Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Oxymatrine Alleviates High-Fat-High-Fructose-Induced Fatty Liver in Rats: Understanding the Molecular Mechanism Through an Untargeted Metabonomics Study
Authors Li H, Wang C, Wang Q , Liu X, Zhang J, Zhang H, Fei W, Zhao H, Ren L
Received 1 August 2023
Accepted for publication 22 November 2023
Published 7 December 2023 Volume 2023:16 Pages 4013—4024
DOI https://doi.org/10.2147/DMSO.S428864
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Muthuswamy Balasubramanyam
Huan Li, Chang Wang, Qing Wang, Xuehua Liu, Juanjuan Zhang, He Zhang, Wenjie Fei, Hang Zhao, Luping Ren
Department of Endocrinology, Hebei General Hospital, Shijiazhuang, Hebei, People’s Republic of China
Correspondence: Luping Ren, Email [email protected]
Objective: Previous studies have shown that oxymatrine (OMT) can improve high-fat-high-fructose-diet-induced non-alcoholic fatty liver disease (NAFLD), and our study aimed to explore its possible metabolic potential mechanisms.
Methods: Wistar rats were fed a high-fat-high-fructose diet for 8 weeks and treated with oxymatrine by gavage for the last 4 weeks. We measured biochemical indicators and pathological changes in each group and used liquid chromatography-mass spectrometry (LC-MS) to analyze changes in metabolites in the serum and liver of the rats.
Results: The results showed that OMT can alleviate the high-fat-high-fructose-induced weight gain and hepatic lipid deposition in rats. Metabolomic analysis showed that the level of eicosapentaenoic acid (EPA) was downregulated and levels of desmosterol and d-galactose were upregulated in livers fed with HFDHFr. The levels of L-isoleucine, L-valine, arachidonic acid (AA), taurocholic acid (TCA), chenodeoxycholyltaurine (TDCA), isocitrate, and glutathione (GSH) were downregulated in the liver, whereas those of linoleic acid (LA), phosphocholine (PC), glycerophosphocholine (GPC), and oxidized glutathione (GSSG) were upregulated in the serum treated with OMT.
Conclusion: In summary, OMT can improve HFDHFr-induced NAFLD, and metabolomic analysis can provide an early warning for the development of NAFLD as well as provide a rationale for therapeutic targets.
Keywords: high-fat-high, fructose, fatty liver, oxymatrine, metabonomics
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a general term for a series of liver diseases closely related to metabolic syndrome (MS), including simple steatosis, non-alcoholic steatohepatitis, and liver fibrosis.1 It is one of the most common liver diseases worldwide because of the changes in the contemporary dietary structure of increased fat and fructose proportions.2,3 Previous studies have shown that a high-fat-high-fructose diet can lead to lipid deposition in the liver of rats.4 The dietary fat and cholesterol are the major drivers of NAFLD development and progression in rats, while fructose acts primarily on the circulating lipid pool; however.5 The pathogenesis of NAFLD has still not been fully elucidated, currently especially in metabolism. The main recognized pathogenesis is the multiple strike theory based on the second strike, which adds complex interactions, including environmental factors such as dietary factors, obesity, microbiota changes, and susceptibility genetic variation, leading to disturbed lipid homeostasis and excessive accumulation of triglycerides and other lipid species in hepatocytes.6–8
Oxymatrine (OMT) is a common colourless columnar crystalline alkaloid isolated mainly from the roots of bitter ginseng, which has a variety of pharmacological effects, including anti-hepatitis virus infection, antioxidant, anti-inflammatory, anti-fibrosis, and anti-tumor properties, and has already been applied in the treatment of hepatitis and type 2 diabetes mellitus (T2DM).9–11 A study by Shi et al indicated that the therapeutic effect of OMT on NAFLD is partly due to the downregulation of the sterol regulatory element binding transcription factor 1 (Srebf1) and the simultaneous upregulation of the Pparα mediated metabolic pathway.12 While the specific mechanisms by which OMT improves NAFLD are still not fully elucidated, especially in metabolism.
Metabolomics is one of the research focuses in the field of omics as an important part of system biology after genomics, transcriptome, and proteomics, in which the research object is all metabolites in vivo.13 Previous studies have explored the changes of small and medium molecular metabolites in serum and liver of NAFLD rats induced by high-fat or high-fructose diets. Xie et al14 showed that the contents of tetradecanoic acid, hexadecanoic acid, and oleic acid in rat liver increased, while the contents of arachidonic acid (AA), eicosapentaenoic acid (EPA), glycine, alanine, aspartic acid, glutamic acid, proline, and other amino acids decreased, indicating enhanced lipogenesis and suppressed utilization of fatty acids due to a high-fat diet. In a study by Hansen et al,15 the serum levels of acetic acid, valine, serine, and methionine were decreased in rats fed a high-fructose diet. These studies provided a possible metabolic-related theoretical basis for the mechanism of NAFLD induced by high-fat or high-fructose diets.
The present clinical prevention and treatment of NAFLD mainly relies on diet control and reasonable exercise.16 It is necessary to develop effective and safe targeted drugs to prevent and treat NAFLD.17 In our study, we established a NAFLD rat model induced by a high-fat-high-fructose diet and used metabolomic analysis to reveal the potential pathogenesis and therapeutic targets of OMT to improve NAFLD, ultimately providing a basis for its further development and wider use.
Materials and Methods
Animal Models and Sample Collection
Fifteen male Wistar rats (seven weeks old, male, purchased from Beijing Weitong Lihua Laboratory Animal Technology Co., Ltd.) were housed in the barrier system of animal experiments at the Clinical Research Center of Hebei Provincial People’s Hospital. All rats were randomly divided into two groups: 5 mice in the control group (NC, normal diet, 20% protein, 70% carbohydrate, and 10% fat, 3.85 kcal/g) and 10 mice in the high-fat-high-fructose group (HFDHFr, 20% protein, 40% carbohydrate, 40% fat, 4.49 kcal/g). After four weeks of feeding, the rats in the HFDHFr group (n = 10) were subdivided into HFDHFr (n = 5) and HFDHFr + OMT (n = 5) groups. The rats in the HFDHFr group were fed with HFDHFr consistently for 4 weeks. The rats in the HFDHFr + OMT group were gavaged with oxymatrine at a dose of 80 mg/kg/day for another four weeks. The feed was purchased from Biopike (Beijing, China). Dosages were selected based on our previous studies.18 Rats in each group were provided with equal amounts of calories and allowed to eat and drink ad libitum every day. Food intake was recorded daily, body weights were measured weekly, and the final weight levels of the three groups were compared at the end of the trial. After four weeks of drug intervention, an intraperitoneal glucose tolerance test (IPGTT) was used to measure blood glucose values at 0, 15, 30, 60, and 120 min time points, and calculate the area under the glucose curve (AUC) was calculated. The mice were fasted overnight, weighed, and anesthetized with 1% pentobarbital sodium (60 mg/kg). Blood was taken from the abdominal aorta and centrifuged at 4 °C at 3000 rpm 4 °C for 20 min, and serum was collected and stored at −80 °C until use. Serum triglyceride (TG), total cholesterol (TC), and alanine aminotransferase (ALT) levels were measured in all three groups. After taking blood from the abdominal aorta, taking out the liver quickly, weighing the liver, dividing it into several pieces of liver tissue, quickly putting it into liquid nitrogen, and then moving it to a −80 °C low-temperature refrigerator for storage as a follow-up test sample. All experiments were performed in accordance followed by the guidelines for the Care and Use of Laboratory Animals and approved by the Hebei General Hospital Ethics Committee.
IPGTT and AUC
The rats were fasted for 8 hours, and then took the blood dropped from the tail tip to the test strip of a Roche rapid glucose meter to measure FBG (blood glucose at 0 point), followed by intraperitoneal injection of 2 g/kg of 50% glucose injection. The tail tip blood glucose values of the IPGTT were measured 30, 60, and 120 min after the glucose injection in the rats using ACCUCHEK Performa blood glucose meter (Roche Diagnostics, Germany). The AUC was calculated, AUCglu = (0’+15’)/8 + (15’+30’)/8 + (30’+60’)/4 + (60’+120)/2.
Measurement of Serum Fasting Insulin
We used a serum antibody sandwich ELISA, according to the instructions provided by the mouse insulin ELISA kit (ALPCO, USA), to determine fasting insulin levels in rats. We set standard wells and sample wells were set on the enzyme-labeled plate, and, respectively, added 10 μL of standard and 10 μL of samples were added to the bottom of the plate. Then, we add 75 μL of enzyme-labeled antibody working solution was added to each well. The plate with the samples was incubated at room temperature, and shaken on a microwell plate oscillator for 2 h covering with a sealer. We washed each well with 350 μL of wash solution and removed the remaining liquid from all wells; which was repeated six times. After adding 100 μL of chromogenic substrate solution, we covered the plate and vortexed with a sealer. Then the samples were incubated with shaking for 15 min at room temperature. Then, 100 μL of stop solution was added to each well to stop the reaction, then we mixed the samples, and determined the absorbance value at 450 nm wavelength using a microplate reader. Finally, the insulin content of the samples was calculated from the absorbance and the standard curves drawn using different concentrations of standards.
Hepatic Tissue Histology
Histological sections stained with hematoxylin and eosin (H&E) and Oil Red O staining were examined under a light microscope and histology to evaluate the changes in the influence of the HFDHFr diet and OMT on liver histology. Liver tissues were fixed in a 4% neutral formaldehyde solution for 24 h, after dehydration with graded ethanol, vitrification with dimethylbenzene, embedded in paraffin embedding, and the tissues were sectioned for H&E staining. Liver tissues were frozen and sliced into 8 μm sections, air-dried giving the air-drying for 30 min and then fixed in 10% neutral formaldehyde for 10–15 min, and washed for Oil Red O staining. Photomicrographs were obtained using a light microscope.
Measurement of Liver TG
Lysis buffer (adding 1 mL) was lysis added buffer into 50 mg of the liver tissue to ensure effective homogenate splitting and lipid extraction. The tissue was pulverised using an electric high speed homogeniser and then left to stand for 10 minutes, the appropriate amount of supernatant was transferred to a 1.5 mL centrifuge tube and centrifuged at room temperature. The supernatant was used to determine for the triglyceride (determination of TG) content in the liver.
Metabonomic Analysis of Serum and Liver
Liquid chromatography-mass spectrometry (LC-MS) analysis was performed by using the OE BioTech instrument (Shanghai, China). Samples of serum and liver samples were stored at −80 °C and thawed at room temperature; 100 μL of serum sample was added to a 1.5 mL Eppendorf tube with 20 μL of L-2-chlorophenylalanine (0.3 mg/mL) dissolved in methanol as an internal standard, and the tube was vortexed for 10s. Subsequently, 300 μL of ice-cold mixture of methanol and acetonitrile (2/1, v/v) was added, and the mixtures were vortexed for 1 min, 30 mg of accurately weighed liver sample was transferred to a 1.5 mL Eppendorf tube. Two small steel balls were added to each tube. 20 μL of L-2-chlorophenylalanine (0.3 mg/mL) dissolved in methanol as an internal standard and 400 mL mixture of methanol and water (4/1, vol/vol) were added to each sample. Samples were stored at −20 °C for 2 min and then grounded at 60 HZ for 2 min. Subsequently, whole samples of serum and liver samples were extracted by ultrasonication for 10 min in an ice-water bath, stored at −20 °C for 30 min, and were centrifuged at 4 °C (13,000 rpm) for 10 min, respectively. The supernatant (300 μL) of supernatant in a glass vial was dried in a freeze-concentration centrifugal dryer. 300 μL mixture of methanol and water (1/4, v/v) was added to each sample, samples vortexed for 30s, extracted by ultrasonication for 3 min in an ice-water bath, and then placed at −20 °C for 2 h. Samples were centrifuged at 4 °C (13,000 rpm) for 10 min. The supernatants (150 μL) from each tube were collected using crystal syringes, filtered through 0.22 μm microfilters, and transferred to LC vials. The vials were stored at −80 °C until LC-MS analysis. QC samples were prepared by mixing aliquots of all samples to be a pooled sample to evaluate the stability of the mass spectrometry system during sample testing. All the chemicals and solvents used were of analytical or HPLC grade. Water, methanol, acetonitrile, and formic acid were purchased from Thermo Fisher Scientific (Thermo Fisher Scientific, Waltham, MA, USA). L-2-chlorophenylalanine was purchased from Shanghai Heng Cchuang Biotechnology Co., Ltd. (Shanghai, China). All the extraction reagents were pre-cooled at −20 °C before use.
An ACQUITY UPLC I-Class system (Waters Corporation, Milford, USA) coupled with a VION IMS QTOF Mass spectrometer (Waters Corporation, Milford, USA) was used to analyze the metabolic profiles in both the ESI positive and ESI negative ion modes. The original liquid chromatography-mass spectrometry (LC-MS) data were processed by using software the Progenesis QI V2.3 (Nonlinear, Dynamics, Newcastle, UK) for baseline filtering, peak identification, integration, retention time correction, peak alignment, and normalization. Use principle component analysis (PCA) to observe the overall distribution among the samples and the stability of the whole analysis process. Orthogonal Partial Least-Squares -Discriminant Analysis (OPLS-DA) was utilized to distinguish metabolites that differed between the groups. To prevent overfitting, 7-fold cross-validation and 200 response permutation testing (RPT) were performed used to evaluate the quality of the model. Variable importance of projection (VIP) values obtained from the OPLS-DA model were used to rank the overall contribution of each variable to group discrimination. Differential metabolites were selected with VIP values greater than 1.0 and p-values less than 0.05. KEGG (https://www.kegg.jp/) database was used to explore related metabolic pathways.
Statistical Analysis
All data were analyzed by SPSS 26.0 software (version 26.0), and figures were generated using GraphPad Prism software (version 8.0). Measurement data with normal distribution or close to normal distribution were expressed by mean ± standard deviation (Mean ± SD), while measurement data with non-normal distribution were expressed by median (quartile). The t-test was used if the mean of two samples was normal and the variance was homogeneous, and the nonparametric rank-sum test was used for non-normal distributions or uneven variances. Comparison among multiple groups were performed using one-way ANOVA, and the differences were statistically significant (P < 0.05).
Results
Comparison of Body Weight, Liver Weight, Food Intake, Fasting Blood Glucose, Fasting Insulin, Serum TG, Serum TC, and Serum ALT
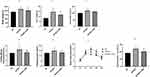
At the end of 8 weeks, the body weight, liver weight, fasting blood glucose level, and fasting insulin level of rats in the HFDHFr group were significantly higher than those in the NC group. And after 4 weeks of OMT administration, the levels of the above indexes decreased, and the difference was statistically significant (Figure 1a, b, d and e). The food intake of HFDHFr group was higher than that of the NC group, while there was no statistically significant difference in the food intake of OMT group compared with both HFDHFr and NC groups (Figure 1c). Serum TG, TC, and ALT levels increased after HFDHFr feeding and decreased after OMT intervention (Table 1).
 |
Table 1 Effect of Oxymatrine on the Serum Parameters in High-Fat-High-Fructose-Diet Fed Rats |
Comparison Between IPGTT and AUC
The blood glucose values at 15 min, 30 min, 60 min, and 120 min were elevated in the HDFHFr group and decreased after the OMT intervention (Figure 1f). The area under the glucose curve (AUC) was increased in the HFDHFr group while decreased in the HFDHFr + OMT group (Figure 1g).
Comparison of Liver TG Content and Liver Histopathology
Liver TG content in the HFDHFr group was significantly higher than that in the NC group, whereas that in the HFDHFr + OMT group was significantly lower than that in the HFDHFr group (Figure 2a).
The H&E staining results of liver samples showed that in the NC group, the liver tissue structure was complete, the liver cells were orderly arranged, the liver lobules were regular, the cytoplasm was uniformly red-stained, and no obvious inflammatory cells or lipid droplets were found. In the HFDHFr group, the structure of the hepatic lobule was disordered, hepatocytes were swollen, a large number of lipid droplets of different sizes were observed in in the cytoplasm, ballooning degeneration was obvious, and a large number of inflammatory cells were infiltrated in the hepatic portal area and hepatic lobule. In the HFDHFr + OMT group, the inflammatory infiltration of hepatocytes was reduced, and degree of steatosis was significantly reduced. The results of liver Oil Red O staining showed that there were blue-stained nuclei in the NC group, and no obvious lipid droplets were found. In the HFDHFr group, a large number of red-stained lipid droplets were deposited, whereas in the HFDHFr + OMT group, lipid droplets were significantly reduced between the NC and HFDHFr groups (Figure 2b).
Based on the increased hepatic TG content in the HFDHFr group and the section staining results suggesting hepatic steatosis, we deduced that the diagnosis of NAFLD in rats in the HFDHFr group was established.
Serum and Liver Metabonomic Analysis
First, the PCA scatterplot of serum and liver showed that QC samples were closely clustered together, indicating that instrument detection stability was good during this experiment (Supplemental Figure 1). In the OPLS-DA model, there was an obvious separation between the groups, indicating a significant difference between the groups. The displacement test showed no overfitting and performance proving that the data were reliable (Supplemental Figures 2 and 3). The differential metabolites among groups were screened according to the criteria that the VIP value of the first principal component of the OPLS-DA model was >1 and the p-value of theT t-test was <0.05. 163 and 352 differential metabolites were detected between HFDHFr and NC groups in serum and liver, respectively. 424 and 534 differential metabolites were detected between HFDHFr+OMT and HFDHFr groups in serum and liver, respectively (Supplemental Table 1).
Based on the thermographic results of the metabolite differences between the groups of serum and liver samples, the first 50 metabolites with the most significant differences were mainly glyceride phospholipid metabolites such as phosphatidylcholine (PC), phosphatidylethanolamine (PE), and other different branch chain forms (Figure 3a and b).
In serum samples, the expression levels of different branch chain forms of glycerophospholipid metabolites were almost the same in the NC group and HFDHFr group, but they were significantly higher in the HFDHFr + OMT group, such as PE (18:1(11Z)/0:0), PE (18:1(9Z)/0:0), PE(0:0/18:2(9Z,12Z)). Compared with NC group, LysoPC (22:6 (4Z, 7Z, 10Z, 13Z, 16Z, 19Z)/0:0), PC (22:6 (4Z, 7Z, 10Z, 13Z, 16Z, 19Z)/0:0), two branched chain forms, were low expressed in HFDHFr group and high expressed in HFDHFr + OMT group, LysoPC (0:0/18:2 (9Z, 12Z)), LysoPC (18:2 (9Z, 12Z)/0:0), LysoPC (18:1 (11Z)) was high expressed in the HFDHFr group in the HFDHFr + OMT group, the expression was low.
In the liver samples, compared to the NC group, the different branch chain forms of most glycerol phospholipids showed low expression in the HDFHFr and HFDHFr + OMT groups, and low expression was more significant in the HFDHFr + OMT group, such as LysoPC (20:4 (8Z, 11Z, 14Z, 17Z)/0:00), PC (20:4 (5Z, 8Z, 11Z, 14Z)/0:00), and PC (22:5 (4Z, 7Z, 10Z, 13Z, 16Z)/0:00). PE (P − 18:0/20:4 (5Z, 8Z, 10E, and 14Z) (12OH [S])) was showed highly expressed in in the HFDHFr group. PE (17:0/22:4 (7Z, 10Z, 13Z, 16Z)), lLysoPC (0:0/18:2 (9Z, 12Z)), PC (18:2 (9Z, 12Z)/0:0), and lLysoPC (18:1 (11Z)) showered highly expressed in in the HFDHFr + OMT group.
We also found that in liver samples, tauoursocholic acid, ADP, FAD, and galactonic acid were highly expressed in the HFDHFr group compared to the NC group, and this trend was reversed after feeding OMT (Figure 4). The expression of eicosapentaenoic acid, oleamideoleamide, and stearoylcarnitine was downregulated, the expression of desmosterol, d-galactose, phosphohydroxypyruvic acid, d-glucuronic acid, and d-maltose were upregulated (Figure 5).
 |
Figure 4 Other metabolites with significant differences among the three groups in liver samples. Red represents high expression and blue represents low expression. |
 |
Figure 5 Metabolites with significant differences among the HFDHFr + OMT group and HFDHFr group in liver samples. Red represents high expression and blue represents low expression. |
Compared to the HFDHFr group, except for the upregulation of L-arginine, most amino acid levels were downregulated in the HFDHFr + OMT group including L-aspartic acid, L-serine, L-isoleucine, L-ornithine, L-valine, L-histidine, and L-glutamate. However, the changes in these metabolites in the serum samples showed an opposite trend to those in the liver samples (Figure 6a and b).
 |
Figure 6 Metabolites with significant differences among the HFDHFr group and NC group in serum samples (a) and liver samples (b). Red represents high expression and blue represents low expression. |
Metabolite Enrichment and Pathway Analysis
The KEGG pathways with significantly enriched differential metabolites are displayed in a bubble diagram (Figure 7a–d). The metabolic pathways significantly affected included citrate cycle (TCA cycle), histidine metabolism, purine metabolism, linoleic acid metabolism, biosynthesis of unsaturated fatty acids, primary bile acid biosynthesis, and sphingolipid signaling pathway.
Discussion
Our study simulated typical Western eating habits and successfully established an NAFLD rat model based according to body weight, liver weight, and histology of rats fed with HFDHFr diet. Our study is the first to observe changes in small-molecule metabolites produced by OMT in the serum and liver of NAFLD rats induced by a high-fat-high-fructose diet and to explore the possible target of OMT to improve NAFLD. Our study found that OMT can significantly reduce liver TG content and improved liver lipid deposition as well as insulin resistance (IR), which is consistent with previous research results, indicating that OMT plays a role in resisting the multiple strike pathogenesis of NAFLD caused by dietary factors. This may be related to the fact that OMT decreases hepatic lipid metabolism in HepG2 cells by regulating miR-182, Inhibiting sterol regulatory element-binding transcription factor 1 (Srebef1) and activating peroxisome proliferator-activated receptor α (Pparα).11
In the metabolism of rats fed with HFDHFr, we found that the levels of desmosterol were upregulated and the level of EPA was downregulated compared to those in the NC group. Desmosterol is an endogenous agonist of the X receptor (LXR), the main regulator of liver lipid metabolism, involved in many pathophysiological processes including inflammation, diabetes (DM), non-alcoholic steatohepatitis (NASH) as an intermediate product of cholesterol synthesis.19 Studies have shown that the desmosterol level in liver is positively correlated with the degree of liver steatosis and inflammation. Increased desmosterol levels can be used as a sign of liver cholesterol metabolism disorder.20 The elevated desmosterol level was consistent with the increased lipid deposition and TG content in the liver in our study. EPA is conducive to weight loss and increasing liver superoxide dismutase activity and the level of glutathione (GSH), thus reducing oxidative stress and improving NAFLD induced by a high-fat-diet.21 Previous studies showed that the level of EPA in the liver of rats fed with high fat and mice fed with high-fructose and high-corn-syrup were significantly decreased, which was consistent with our results.14,22 Therefore, the development of NAFLD induced by high-fat-high-fructose may be related to interfering with cholesterol metabolism and decreasing EPA levels. Desmosterol and EPA may be used as biochemical markers of NAFLD consequently.
The levels of branched chain amino acids (BCAAs) in the liver, including L-isoleucine and L-valine, were significantly decreased after treatment. BCAAs can mediate the activation of several important liver metabolic signal transduction pathways from insulin signal transduction to glucose regulation, including improving insulin resistance.23 It was found that BCAAs levels were elevated in patients with NAFLD and in people with high fat density, and plasma BCAAs levels were positively correlated with intrahepatic lipid content.24,25 While the causal relationship between BCAAs and NAFLD is still open to debate. One study suggested that the observed link between BCAAs and NAFLD may be confounded by factors such as insulin resistance, which increases circulating amino acid levels by increasing muscle protein catabolism. However, some preclinical models support a causal role for BCAAs in the development of NAFLD. For example, overexpression of phosphatase PPM1K reduces circulating BCAAs, which reduces hepatic steatosis in mice.26 Previous study showed that the BCAAs level in liver of NAFLD rats fed with a high-fat diet is increased, and then significantly decreased after the intervention of Eurycoma longifolia, a kind of obesity drug, which may be related to the improvement of IR, the increase of glucose oxidation, and the reduction of circulating fatty acids.27 Although there was no significant difference in BCAAs level between the HFDHFr group and NC group in our study, which may have a bearing on the difference of forage compounding or animal model, the BCAAs levels were obviously decreased after OMT treatment, which may herald the improvement of IR and liver lipid deposition.
Arachidonic acid (AA), an important precursor for the synthesis of inflammatory mediators, plays a role in the development of metabolic diseases such as NAFLD and correlates with the severity of NAFLD.28 Elevated AA levels can be an early indicator of inflammation induced by a high-fat diet, which can be converted to leukotrienes, among others, via the lipoxygenase (LOX) pathway, driving the progression of NAFLD to NASH.29,30 OMT can reduce the production of inflammatory factors such as PGE2 (prostaglandin E2) and TNF-α (tumor necrosis factor-α) and can downregulate TLR4 (toll-like receptor 4)/NF-κB downregulation to exert anti-inflammatory effects.11 The decrease in AA levels after OMT intervention may predict that OMT may promote its anti-inflammatory effects by restoring AA metabolism to alleviate NAFLD.
Dysfunctional citric acid cycle (TCA cycle) plays a role in the pathogenesis of NAFLD, and intracellular lipid accumulation in hepatocytes can lead to dysregulation of TCA cycle activity and associated alterations in metabolite levels, whereas sustained TCA cycle activity in turn accelerates oxidative stress and inflammation and exacerbates hepatic mitochondrial dysfunction.31,32 It was found that hyperactivation of the tricarboxylic acid cycle in high-fructose corn syrup-fed mice may contribute to the exacerbation of steatosis during HFD-HFCS-induced NAFLD.33 TCA cycle dysregulation was also observed in high-fat diet mice.34 Hyperactivation of the TCA cycle after the HFDHFr diet in the present study was reversed after OMT intervention, suggesting that OMT may ameliorate NAFLD by reverting to the pathway of dysregulated TCA cycle metabolism.
Histidine metabolism is associated with NAFLD. Elevated levels of histidine correlate with the severity of NAFLD.35 Increased levels of its metabolite histamine lead to elevated levels of PARP-1 and IL-1β, as well as MAO-A, total MAO, CRP, and AST/ALT, causing liver injury.36 In addition, histamine may regulate metabolic and inflammatory processes in other organs targeted by metabolic syndrome. Two potential mechanisms associated with histamine receptors may attenuate liver injury during the initial and progressive stages of NAFLD in a manner involving the regulation of cholesterol and bile acid metabolism.37,38 In an untargeted metabolomic analysis of nonobese NAFLD patients, levels of the histamine synthesis precursor histidine were elevated, and levels of the histamine receptor agonist histamine-trifluoromethyl-toluidide were decreased.24 In contrast, in the present study, histidine metabolism was disturbed in the HFDHFr group with elevated histidine levels and increased histamine, and histamine and histamine levels decreased after OMT intervention. OMT may improve NAFLD by downregulating histamine levels and attenuating liver injury.
Hypoxanthine levels are increased in the NAFLD population and are diet-independent.24 Impaired purine metabolism due to increased levels of hypoxanthine metabolites was detected in high-fat-fed rabbits, and the increase in hypoxanthine was associated with increased oxidative stress in hepatocytes.39 Our study found that both hypoxanthine and xanthine levels decreased after OMT administration, suggesting that OMT may improve NAFLD by reducing hypoxanthine levels and repairing purine metabolism to alleviate oxidative stress.
As an essential fatty acid that cannot be synthesized in vivo, linoleic acid (LA) is essential for health because of many beneficial effects including improving liver lipid deposition and IR. Studies have shown that the LA level in the serum of NAFLD patients decreases, and the degree of decline is positively related to the severity of NAFLD and negatively related to the level of serum anhydrase.40,41 The LA metabolite 9-hydroxy-octadecadienoic (9-HODE) increases PPARγ expression.42,43 PPARγ is a known regulator of glucose homeostasis and lipid metabolism and plays a role in the development of NAFLD.44 9-HODE decreased in Rats after high-fat diet and increased after Jiangzhi intervention, which is similar to the results of the present study.29 Our study found that the levels of both LA and its metabolite 9-HODE were elevated after OMT administration, suggesting that OMT may contribute to the amelioration of NAFLD by modulating the levels of LA and its metabolites, thereby affecting the expression of PPARγ.
This study is the first to observe the effects of OMT on serum and liver metabolism in NAFLD rats induced by high-fat-high-fructose-diet, providing a new theoretical basis for the mechanism and treatment of NAFLD. However, there were still several deficiencies: 1. The number of samples was small, and the sex was single, without an impact on female rats; 2. The levels of inflammatory factors and oxidative stress markers were not detected in this study, and these problems will be considered in future studies.
Conclusion
Oxymatrine can improve NAFLD induced by a high-fat-high-fructose diet in rats by possible mechanism of affecting amino acids, purine metabolism, and other metabolic pathways in the serum and liver. Desmosterol EPA and d-galactose levels may serve as biochemical markers of NAFLD and provide an early warning, whereas L-isoleucine, L-valine, AA, TCA, TDCA, isocitrate, GSH, LA, PC, GPC, and GSSG may be used as biomarkers for the efficacy of OMT in the treatment of NAFLD and provide a theoretical basis and new ideas for further therapy.
Acknowledgments
Huan Li wishes to thank the senior fellow apprentice of He Zhang, classmates of Chang Wang, and Qing Wang, junior brother apprentice of Xuehua Liu, and junior sister apprentice of Juanjuan Zhang for their assistance with the experiment. In addition, I especially wish to thank the teachers of Wenjie Fei, Hang Zhao, and Luping Ren at the Hebei Key Laboratory of Metabolic Diseases for their careful guidance on the experiment.
Disclosure
No potential conflict of interest was reported by the authors.
References
1. Pan J, Zhou W, Xu R, Xing L, Ji G, Dang Y. Natural PPARs agonists for the treatment of nonalcoholic fatty liver disease. Biomed Pharmacother. 2022;151:113127. doi: 10.1016/j.biopha.2022.113127
2. Alwahsh SM, Gebhardt R. Dietary fructose as a risk factor for non-alcoholic fatty liver disease (NAFLD). Arch Toxicol. 2017;91(4):1545–1563. doi: 10.1007/s00204-016-1892-7
3. Longato L. Non-alcoholic fatty liver disease (NAFLD): a tale of fat and sugar? Fibrogenesis Tissue Repair. 2013;6(1):14. doi: 10.1186/1755-1536-6-14
4. Kamboj P, Sarkar S, Gupta SK, Bisht N, Kumari D, Alam MJ. Methanolic extract of Lysimachia Candida Lindl. Prevents high-fat high-fructose-induced fatty liver in rats: understanding the molecular mechanism through untargeted metabolomics study. Front Pharmacol. 2021;12:653872. doi: 10.3389/fphar.2021.653872
5. Jensen VS, Hvid H, Damgaard J, Nygaard H, Ingvorsen C, Wulff EM. Dietary fat stimulates development of NAFLD more potently than dietary fructose in Sprague-Dawley rats. Diabetol Metab Syndr. 2018;10:4. doi: 10.1186/s13098-018-0307-8
6. Arab JP, Arrese M, Trauner M. Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annu Rev Pathol. 2018;13:321–350. doi: 10.1146/annurev-pathol-020117-043617
7. Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65(8):1038–1048. doi: 10.1016/j.metabol.2015.12.012
8. Pafili K, Roden M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol Metab. 2021;50:101122.
9. Hua S, Gu M, Wang Y, Ban D, Ji H. Oxymatrine reduces expression of programmed death-ligand 1 by promoting DNA demethylation in colorectal cancer cells. Clin Transl Oncol. 2021;23(4):750–756. doi: 10.1007/s12094-020-02464-x
10. Zhu YX, Hu HQ, Zuo ML, Mao L, Song GL, Li TM. Effect of oxymatrine on liver gluconeogenesis is associated with the regulation of PEPCK and G6Pase expression and AKT phosphorylation. Biomed Rep. 2021;15(1):56. doi: 10.3892/br.2021.1432
11. Huan DQ, Hop NQ, Son NT. Oxymatrine: a current overview of its health benefits. Fitoterapia. 2023;168:105565. doi: 10.1016/j.fitote.2023.105565
12. Shi LJ, Shi L, Song GY, Zhang HF, Hu ZJ, Wang C. Oxymatrine attenuates hepatic steatosis in non-alcoholic fatty liver disease rats fed with high fructose diet through inhibition of sterol regulatory element binding transcription factor 1 (Srebf1) and activation of peroxisome proliferator activated receptor alpha (Pparalpha). Eur J Pharmacol. 2013;714(1–3):89–95. doi: 10.1016/j.ejphar.2013.06.013
13. Son JW, Shoaie S, Lee S. Systems biology: a multi-omics integration approach to metabolism and the microbiome. Endocrinol Metab (Seoul). 2020;35(3):507–514. doi: 10.3803/EnM.2020.303
14. Xie Z, Li H, Wang K, Lin J, Wang Q, Zhao G. Analysis of transcriptome and metabolome profiles alterations in fatty liver induced by high-fat diet in rat. Metabolism. 2010;59(4):554–560. doi: 10.1016/j.metabol.2009.08.022
15. Hansen M, Baunsgaard D, Autrup H, Vogel UB, Moller P, Lindecrona R. Sucrose, glucose and fructose have similar genotoxicity in the rat colon and affect the metabolome. Food Chem Toxicol. 2008;46(2):752–760. doi: 10.1016/j.fct.2007.09.110
16. Abd ES, El-Den AE. Non-alcoholic fatty liver disease: the diagnosis and management. World J Hepatol. 2015;7(6):846–858. doi: 10.4254/wjh.v7.i6.846
17. Wang Y, Wu J, Shi A. Literature review on the use of herbal extracts in the treatment of non- alcoholic fatty liver disease. Endocr Metab Immune Disord Drug Targets. 2022;22(11):1123–1145. doi: 10.2174/1871530322666220408123746
18. Zhang H, Yang L, Wang Y, Huang W, Li Y, Chen S. Oxymatrine alleviated hepatic lipid metabolism via regulating miR-182 in non-alcoholic fatty liver disease. Life Sci. 2020;257:118090. doi: 10.1016/j.lfs.2020.118090
19. Muller C, Hank E, Giera M, Bracher F. Dehydrocholesterol reductase 24 (DHCR24): medicinal chemistry, pharmacology and novel therapeutic options. Curr Med Chem. 2022;29(23):4005–4025. doi:10.2174/0929867328666211115121832
20. Simonen M, Mannisto V, Leppanen J, Kaminska D, Karja V, Venesmaa S. Desmosterol in human nonalcoholic steatohepatitis. Hepatology. 2013;58(3):976–982. doi: 10.1002/hep.26342
21. Hirotani Y, Ozaki N, Tsuji Y, Urashima Y, Myotoku M. Effects of eicosapentaenoic acid on hepatic dyslipidemia and oxidative stress in high fat diet-induced steatosis. Int J Food Sci Nutr. 2015;66(5):569–573. doi: 10.3109/09637486.2015.1042848
22. Bhat SF, Pinney SE, Kennedy KM, McCourt CR, Mundy MA, Surette MG. Exposure to high fructose corn syrup during adolescence in the mouse alters hepatic metabolism and the microbiome in a sex-specific manner. J Physiol. 2021;599(5):1487–1511. doi: 10.1113/JP280034
23. Lake AD, Novak P, Shipkova P, Aranibar N, Robertson DG, Reily MD. Branched chain amino acid metabolism profiles in progressive human nonalcoholic fatty liver disease. Amino Acids. 2015;47(3):603–615. doi: 10.1007/s00726-014-1894-9
24. Demirel M, Koktasoglu F, Ozkan E, Dulun AH, Gul AZ, Sharifov R. Mass spectrometry-based untargeted metabolomics study of non-obese individuals with non-alcoholic fatty liver disease. Scand J Gastroenterol. 2023;58(11):1344–1350. doi: 10.1080/00365521.2023.2225667
25. Amanatidou AI, Mikropoulou EV, Amerikanou C, Milanovic M, Stojanoski S, Bjelan M. Plasma amino acids in NAFLD patients with obesity are associated with steatosis and fibrosis: results from the MAST4HEALTH study. Metabolites. 2023;13:8. doi:10.3390/metabo13080959
26. Gagnon E, Manikpurage HD, Mitchell PL, Girard A, Gobeil E, Bourgault J. Large-scale metabolomic profiling and incident non-alcoholic fatty liver disease. iScience. 2023;26(7):107127. doi: 10.1016/j.isci.2023.107127
27. Zhang D, Zheng W, Li X, Liang G, Ye N, Liu Y. Investigation of obesity-alleviation effect of eurycoma longifolia on mice fed with a high-fat diet through metabolomics revealed enhanced decomposition and inhibition of accumulation of lipids. J Proteome Res. 2021;20(5):2714–2724. doi: 10.1021/acs.jproteome.1c00015
28. Sonnweber T, Pizzini A, Nairz M, Weiss G, Tancevski I. Arachidonic acid metabolites in cardiovascular and metabolic diseases. Int J Mol Sci. 2018;19(11):3285. doi: 10.3390/ijms19113285
29. Wang L, Zhi Y, Ye Y, Zhang M, Mai Z, Xia W. Metabolomic analysis identifies the regulation of lipid metabolism pathway as potential mechanisms of Jiangzhi decoction against non-alcoholic fatty liver disease. J Pharm Pharmacol. 2023;75(10):1366–1377. doi: 10.1093/jpp/rgad067
30. Sztolsztener K, Chabowski A, Harasim-Symbor E, Bielawiec P, Konstantynowicz-Nowicka K. Arachidonic acid as an early indicator of inflammation during non-alcoholic fatty liver disease development. Biomolecules. 2020;10:8. doi: 10.3390/biom10081133
31. Chashmniam S, Mirhafez SR, Dehabeh M, Hariri M, Azimi NM, Nobakht MGB. A pilot study of the effect of phospholipid curcumin on serum metabolomic profile in patients with non-alcoholic fatty liver disease: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2019;73(9):1224–1235. doi: 10.1038/s41430-018-0386-5
32. Muyyarikkandy MS, McLeod M, Maguire M, Mahar R, Kattapuram N, Zhang C. Branched chain amino acids and carbohydrate restriction exacerbate ketogenesis and hepatic mitochondrial oxidative dysfunction during NAFLD. FASEB J. 2020;34(11):14832–14849. doi: 10.1096/fj.202001495R
33. Papadopoulos G, Legaki AI, Georgila K, Vorkas P, Giannousi E, Stamatakis G. Integrated omics analysis for characterization of the contribution of high fructose corn syrup to non-alcoholic fatty liver disease in obesity. Metabolism. 2023;144:155552. doi: 10.1016/j.metabol.2023.155552
34. Sun R, Xu D, Wei Q, Zhang B, Aa J, Wang G. Silybin ameliorates hepatic lipid accumulation and modulates global metabolism in an NAFLD mouse model. Biomed Pharmacother. 2020;123:109721. doi: 10.1016/j.biopha.2019.109721
35. Fotakis C, Kalafati IP, Amanatidou AI, Andreou V, Matzapetakis M, Kafyra M. Serum metabolomic profiling unveils distinct sex-related metabolic patterns in NAFLD. Front Endocrinol (Lausanne). 2023;14:1230457. doi: 10.3389/fendo.2023.1230457
36. Yang JH, Byeon EH, Kang D, Hong SG, Yang J, Kim DR. Fermented soybean paste attenuates biogenic amine-induced liver damage in obese mice. Cells. 2023;12:5. doi: 10.3390/cells12050822
37. Yamada S, Tanimoto A, Sasaguri Y. Critical in vivo roles of histamine and histamine receptor signaling in animal models of metabolic syndrome. Pathol Int. 2016;66(12):661–671. doi: 10.1111/pin.12477
38. Yamada S, Guo X, Wang KY, Tanimoto A, Sasaguri Y. Novel function of histamine signaling via histamine receptors in cholesterol and bile acid metabolism: histamine H2 receptor protects against nonalcoholic fatty liver disease. Pathol Int. 2016;66(7):376–385. doi: 10.1111/pin.12423
39. Toledo-Ibelles P, Gutierrez-Vidal R, Calixto-Tlacomulco S, Delgado-Coello B, Mas-Oliva J. Hepatic accumulation of hypoxanthine: a link between hyperuricemia and nonalcoholic fatty liver disease. Arch Med Res. 2021;52(7):692–702. doi: 10.1016/j.arcmed.2021.04.005
40. Hegazy M, Elsayed NM, Ali HM, Hassan HG, Rashed L. Diabetes mellitus, nonalcoholic fatty liver disease, and conjugated linoleic acid (Omega 6): what is the link? J Diabetes Res. 2019;2019:5267025. doi: 10.1155/2019/5267025
41. Pertiwi K, Kupers LK, Geleijnse JM, Zock PL, Wanders AJ, Kruger HS. Associations of linoleic acid with markers of glucose metabolism and liver function in South African adults. Lipids Health Dis. 2020;19(1):138. doi: 10.1186/s12944-020-01318-3
42. Naughton SS, Mathai ML, Hryciw DH, McAinch AJ. Linoleic acid and the pathogenesis of obesity. Prostaglandins Other Lipid Mediat. 2016;125:90–99. doi: 10.1016/j.prostaglandins.2016.06.003
43. Marangoni F, Agostoni C, Borghi C, Catapano AL, Cena H, Ghiselli A. Dietary linoleic acid and human health: focus on cardiovascular and cardiometabolic effects. Atherosclerosis. 2020;292:90–98. doi: 10.1016/j.atherosclerosis.2019.11.018
44. Chen H, Tan H, Wan J, Zeng Y, Wang J, Wang H. PPAR-gamma signaling in nonalcoholic fatty liver disease: pathogenesis and therapeutic targets. Pharmacol Ther. 2023;245:108391. doi: 10.1016/j.pharmthera.2023.108391
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.