic tissue (scar),22 that can occur due to MI, ischemic cardiomyopathy, ventricular hypertrophy, and heart valve disease.24,25 A decrease in ejection fraction is caused by changes in myocardial function arising due to scarring from the contractile myocardium to non‐contractile myocardium.24 Clinical manifestations of idiopathic ventricular arrhythmias are variable, ranging from mild asymptomatic PVC to persistent VT. PVC in structural heart disease is associated with structural changes in the heart contributed by ischemic heart disease, heart valve disease, cardiomyopathy, and ventricular hypertrophy.26,27 Therefore, availability of information about the location of PVC origin in the right or left heart based on the ECG studies will be beneficial not only for EPS procedures but will also assist in better evaluation and patient management.
In a study conducted by Latchamsetty et al28 in 2015, the duration for EPS and PVC ablation was found to be 198±115 minutes, average fluoroscopy time was 30±24 minutes, and average radiofrequency energy delivery time was 12±11 minutes. The lowest time required for procedure and fluoroscopy in the case of RVOT PVC was 157±97 minutes and 20±17 minutes, respectively (P<0.01). Patients with PVC originating from papillary muscles and the epicardium required the longest procedure time of 249±109 minutes and 249±117 minutes, respectively. In addition to this, a longer fluoroscopic time of 40±21 minutes and 48±27 minutes were also required in the case of PVC arising from papillary muscles and epicardium, respectively, as compared to the patients with PVC originating from RVOT or LVOT (P<0.01). Patients with PVC arising from papillary muscle involved the longest radiofrequency time, 26±19 minutes, as compared to the others (P<0.01). Patients with a single PVC configuration were characterized by fastest procedure, fluoroscopy, and radiofrequency time as compared to multiple PVC (P<0.01). In terms of outcomes of EPS and ablation, the highest and lowest success rates for ablation were observed in RVOT PVC (93%) and epicardial PVC (67%), respectively. It was reported that complications of electrophysiology and ablation studies were tamponade (0.8%), pseudoaneurysm (0.7%), hematoma (0.34%), arteriovenous fistula (0.25%), and atrioventricular (AV) block (0.1%). The complications were frequent in epicardial PVC ablation (4.2%), while complications were a rare event in RVOT PVC (2.1%).28 Thus, all these findings suggest that even a slight knowledge regarding the location of PVC before the EPS procedure and ablation will be highly instrumental in estimating the duration of the procedure, duration of radiation exposure, outcome, and complications. This will further assist in improving the strategy and patient education.
Prediction of the origin of PVC before EPS procedures and ablation is particularly crucial in terms of strategies devised for vascular access. PVC from the right heart is accessed through a vein, while PVC from the left heart is accessed via an artery. The Park algorithm for prediction of origin of PVC from left or right heart showed 95% sensitivity, 100% specificity, 100% positive predictive value, 87.5% negative predictive value, and 96% accuracy as studied using cross-tabulation and Fisher’s exact test (P<0.001). Thus, all these findings highlight the suitability of the Park algorithm in predicting the origin of PVC from the right or left side of the heart.
Conclusion
Most PVCs with or without structural heart disease originated from the right heart, especially in the OTs. The suitability of the Park algorithm in determining the origin of PVC was quite accurate. Statistical analysis established high sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the Park algorithm, and thus the algorithm can be used to distinguish PVC arising from the right or left heart.
Despite all these features, the application of the Park algorithm in determining PVC origin suffers from certain limitations. So, far, the Park algorithm has not been able to determine PVC origination from specific locations such as OT or non-outflow tract. The present study did not include all the PVC locations and thus it cannot be tested statistically. Besides this, no relationship can be established between ischemic PVC and location of PVC origin, owing to the absence of angiographic data of patients included in the study.
Compliance with Ethical Standards
All the procedures performed in the study were approved by the Ethics committee of the Biomedical Research Ethics Commission in humans at the Medical Faculty of Hasanuddin University and Dr. Wahidin Sudirohusodo hospital, Makassar, Indonesia (file number UH19050331) and conducted in accordance with the guidelines of the 1975 declaration of Helsinki. All subjects included in the study were pre-informed about all the procedures and signed a patient consent form which was collected as per the comprehensive agreement method of the hospital. The patients’ anonymity was protected throughout.
Acknowledgments
The authors would like to express their sincere gratitude towards the data management team at Integrated Heart Center Wahidin Sudirohusodo Hospital, Makassar, Indonesia for their cooperation while obtaining and extracting patients’ data for the study.
Disclosure
The authors declare no conflict of interest.
References
1. Ahn M-S. Current concepts of premature ventricular contractions. J Lifestyle Medicine. 2013;3(1):26.
2. Aliot EM, Stevenson WG, Almendral-Garrote JM, et al. EHRA/HRS expert consensus on catheter ablation of ventricular arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Europace European Pacing Arrhythmias Cardiac Electrophysiol. 2009;11(6):771–817.
3. Bhakta D, Miller JM. Principles of electroanatomic mapping. Indian Pacing Electrophysiol J. 2008;8(1):32.
4. Lin D, Ilkhanoff L, Gerstenfeld E, et al. Twelve-lead electrocardiographic characteristics of the aortic cusp region guided by intracardiac echocardiography and electroanatomic mapping. Heart Rhythm. 2008;5(5):663–669. doi:10.1016/j.hrthm.2008.02.009
5. Betensky BP, Park RE, Marchlinski FE, et al. The V2 transition ratio: a new electrocardiographic criterion for distinguishing left from right ventricular outflow tract tachycardia origin. J Am Coll Cardiol. 2011;57(22):2255–2262. doi:10.1016/j.jacc.2011.01.035
6. Park K-M, Kim Y-H, Marchlinski FE. Using the surface electrocardiogram to localize the origin of idiopathic ventricular tachycardia. Pacing Clin Electrophysiol. 2012;35(12):1516–1527. doi:10.1111/j.1540-8159.2012.03488.x
7. Akdemir B, Yarmohammadi H, Alraies MC, Adkisson WO. Premature ventricular contractions: reassure or refer? Cleveland Clinic J Medicine. 2016;83(7):525–530. doi:10.3949/ccjm.83a.15090
8. Issa Z, M=iller JM, Zipes DP. Clinical arrhythmology and electrophysiology: a companion to braunwald’s heart disease e-book: expert consult: online and Print. Elsevier Health Sci. 2012.
9. De Chillou C, Lacroix D, Klug D, et al. Isthmus characteristics of reentrant ventricular tachycardia after myocardial infarction. Circulation. 2002;105(6):726–731. doi:10.1161/hc0602.103675
10. Amir M, Mappangara I, Setiadji R, Zam SM. Characteristics and prevalence of premature ventricular complex: a telemedicine study. Cardiology Research. 2019;10(5):285. doi:10.14740/cr887
11. Brodsky M, Wu D, Denes P, Kanakis C, Rosen KM. Arrhythmias documented by 24 hour continuous electrocardiographic monitoring in 50 male medical students without apparent heart disease. Am J Cardiol. 1977;39(3):390–395. doi:10.1016/S0002-9149(77)80094-5
12. Kostis JB, McCrone K, Moreyra A, et al. Premature ventricular complexes in the absence of identifiable heart disease. Circulation. 1981;63(6):1351–1356. doi:10.1161/01.CIR.63.6.1351
13. Reynolds MR, Gunnarsson CL, Hunter TD, et al. Health outcomes with catheter ablation or antiarrhythmic drug therapy in atrial fibrillation: results of a propensity-matched analysis. Circ Cardiovasc Qual Outcomes. 2012;5(2):171–181.
14. Lin C-Y, Chang S-L, Lin Y-J, et al. An observational study on the effect of premature ventricular complex burden on long-term outcome. Medicine. 2017;96:1.
15. Langendorf R, Pick A, Winternitz M. Mechanisms of intermittent ventricular bigeminy: i. appearance of ectopic beats dependent upon length of the ventricular cycle, the “rule of bigeminy.”. Circulation. 1955;11(3):422–430. doi:10.1161/01.CIR.11.3.422
16. Duffee DF, Shen W-K, Smith HC. Suppression of frequent premature ventricular contractions and improvement of left ventricular function in patients with presumed idiopathic dilated cardiomyopathy. Mayo Clin Proc. 1998;73(5):430–433. doi:10.1016/S0025-6196(11)63724-5
17. Niwano S, Wakisaka Y, Niwano H, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart. 2009;95(15):1230–1237. doi:10.1136/hrt.2008.159558
18. Lown B, Wolf M. Approaches to sudden death from coronary heart disease. Circulation. 1971;44(1):130–142. doi:10.1161/01.CIR.44.1.130
19. Tada H, Tadokoro K, Ito S, et al. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm. 2007;4(1):7–16. doi:10.1016/j.hrthm.2006.09.025
20. Wang Z, Zhang H, Peng H, et al. Voltage combined with pace mapping is simple and effective for ablation of noninducible premature ventricular contractions originating from the right ventricular outflow tract. Clin Cardiol. 2016;39(12):733–738. doi:10.1002/clc.22598
21. Deng Y, Naeini PS, Razavi M, Collard CD, Tolpin DA, Anton JM. Anesthetic management in radiofrequency catheter ablation of ventricular tachycardia. Texas Heart Institute J. 2016;43(6):496–502. doi:10.14503/THIJ-15-5688
22. Bunch TJ, Day JD. Right meets left: a common mechanism underlying right and left ventricular outflow tract tachycardias. J Cardiovasc Electrophysiol. 2006;17(10):1059–1061. doi:10.1111/j.1540-8167.2006.00577.x
23. Christoph M, Wunderlich C, Moebius S, et al. Fluoroscopy integrated 3D mapping significantly reduces radiation exposure during ablation for a wide spectrum of cardiac arrhythmias. Ep Europace. 2015;17(6):928–937. doi:10.1093/europace/euu334
24. Turkbey EB, Nacif MS, Guo M, et al. Prevalence and correlates of myocardial scar in a US cohort. JAMA. 2015;314(18):1945–1954. doi:10.1001/jama.2015.14849
25. Musa TA, Treibel TA, Vassiliou VS, et al. Myocardial scar and mortality in severe aortic stenosis: data from the BSCMR valve consortium. Circulation. 2018;138(18):1935–1947. doi:10.1161/CIRCULATIONAHA.117.032839
26. DeMaria AN, Amsterdam EA, Vismara LA, Neumann A, Mason DT. Arrhythmias in the mitral valve prolapse syndrome: prevalence, nature, and frequency. Ann Intern Med. 1976;84(6):656–660. doi:10.7326/0003-4819-84-6-656
27. Ruberman W, Weinblatt E, Goldberg JD, Frank CW, Shapiro S. Ventricular premature beats and mortality after myocardial infarction. New England J Med. 1977;297(14):750–757. doi:10.1056/NEJM197710062971404
28. Latchamsetty R, Yokokawa M, Morady F, et al. Multicenter outcomes for catheter ablation of idiopathic premature ventricular complexes. JACC. 2015;1(3):116–123.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.