Back to Journals » Journal of Inflammation Research » Volume 16
Pan-Immune-Inflammatory Value in Patients with Non-Small-Cell Lung Cancer Undergoing Neoadjuvant Immunochemotherapy
Authors Zhai WY, Duan FF , Lin YB, Lin YB, Zhao ZR, Wang JY, Rao BY, Zheng L , Long H
Received 22 April 2023
Accepted for publication 1 August 2023
Published 8 August 2023 Volume 2023:16 Pages 3329—3339
DOI https://doi.org/10.2147/JIR.S418276
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Wen-Yu Zhai,1,2,* Fang-Fang Duan,3,* Yao-Bin Lin,1,2,* Yong-Bin Lin,1,2 Ze-Rui Zhao,1,2 Jun-Ye Wang,1,2 Bing-Yu Rao,1,2 Lie Zheng,4 Hao Long1,2
1Department of Thoracic Surgery, State Key Laboratory of Oncology in Southern China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-Sen University Cancer Center, Guangzhou, People’s Republic of China; 2Lung Cancer Research Center, Sun Yat-Sen University, Guangzhou, People’s Republic of China; 3Department of Medical oncology, State Key Laboratory of Oncology in Southern China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-Sen University Cancer Center, Guangzhou, People’s Republic of China; 4Medical Imaging Division, Department of Medical Imaging and Interventional Radiology, State Key Laboratory of Oncology in Southern China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-Sen University Cancer Center, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hao Long, State Key Laboratory of Oncology in Southern China, Collaborative Innovation Center for Cancer Medicine, and Department of Thoracic Surgery, Sun Yat-Sen University Cancer Center, 651 Dongfeng Road East, Guangzhou, Guangdong, 510060, People’s Republic of China, Email [email protected] Lie Zheng, State Key Laboratory of Oncology in Southern China, Collaborative Innovation Center for Cancer Medicine, and Medical Imaging Division, Department of Medical Imaging and Interventional Radiology, Sun Yat-Sen University Cancer Center, 651 Dongfeng Road East, Guangzhou, Guangdong, 510060, People’s Republic of China, Email [email protected]
Background: We aimed to investigate the predictive value of a systematic serum inflammation index, pan-immune-inflammatory value (PIV), in pathological complete response (pCR) of patients treated with neoadjuvant immunotherapy to further promote ideal patients’ selection.
Methods: The clinicopathological and baseline laboratory information of 128 NSCLC patients receiving neoadjuvant immunochemotherapy between October 2019 and April 2022 were retrospectively reviewed. We performed least absolute shrinkage and selection operator (LASSO) algorithm to screen candidate serum biomarkers for predicting pCR, which further entered the multivariate logistic regression model to determine final biomarkers. Accordingly, a diagnostic model for predicting individual pCR was established. Kaplan–Meier method was utilized to estimate curves of disease-free survival (DFS), and the Log rank test was analyzed to compare DFS differences between patients with and without pCR.
Results: Patients with NSCLC heterogeneously responded to neoadjuvant immunotherapy, and those with pCR had a significant longer DFS than patients without pCR. Through LASSO and the multivariate logistic regression model, PIV was identified as a predictor for predicting pCR of patients. Subsequently, a diagnostic model integrating with PIV, differentiated degree and histological type was constructed to predict pCR, which presented a satisfactory predictive power (AUC, 0.736), significant agreement between actual and our nomogram-predicted pathological response.
Conclusion: Baseline PIV was an independent predictor of pCR for NSCLC patients receiving neoadjuvant immunochemotherapy. A significantly longer DFS was achieved in patients with pCR rather than those without pCR; thus, the PIV-based diagnostic model might serve as a practical tool to identify ideal patients for neoadjuvant immunotherapeutic guidance.
Keywords: non-small-cell lung cancer, pan-immune-inflammatory value, neoadjuvant immunochemotherapy, pathological complete response, survival benefits
Introduction
More than 35% of patients with non-small-cell lung cancer (NSCLC) are diagnosed with locally advanced stage disease.1 Although patients with NSCLC usually undergo radical resection, nearly half of them remain to experience recurrence and ultimately succumb to the disease.2,3 With respect to patients with resectable, locally advanced stage NSCLC, neoadjuvant immune checkpoint inhibitors (ICIs) plus chemotherapy have been explored to induce an unexpectedly high pathological response rate and recommended to be used in clinical settings in the National Comprehensive Cancer Network (NCCN) guideline. The prospective, multicenter, open-label, randomized stage III trial Checkmate 816 has reported that the pathological complete response (pCR) of patients with resectable stage IB-IIIA NSCLC receiving neoadjuvant nivolumab plus chemotherapy was nearly 11 times higher than that of patients only treated with adjuvant chemotherapy (P < 0.0001), which suggested the possible short-term benefits of neoadjuvant immunotherapy-based treatment for patients with NSCLC. Moreover, its latest long-term follow-up results presented significantly better event-free survival (EFS) benefits from neoadjuvant immunochemotherapy than from chemotherapy alone (31.6 months vs 20.8 months, P = 0.0052). In addition, subgroup analysis further favored nivolumab plus chemotherapy rather than sole chemotherapy for better pCR and longer EFS, regardless of the tumor stage, programmed cell death ligand 1 (PD-L1) expression level, and histological type.4
Major pathological response (MPR) is characterized by the presence of no more than 10% residual tumor cells in both the primary tumor bed and all sampled regional lymph nodes after neoadjuvant therapy and surgery, and the pCR is diagnosed in the absence of residual cancer cells in the lung mass from the surgical specimen and all sampled regional lymph nodes following the completion of neoadjuvant treatment and resection. MPR and pCR are usually used as surrogate endpoints in clinical trials on neoadjuvant immunotherapy, which is a strong predictor of long-term survival.5,6 As reported in previous clinical trials, the pCR rates after neoadjuvant immunotherapy for patients with NSCLC range from 4.9% to 63%;7–9 ie, most patients with NSCLC fail to respond positively to ICIs in the neoadjuvant setting. Meanwhile, immune-related adverse events are common.10 PD-L1 and tumor mutational burden are the most common potential predictor for responses to ICIs, but their application was limited due to their unsatisfactory predictive accuracies and high costs.11 Hence, the exploitation of novel, effective, and practical biomarkers at a low cost to select ideal patients who could benefit from neoadjuvant immunotherapy is necessary and meaningful.
It is widely acknowledged that serum inflammation indexes are closely associated with the systemic immune-inflammatory status of patients with lung cancer.12–14 Some serum inflammation indexes, such as neutrophil-to-lymphocyte ratio (NLR), monocyte–lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR), and prognostic nutrition index (PNI), have been explored as biomarkers for the efficacy of immunotherapy for patients with advanced NSCLC.13,15,16 However, their predictive value in neoadjuvant immunotherapy for resectable NSCLC patients is poorly elucidated. Only one study analyzed a subpart of their total cohort and further found that NLR was related to the pathological response to neoadjuvant immunochemotherapy.17 Recently, a novel, systematic serum inflammation index, the pan-immune-inflammatory value (PIV), was shown to exhibit a stronger predictive performance than other serum indexes in the salvage of metastatic colorectal and breast cancer.18,19 Meanwhile, the correlation between PIV and the pathological response to neoadjuvant immunotherapy in NSCLC remained unclear.
In the current study, we analyzed a real-world NSCLC cohort treated with neoadjuvant immunochemotherapy to investigate the diagnostic value of PIV in predicting pCR. In addition, we developed a model integrating PIV with other clinicopathological features to predict pCR after neoadjuvant immunochemotherapy for patients with NSCLC.
Materials and Methods
Patients
This study was conducted in accordance with the recommendations of the Declaration of Helsinki and was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center (SYSUCC) (No. B2022-445-01). The requirement for written informed consent from patients was waived because this was a retrospective study, and the medical data of patients were handled with confidentiality without any intervention.
In the current study, patients with NSCLC who underwent neoadjuvant immunotherapy and subsequently completed surgery between October 2019 and April 2022 at SYSUCC were screened retrospectively. Key inclusion criteria included (1) clinical diagnosis of stage IIA-IIIB (cT1-4N0-3) NSCLC according to the eighth edition of the American Joint Committee on Cancer (AJCC, 2010) criteria by biopsy pathology; (2) administration of at least 2 cycles of neoadjuvant immunochemotherapy; (3) complete pretreatment laboratory data of peripheral blood counts and albumin; (4) resection specimens subjected to pathological assessment after completion of neoadjuvant immunotherapy. Besides, ICIs are largely ineffective for NSCLC patients with epidermal growth factor receptor (EGFR) gene mutation and anaplastic lymphoma kinase (ALK) gene rearrangement, and several large clinical trials, such as Checkmate 816 and NADIM, both excluded patients with EGFR mutation and ALK rearrangement. Therefore, patients with EGFR mutation and ALK rearrangement were also excluded from the current study.
Data Collection and Definition of Serum Inflammation Indexes
We manually retrieved patients’ laboratory data measured one week before neoadjuvant treatment from the electronic medical record system of SYSUCC, including the counts of neutrophils (109/L, N), monocytes (109/L, M), platelets (109/L, P), lymphocytes (109/L, L), and serum albumin concentrations (105/L, A). The specific serum inflammation indexes were calculated as follows: PIV = N*M*P/,18,19 NLR = N/L,20 MLR = M/L,21 PLR = P/L,20 platelet–albumin ratio (PAR) = P/A,22 systemic immune inflammation index (SII) = P*NLR,23 PNI = A+5*L,24 systemic inflammation response index (SIRI) = N*ML.25,26 According to the cutoff value determined by the median value, continuous serum inflammation index parameters were classified as categorical variables (high vs low), namely, PIV (371.97), NLR (2.51), MLR (0.26), PLR (157.50), PAR (6.70), SII (389.55), PNI (52.33), and SIRI (1.35). Moreover, other clinicopathological factors, such as gender, age, histological type, cT stage, cN stage, smoking history, and differentiation degree, were also collected from the electronic medical record system of SYSUCC. The disease in all patients in this study was restaged according to the eighth edition AJCC criteria.
Pathological Assessment After Neoadjuvant Immunotherapy
The sample pathology was assessed by two professional pathologists based on multidisciplinary recommendations from the International Association for Lung Cancer Research.27 pCR is defined as the absence of residual tumor cells in the primary tumor bed and all sampled lymph nodes after neoadjuvant therapy and surgery. MPR is defined as the presence of no more than 10% residual cancer cells within the primary tumor bed, which is same as the Checkmate 159 study.28
Follow-Up of Patients and Study Endpoints
Patients were followed up every 3 months postoperatively with blood examination, and chest and abdominal computed tomography (CT), positron emission tomography, and brain magnetic resonance imaging (MRI) were performed if necessary. The main study endpoint is pCR, and the secondary study endpoint is disease-free survival (DFS), which is defined as the time interval from the date of surgery to the date of the first event recurrence.
Statistical Analysis
Continuous variables were presented as median values with interquartile ranges (IQR). We used Student’s t-test to compare continuous variables with normal distribution and Mann–Whitney U-test to compare continuous variables which do not fit normal distribution. Categorical variables were listed as count (percentage) and compared using the chi-squared test or Fisher’s exact test. Considering too many variables and multicollinearity among variables will weakening statistical power of multivariate Logistics model, the least absolute shrinkage and selection operator (LASSO) algorithm was used via the “glmnet” R package to eliminate possible multicollinearity and narrow down candidate diagnostic serum inflammation indexes. LASSO can compress the regression coefficients of some unnecessary variables to zero and then remove them from the model to achieve the purpose of variable screening, ensuring the simplicity and stability of the model. It also realizes the combination and optimization of the characteristics of optimal subset and ridge regression. When applied to variable screening, LASSO can be used no matter it is continuous, binary, or multi-classification, and it is effective in processing high-dimensional small sample data and solving multicollinearity problems. At the same time, there are several points to note in the analysis process of LASSO. First, LASSO may inevitably produce some deviation in the process of coefficient compression of variables; second, when there are highly correlated features in a set of predictors, LASSO can only select one of the features, resulting in an inaccurate result, or when the number of variables is much larger than the number of samples, LASSO’s fitting effect is poor. Finally, we performed multivariate logistic regression analysis to determine the independent diagnostic serum factors for predicting pCR. Based on the results of the multivariate logistic regression analysis, a diagnostic model for predicting pCR from neoadjuvant immunotherapy was subsequently developed and graphically presented as a nomogram using the “rms” package of R software, according to which, each subtype within these variables would be scored on the point scale, by adding up the total score and locating it on the total point scale, we could draw a straight line down to determine the estimated probability of pCR. Subsequently, we internally assessed the predictive accuracy of this nomogram through the area under the curve (AUC) of the receiver operating characteristic (ROC) and calibration curves, whose X-axis and the Y-axis respectively present the predicted and actual pCR probability, and the 45-degree dashed lines through the coordinate origin represent the excellent calibration models. In addition, the clinical value of our nomogram was evaluated by decision curve analysis (DCA), which could assess the net benefit of NSCLC patients from neoadjuvant immunotherapy. Survival curves for DFS were estimated using the Kaplan–Meier method and compared using the Log rank test. Statistical analyses in our study were performed using R software (version 4.0.1, Vanderbilt University, Nashville, TN, USA). A two-tailed P value <0.05 was considered statistically significant.
Results
Clinicopathological Characteristics of Patients
A total of 128 patients with NSCLC were considered eligible for this study, among them, 91 cases had lung squamous carcinoma (LUSC), 21 patients had lung adenocarcinoma (LUAD), 9 patients had lung lymphoepithelioma-like carcinoma (LELC), 4 patients had adenosquamous carcinoma, and 3 patients had large cell neuroendocrine carcinoma. As listed in Table 1, the median age of total patients was 61 years old. Before neoadjuvant therapy, 113 patients were diagnosed with lymph node metastasis, and 83 of them exhibited the pathological absence of cancer cells in all lymph node samples after surgery. Fifty-one patients achieved pCR after neoadjuvant immunotherapy, and they showed poorer differentiation and tumor undifferentiation (P = 0.012) and a higher proportion of LUSC (P = 0.042).
 |
Table 1 Patients’ Characteristic |
ICIs used in NSCLC patients in this study were conducted every 21 days, including Nivolumab (240 mg), Pembrolizumab (200 mg), Tislelizumab (200 mg), Toripalimab (240 mg), Sintilimab (200 mg), Camrelizumab (200 mg), and durvalumab (1000 mg). The most common neoadjuvant regimens included Tislelizumab plus albumin-bound paclitaxel plus platinum (44, 34.4%). Details of the immunochemotherapy regimen were shown in Supplementary Table.
Identification of Diagnostic Serum Biomarkers for pCR
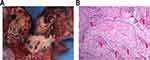
Figure 1 illustrates the pathological response after neoadjuvant immunochemotherapy. A flow chart displaying the identification of diagnostic biomarkers for pCR is provided in Figure 2. First, we performed the LASSO regression algorithm to screen the most valuable diagnostic serum markers without multicollinearity. Among the 13 candidate factors without zero coefficients at the optimal value −3.43 of log(e)λ (Supplementary Figure 1A and B), PIV, differentiated degree, histological type, and smoking history were selected for subsequent multivariate logistics regression model, which showed that high PIV (P = 0.030, OR 2.395; 95% CI 1.087–5.278), differentiated degree (P = 0.001, OR 3.100; 95% CI 1.588–6.167), and histological type (LUSC versus others, P = 0.003, OR 0.103; 95% CI 0.023–0.457) were the independent diagnostic biomarkers for pCR (Table 2).
 |
Table 2 Multivariate Logistic Regression Analyses for pCR |
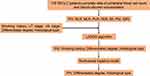 |
Figure 2 Flowchart of the study design. |
Development of a Nomogram for pCR Based on Diagnostic Serum Biomarkers
Integrating above three independent indicators derived from multivariate logistic regression analysis, ie, PIV, differentiated degree, and histological type, we established a diagnostic nomogram to predict pCR after neoadjuvant immunochemotherapy for patients with NSCLC (Figure 3A). In this nomogram, each factor was assigned a score in the point line, and a total score was assigned for each patient with NSCLC. By identifying the patient’s total score on the pCR scale, the probability of pCR in each individual could be predicted.
Assessment of the Predictive Performance of the Diagnostic Model
We estimated the AUC of the ROC curve of this nomogram to evaluate its diagnostic performance (Figure 3B), which presented a good diagnostic efficacy with a high AUC value of 0.736. The Y-axis of the calibration curve shows the observed pathological response, and its X-axis shows the nomogram predicted response. The calibration curve (Figure 3C) exhibited good agreement between the virtual and predicted probabilities of the pathological response, which indicated the satisfactory predictive performance of our nomogram. In addition, we prepared DCA plots and demonstrated that NSCLC patients treated with neoadjuvant immunotherapy could obtain a net benefit from our diagnostic nomogram (Figure 3D).
The Prognostic Value of pCR for Survival
The median follow-up time of our study was 7.6 months (interquartile range: 3.2 to 16.1 months). Fifteen patients suffered from tumor recurrence after resection, including 13 non-pCR and 2 pCR. The survival curve revealed that patients with pCR had apparently better DFS than those without pCR (Figure 4). However, other clinicopathological characteristics, such as age, sex, MPR, and PIV, failed to show prognostic value for patients receiving neoadjuvant immunotherapy (Supplementary Figure 2). Further, a multivariate Cox analysis including age, sex, cT stage, cN stage, pCR, histological type, differentiated degree and PIV also demonstrated that only pCR was the independent prognostic factor for patients with neoadjuvant immunotherapy (P = 0.027, HR 0.166; 95% CI 0.034–0.817) (Supplementary Figure 3).
 |
Figure 4 Disease-free survival for patients with pCR and non-pCR. |
Discussion
Based on findings from this real-world cohort, PIV was determined to be an independent diagnostic biomarker of pCR for patients with NSCLC receiving neoadjuvant immunochemotherapy. Additionally, we further developed a diagnostic model integrating PIV with the differentiated degree and histological type to predict individual possibility of pCR, which presented a good predictive performance. Subsequently, the survival analysis revealed that pCR was the only prognostic factor for DFS.
Inflammation is a key characteristic of tumors and is closely related to anti-tumor immunology.29 On the one hand, the expression pattern of inflammation-related genes, cytokines, and inflammatory proteins affects the tumor immune microenvironment by influencing local inflammation.30,31 On the other hand, the systemic inflammatory status plays an important role in the survival and immunotherapy of cancers.32 Based on the above theories, an increasing number of studies have focused on the potential impact of serum inflammation indexes on survival and immunotherapy. Diem et al reviewed data from 52 patients with metastatic or non-resectable NSCLC treated with nivolumab, and they showed that NLR and PLR were negative prognostic factors for overall survival (OS).12 Another retrospective study, which included data from 86 patients with metastatic renal cell carcinoma and 61 patients with metastatic NSCLC receiving nivolumab, demonstrated that an increased NLR at 6 weeks was negatively associated with the OS and progression-free survival (PFS).33 Sun et al collected data from 79 and 89 patients resectable NSCLC who underwent neoadjuvant immunotherapy and neoadjuvant chemotherapy, respectively, and deduced that the baseline NLR before treatment was a predictor of both pCR and DFS.17
NLR only considers neutrophils and lymphocytes but does not include platelets and monocytes, whereas PIV integrates all of them. Therefore, PIV might be a more comprehensive reflection of the systemic inflammation status and produce a high predictive accuracy, which has been explored in several studies.18,19 Gambichler et al retrospectively analyzed data of 49 patients with stage I–III Merkel cell carcinoma and found that PIV was a predictor for tumor recurrence.34 With respect to the use of ICIs for anti-tumor therapy, the predictive value of PIV in survival cancers has been acknowledged. Corti et al reviewed the data of 163 patients with metastatic colorectal cancer treated with immunotherapy and revealed that patients with high PIV had poorer survival. However, an early increase in PIV could yield clinical benefits.35 In the current study, we showed that a high pretreatment PIV was a positive biomarker of pCR for patients with NSCLC who underwent neoadjuvant immunochemotherapy. This is the first study to explore the predictive role of PIV in neoadjuvant immunotherapy for patients with NSCLC.
In clinical practice, a higher pCR rate was observed in patients with LUSC than that in LUAD patients, similar to the results obtained in this study. Two previously published Phase II clinical trials also demonstrated that LUSC patients tended to have a higher MPR and pCR rate than patients with LUAD.7,36 Another retrospective study collected data from 2064 patients with lung cancer from the National Cancer Database and showed that LUSC patients showed greater benefits from immunochemotherapy.37 With respect to patients with non-squamous-adenocarcinoma NSCLC, the pathological response is rarely reported. In this study, 17 patients were pathologically diagnosed with non-squamous-adenocarcinoma NSCLC, and only 3 of them exhibited pCR, including 2 patients with LELCs and 1 patient with large cell neuroendocrine carcinoma. Additionally, non-squamous-adenocarcinoma was found to be negatively associated with pCR in the multivariate logistic analysis. For these conditions, it is essential to explore a more effective regimen to improve the pathological response rate.
However, some limitations of the present study should be considered. First, this was a single-center retrospective study. Although we reported the largest real-world cohort to date, the sample size is considerably limited, and selection bias is inevitable; hence, the validation of our results in large, multicenter, prospective cohorts is warranted. Second, we failed to obtain information on PD-LI expression and tumor mutation burden (TMB). Substantial studies have demonstrated the strong association between PD-L1 expression/TMB and response of immunotherapy in pan-cancer including NSCLC. Combined with the PD-L1 expression and TMB data, this nomogram might exhibit a better predictive performance for pCR. Unfortunately, most pre-treatment biopsy specimens in this study were obtained from primary hospitals, which did not have the infrastructure to conduct immune-histochemical staining for PD-L1 and next generation sequencing for TMB. Finally, our preliminary follow-up showed that patients with NSCLC with pCR had a significantly longer DFS, ie, pCR was a possible predictor for long-term survival benefits, which is in line with the findings of the Checkmate 816 trial.4 Whereas although PIV was a predictor for pCR, it failed to present a prognostic value for DFS, which might be explained by the brief follow-up. Therefore, we will continue with the follow-up and look forward to the long-term results.
Conclusion
We parsed the positively predictive value of pre-treatment serum PIV in pCR of patients with NSCLC treated with neoadjuvant immunotherapy in this study. Subsequently, a diagnostic nomogram integrating with serum PIV, differentiated degree and histological type for predicting pCR in NSCLC patients was developed and evaluated with a good predictive performance. Therefore, our nomogram based on PIV might serve as a practical tool to identify ideal patients with NSCLC most suitable for neoadjuvant immunotherapy, but further validation of the findings in future is necessary.
Data Sharing Statement
The key raw data have been deposited into the Research Data Deposit (http://www.researchdata.org.cn), with the Approval number RDDA2022679462 and the datasets used in this study are publicly available.
Ethics Approval and Informed Consent
This study was conducted in accordance with the recommendations of the Declaration of Helsinki and was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center (SYSUCC) (No. B2022-445-01). The requirement for written informed consent from patients was waived because this was a retrospective study, and the medical data of patients were handled with confidentiality without any intervention.
Acknowledgments
Wen-Yu Zhai, Fang-Fang Duan, and Yao-Bin Lin are co-first authors for this study. We thank Bullet Edits Limited for the English editing of the manuscript and Dr. Jietian Jin and Dr. Cong Li for pathological assessment.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Natural Science Foundation of Guangdong Province of China (Grant Numbers. 2019A1515011601).
Disclosure
The authors declare that there is no competing interests.
References
1. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) Edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. doi:10.1016/j.jtho.2015.09.009
2. Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res. 2014;3(4):242–249. doi:10.3978/j.issn.2218-6751.2013.12.05
3. Taylor MD, Nagji AS, Bhamidipati CM, et al. Tumor recurrence after complete resection for non-small cell lung cancer. Ann Thorac Surg. 2012;93(6):1813–1820. doi:10.1016/j.athoracsur.2012.03.031
4. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386:1973–1985. doi:10.1056/NEJMoa2202170
5. Melek H, Çetinkaya G, Özer E, et al. Pathological complete response after neoadjuvant/induction treatment: where is its place in the lung cancer staging system? Eur J Cardiothorac Surg. 2019;56(3):604–611. doi:10.1093/ejcts/ezz044
6. Martinez-Meehan D, Lutfi W, Dhupar R, et al. Factors associated with survival in complete pathologic response non-small cell lung cancer. Clin Lung Cancer. 2020;21(4):349–356. doi:10.1016/j.cllc.2020.03.003
7. Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, Phase 2 trial. Lancet Oncol. 2020;21(6):786–795. doi:10.1016/S1470-2045(20)30140-6
8. Rothschild SI, Zippelius A, Eboulet EI, et al. SAKK 16/14: durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small-cell lung cancer-A multicenter single-arm phase II trial. J Clin Oncol. 2021;39(26):2872–2880. doi:10.1200/JCO.21.00276
9. Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(11):1413–1422. doi:10.1016/S1470-2045(20)30453-8
10. Chen Y, Wen S, Xia J, et al. Association of dynamic changes in peripheral blood indexes with response to PD-1 inhibitor-based combination therapy and survival among patients with advanced non-small cell lung cancer. Front Immunol. 2021;2021:12.
11. Insa A, Martín-Martorell P, Di Liello R, et al. Which treatment after first line therapy in NSCLC patients without genetic alterations in the era of immunotherapy? Crit Rev Oncol Hematol. 2022;169:103538. doi:10.1016/j.critrevonc.2021.103538
12. Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7. doi:10.1016/j.lungcan.2017.01.013
13. Russo A, Russano M, Franchina T, et al. Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Outcomes with Nivolumab in Pretreated Non-Small Cell Lung Cancer (NSCLC): a large retrospective multicenter study. Adv Ther. 2020;37(3):1145–1155. doi:10.1007/s12325-020-01229-w
14. Cannon NA, Meyer J, Iyengar P, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-small-cell lung cancer. J Thorac Oncol. 2015;10(2):280–285. doi:10.1097/JTO.0000000000000399
15. Peng L, Wang Y, Liu F, et al. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother. 2020;69(9):1813–1822. doi:10.1007/s00262-020-02585-w
16. Sekine K, Kanda S, Goto Y, et al. Change in the lymphocyte-to-monocyte ratio is an early surrogate marker of the efficacy of nivolumab monotherapy in advanced non-small-cell lung cancer. Lung Cancer. 2018;124:179–188. doi:10.1016/j.lungcan.2018.08.012
17. Sun X, Feng Y, Zhang B, et al. The role of neutrophil-to-lymphocyte ratio in predicting pathological response for resectable NSCLC treated with neoadjuvant chemotherapy combined with PD-1 checkpoint inhibitors. Cancer Res Treat. 2021;54:1017–1029. doi:10.4143/crt.2021.1007
18. Fucà G, Guarini V, Antoniotti C, et al. The pan-immune-inflammation value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br J Cancer. 2020;123(3):403–409. doi:10.1038/s41416-020-0894-7
19. Ligorio F, Fucà G, Zattarin E, et al. The pan-immune-inflammation-value predicts the survival of patients with human epidermal growth factor receptor 2 (HER2)-positive advanced breast cancer treated with first-line taxane-trastuzumab-pertuzumab. Cancers. 2021;13(8). doi:10.3390/cancers13081964
20. Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. doi:10.1016/j.lungcan.2017.07.024
21. Zheng L, Xiong A, Wang S, et al. Decreased monocyte-to-lymphocyte ratio was associated with satisfied outcomes of first-line PD-1 inhibitors plus chemotherapy in stage IIIB-IV non-small cell lung cancer. Front Immunol. 2023;14:1094378. doi:10.3389/fimmu.2023.1094378
22. Guo M, Sun T, Zhao Z, Ming L. Preoperative platelet to albumin ratio predicts outcome of patients with non-small-cell lung cancer. Ann Thorac Cardiovasc Surg. 2021;27(2):84–90. doi:10.5761/atcs.oa.20-00090
23. Tong YS, Tan J, Zhou XL, Song YQ, Song YJ. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J Transl Med. 2017;15(1):221. doi:10.1186/s12967-017-1326-1
24. Johannet P, Sawyers A, Qian Y, et al. Baseline prognostic nutritional index and changes in pretreatment body mass index associate with immunotherapy response in patients with advanced cancer. J Immunother Cancer. 2020;8(2):e001674. doi:10.1136/jitc-2020-001674
25. Qi Q, Zhuang L, Shen Y, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122(14):2158–2167. doi:10.1002/cncr.30057
26. Hua X, Duan F, Zhai W, et al. A novel inflammatory-nutritional prognostic scoring system for patients with early-stage breast cancer. J Inflamm Res. 2022;15:381–394. doi:10.2147/JIR.S338421
27. Travis WD, Dacic S, Wistuba I, et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol. 2020;15(5):709–740. doi:10.1016/j.jtho.2020.01.005
28. Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–1986. doi:10.1056/NEJMoa1716078
29. Song JM, Qian X, Teferi F, Pan J, Wang Y, Kassie F. Dietary diindolylmethane suppresses inflammation-driven lung squamous cell carcinoma in mice. Cancer Prev Res. 2015;8(1):77–85. doi:10.1158/1940-6207.CAPR-14-0245
30. Ben-Baruch A. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol. 2006;16(1):38–52. doi:10.1016/j.semcancer.2005.07.006
31. Zhai WY, Duan FF, Chen S, et al. A novel inflammatory-related gene signature based model for risk stratification and prognosis prediction in lung adenocarcinoma. Front Genet. 2021;12:798131. doi:10.3389/fgene.2021.798131
32. Zhang Q, Song MM, Zhang X, et al. Association of systemic inflammation with survival in patients with cancer cachexia: results from a multicentre cohort study. J Cachexia Sarcopenia Muscle. 2021;12(6):1466–1476. doi:10.1002/jcsm.12761
33. Simonaggio A, Elaidi R, Fournier L, et al. Variation in neutrophil to lymphocyte ratio (NLR) as predictor of outcomes in metastatic renal cell carcinoma (mRCC) and non-small cell lung cancer (mNSCLC) patients treated with nivolumab. Cancer Immunol Immunother. 2020;69(12):2513–2522. doi:10.1007/s00262-020-02637-1
34. Gambichler T, Said S, Abu Rached N, et al. Pan-immune-inflammation value independently predicts disease recurrence in patients with Merkel cell carcinoma. J Cancer Res Clin Oncol. 2022;148:3183–3189. doi:10.1007/s00432-022-03929-y
35. Corti F, Lonardi S, Intini R, et al. The pan-immune-inflammation value in microsatellite instability-high metastatic colorectal cancer patients treated with immune checkpoint inhibitors. Eur J Cancer. 2021;150:155–167. doi:10.1016/j.ejca.2021.03.043
36. Zhao ZR, Yang CP, Chen S, et al. Phase 2 trial of neoadjuvant toripalimab with chemotherapy for resectable stage III non-small-cell lung cancer. Oncoimmunology. 2021;10(1):1996000. doi:10.1080/2162402X.2021.1996000
37. Tuminello S, Alpert N, Veluswamy R, et al. Modulation of chemoimmunotherapy efficacy in non-small cell lung cancer by sex and histology: a real-world, patient-level analysis. BMC Cancer. 2022;22(1):80. doi:10.1186/s12885-022-09187-y
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.