Back to Journals » OncoTargets and Therapy » Volume 14
Oral PI3K-δ,γ Inhibitor for the Management of People with Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma: A Narrative Review on Duvelisib
Authors Shah A , Barrientos JC
Received 17 July 2020
Accepted for publication 5 March 2021
Published 25 March 2021 Volume 2021:14 Pages 2109—2119
DOI https://doi.org/10.2147/OTT.S189032
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Takuya Aoki
Ankit Shah,1 Jacqueline C Barrientos2
1Division of Hematology-Oncology, Department of Medicine at Rutgers New Jersey Medical School, Newark, NJ, USA; 2CLL Research and Treatment Center, Division of Hematology-Oncology, Department of Medicine at Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, USA
Correspondence: Jacqueline C Barrientos Tel +1-516-470-4050
Fax +1-516-470-4250
Email [email protected]
Abstract: The development of highly effective targeted therapies has led to a new treatment paradigm in patients with chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL). Despite these advances, many patients will eventually require alternative treatment strategies due to the emergence of tolerability issues or resistance to these novel agents. Duvelisib is a first-in-class, potent oral agent with dual inhibitor activity against the δ and γ isoforms of phosphoinositide 3-kinase (PI3Kδ and PI3Kγ), which are specific to the hematopoietic system. Dysregulation of the PI3K/PTEN/AKT/mTOR pathway has been implicated in cancer cell growth, survival and metabolism and has been the subject of cancer drug development in recent years. Duvelisib demonstrated activity in CLL/SLL in early trials, leading to further evaluation in the Phase 3 DUO trial that compared duvelisib against ofatumumab in patients with relapsed/refractory CLL/SLL. This trial led to the Food and Drug Administration (FDA) approval for the treatment of adult patients with CLL/SLL after at least two prior lines of therapy. The major reason for therapy discontinuation is the development of serious adverse events, which include severe infections and diarrhea/colitis, precluding its widespread use. Ongoing clinical trials are evaluating duvelisib in combination strategies and with alternate dosing schedules in patients with CLL/SLL. With close monitoring, duvelisib can be a promising drug for the treatment of patients with relapsed or refractory CLL/SLL. This review summarizes the relevant clinical data from recent clinical advances in CLL and aims to interpret the duvelisib trials while exploring strategies to improve its use and adverse event management in the era of novel targeted agents.
Keywords: duvelisib, CLL/SLL, PI3K, relapsed/refractory
Introduction
Chronic Lymphocytic Leukemia (CLL) and Small Lymphocytic Lymphoma (SLL) are considered to be different presentations of the same mature B-cell neoplasm.1,2 In 2020, the Surveillance, Epidemiology and End Result program (SEER) database estimated 21,040 new cases of CLL in the United States with 4060 deaths attributed to this disease.3 This translates into an incidence of CLL in the United States of approximately 5.0 per 100,000 men or women with a lifetime risk of 0.6%. CLL is largely considered a disease of the elderly with a median age at diagnosis of 70 and affects men approximately twice as often as women.3 While several novel therapies have been developed for the treatment of CLL in recent years, most patients with CLL ultimately progress or relapse requiring therapy.4,5
The natural history of CLL/SLL is variable and outcomes are influenced by patient characteristics, clinical factors at the time of diagnosis, and the genetics of the tumor. Given the heterogeneity in clinical outcomes in CLL, not all patients require therapy at the time of diagnosis and clinical decision-making is centered on selecting optimal therapy taking into consideration the patient and the disease. Clinical factors associated with worse prognosis include advanced age, male sex, and clinical Rai or Binet stage.6 Genetic abnormalities associated with a poor prognosis in CLL include presence of del (11q), del (17p) or alterations in TP53 gene, and unmutated immunoglobulin heavy chain variable region (IGHV).7–13
Current Treatment Approaches in CLL
The majority of patients with a CLL diagnosis do not require therapy at the time of initial diagnosis and active surveillance is appropriate. The international workshop on CLL (iwCLL) has proposed criteria for the initiation of therapy and these recommendations highlight the importance of a treatment approach guided by symptoms or signs of disease progression.14 When therapy is indicated, there are several treatment options available for both the front-line and relapsed/refractory (R/R) setting. In the treatment-naive setting, in no particular order, continuous daily dosing with either Bruton’s tyrosine kinase inhibitor (BTKi), ibrutinib or acalabrutinib, or fixed-duration therapy for 12 months with the BCL-2 inhibitor (BCL2i), venetoclax, given in combination with the monoclonal antibody obinutuzumab are Food and Drug Administration (FDA)-approved and preferred regimens for the treatment of CLL with or without del(17p).
Ibrutinib as monotherapy and in combination with both rituximab and obinutuzumab established efficacy in the treatment of CLL based on the findings of several clinical trials.15–21 One of the concerns is the potential toxicity that can occur as a result of indefinite treatment. The risk of atrial fibrillation along with the bleeding risk, require careful selection of candidates for this therapy.22 Furthermore, the risk of atrial fibrillation on ibrutinib therapy appears to be increased in patients with prior history of atrial fibrillation and age > 65, the latter reflecting the majority of the overall CLL/SLL population.23 Importantly, there is a risk of developing hypertension over time, which can contribute to the risk for cardiac arrhythmias.
Acalabrutinib, a highly-selective, covalent, irreversible BTKi, has also received recent approval for the treatment of CLL based on initial findings from the ELEVATE-TN study and the ASCEND trial. In the ELEVATE-TN trial, both acalabrutinib monotherapy and acalabrutinib combined with obinutuzumab significantly prolonged median progression-free survival (PFS) compared to combination chlorambucil-obinutuzumab therapy in the front-line setting.24 In the ASCEND trial, acalabrutinib demonstrated superior PFS compared to investigator’s choice of idelalisib or bendamustine in combination with rituximab in previously treated CLL/SLL patients.25 Together these two trials demonstrate the effectiveness of acalabrutinib for the treatment of CLL, however, similar to ibrutinib-based therapy, treatment with acalabrutinib carries a risk of atrial fibrillation (~3%) and bleeding (grade 3 or higher, ~2%) and requires continuous therapy until disease progression.24 A recent press release from the phase 3 ELEVATE R/R trial demonstrated that compared to ibrutinib, acalabrutinib met the study’s primary endpoint of non-inferior PFS with statistically lower incidence of atrial fibrillation.26 Data from this trial are not yet available to determine how this will impact further guidance.
Venetoclax, a BCL2i, is also indicated for the first-line therapy of CLL in combination with obinutuzumab based on outcomes from the CLL14 trial, which showed a 3-year PFS of 81.9%.27,28 At a median follow-up of 39.6 months, the median PFS was not reached for the patients treated with venetoclax. A major advantage to this approach is that therapy is limited to 12 months, with the majority of patients being free of disease 2 years post completion of time-limited therapy.28 Given its mechanism of action of inhibiting the anti-apoptotic protein BCL-2, venetoclax use carries a risk of tumor lysis syndrome especially at the beginning and requires risk-adapted titration and monitoring per the manufacturer’s guidelines.29
While chemo-immunotherapy (CIT) use has decreased in the front-line setting, fludarabine-cyclophosphamide-rituximab (FCR) remains an option for select young, fit patients with mutated IGHV who do not have a 17p deletion or TP53 mutation. This finding is supported by long-term follow up from the original phase 2 single-arm study of FCR and from the phase 3 CLL8 trial which show that prolonged remissions, and potentially “cures”, are possible.30–34 E1912 trial compared FCR against ibrutinib-rituximab in newly diagnosed CLL patients under the age of 70 without del (17p). Although patients treated with ibrutinib-rituximab had a superior PFS and overall survival (OS), in the subset of patients with mutated IGHV, there was no statistically significant difference in 3-years PFS.20 FCR, however, was associated with a higher rate of infectious complications, including neutropenic fever, and has been associated with an increased risk of treatment-related acute myeloid leukemia and myelodysplastic syndrome.20,35 While FCR has the advantages of fixed duration (6 cycles) and potential for cure, this needs to be weighed against the possible treatment-related toxicities when considering this regimen.
In the relapsed or refractory setting, there are several therapeutic options including any of the first-line regimens not previously administered, the PI3Kδ inhibitor, idelalisib, with or without rituximab, the PI3Kγ,δ inhibitor, duvelisib, and CIT. CIT use, however, is generally less favored in light of the availability of more effective and safer targeted agents such as an alternate tyrosine kinase inhibitor.
The optimal sequencing of therapies in the R/R setting remains unknown at this time as randomized trials evaluating these novel agents after prior BTKi or BCL2 inhibitor therapy in the front-line setting are not available. Importantly, patients previously treated with BTKi, PI3Ki or BCL2i were ineligible to participate in six landmark trials for relapsed/refractory disease with novel agents (RESONATE, ASCEND, M13-982, MURANO, Study 116, DUO).16,25,36–39 As a result, decisions regarding subsequent therapy require taking into consideration the patient’s age and comorbidities, their response to prior therapies and the reasons for discontinuation (progression while on therapy vs relapse post completion of therapy vs intolerance), and the genetics of the CLL/SLL at the time of relapse (eg presence or absence of mutated TP53).40 Unfortunately, despite the panoply of options, patients with a CLL diagnosis may relapse making effective therapeutic options that target distinct pathways a necessity.
Duvelisib: A First-in-Class PI3K-δ,γ Inhibitor for the Treatment of Relapsed/Refractory CLL
The PI3K family of kinases plays an important role in intracellular signaling in response to growth factors, cytokines and chemokines, typically through activation of the PI3K, protein kinase B (AKT), and mammalian target of rapamycin (mTOR) pathway. Deregulation of this pathway has been implicated in cancer cell growth, survival and metabolism and has been the subject of cancer drug development in recent years. Specifically, novel agents have been developed to target certain isoforms of Class I PI3K (α, ß, γ and δ), which appears to be more integral to oncogenesis than PI3K classes II and III.41,42 While activating PI3KA mutations are often seen in solid tumor malignancies (eg 40% of hormone-receptor positive, HER-2 negative metastatic breast cancers), these alterations are rarely seen in B-cell malignancies.41,43 Rather, PI3K activation in B-cell disorders occurs primarily through receptor tyrosine kinase-mediated signaling, such as via B-cell receptor (BCR) activation. While PI3Kα and PI3Kß isoforms are ubiquitously expressed, PI3Kδ and PI3Kγ are specific to the hematopoietic system.44 Pre-clinical studies have demonstrated that the PI3Kδ isoform, which is selectively expressed by leukocytes, is crucial for B-cell survival and migration, making it an attractive target for therapies. PI3Kγ, in contrast, is minimally expressed on B-cells, and instead is expressed on T-cells and myeloid cells. The rationale for targeting the γ isoform of PI3K is that it may reduce cytokine production by T-cells and macrophages in the tumor microenvironment, which may be contributing to neoplastic B-cell survival.41,45,46
There are currently 4 FDA-approved PI3K inhibitors for the treatment of B-cell malignancies, idelalisib, copanlisib, duvelisib, and umbralisib. Idelalisib is an oral, highly selective inhibitor of PI3Kδ, copanlisib is an intravenous pan-class I PI3K inhibitor with predominant activity against the PI3Kδ and PI3Kα isoforms, duvelisib (IPI-145) is a dual inhibitor against PI3Kδ and PI3Kγ, and umbralisib is a dual inhibitor of PI3Kδ and CK1ε. The inhibitory concentrations (IC50 expressed in nanomolar) of these PI3K inhibitors are shown in Table 1.
 |
Table 1 IC50 of Select PI3K Inhibitors Used in Hematologic Malignancies |
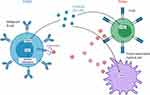
Duvelisib’s structure is similar to that of idelalisib, however, duvelisib has unique specificity (see Table 1) and pharmacokinetic properties including a longer target residence time.47,48 In pre-clinical studies, exposure of CLL cells activated by plate-bound anti-immunoglobulin M to duvelisib resulted in abrogation of BCR-mediated downstream AKT and ERK signaling, direct cytotoxicity to CLL B-cells, and diminished T-cell production of pro-survival cytokines49,50 (Figure 1). In vitro, duvelisib appeared to successfully overcome resistance due to the point mutation BTK C481S, a frequently implicated resistance mechanism to ibrutinib therapy. Additionally, mouse models showed dual inhibition of PI3K δ and γ isoforms resulted in a greater decrease in the number of CLL B-cells in the spleen compared to PI3Kδ inhibition alone.51 These direct and indirect anti-neoplastic effects of duvelisib provided the basis for subsequent early-phase clinical trials in advanced hematologic malignancies.
Clinical Efficacy of Duvelisib in CLL
Duvelisib (Copiktra®) is currently FDA-approved for the treatment of relapsed or refractory CLL/SLL or FL after at least two prior therapies and has been granted orphan drug designation for the treatment of T-cell lymphoma. Duvelisib was first studied in IPI-145-02 trial, a phase 1, open label, dose-escalation study in patients with advanced hematologic malignancies including indolent and aggressive non-Hodgkin’s lymphoma (27%), T-cell lymphoma (17%), aggressive R/R and treatment naive (TN) CLL (35%), and other hematologic malignancies (21%).52 In this study, the maximum tolerated dose was determined to be 75mg twice daily (BID) based on 2 dose-limiting toxicities occurring at the 100mg dose. Doses of 25mg or 75mg both resulted in a rapid inhibition of p-AKT with near-complete inhibition of CLL proliferation at the start of the second cycle of therapy. Furthermore, a subset of serum cytokine and chemokine levels associated with the B-cell tumor microenvironment were decreased following therapy exposure compared to baseline. The overall response rate (ORR) in patients with R/R CLL and TN CLL was 56% and 83%, respectively, with a median PFS of 15.7 months in the R/R cohort across all dose levels and not reached in the TN cohort who only received duvelisib 25mg BID. OS was not reached in either cohort. In subgroup analysis, duvelisib was also efficacious in patients with high-risk biology such as those with 17p deletion or TP53 mutation (ORR = 46%) and those with unmutated IGHV (ORR = 51%). Given clinical activity at 25mg BID and no further suppression in pAKT and CLL proliferation at higher doses, this dose was selected for subsequent phase II and III studies.52,53
IPI 145–02 trial was followed by the phase 2 DYNAMO and phase 3 DUO trials. The DYNAMO trial enrolled patients with R/R indolent non-Hodgkin’s lymphoma (iNHL), including SLL, follicular lymphoma (FL) and marginal zone lymphoma (MZL), to receive duvelisib at a dose of 25mg BID. Patients had to have disease refractory to both rituximab and chemotherapy (including a purine analogue or alkylating agent) or radioimmunotherapy. Patients previously treated with BTKi or an alternate PI3Kδ inhibitor were excluded from participation. In the overall treatment cohort the ORR was 47%, meeting the pre-specified threshold of 30% as its primary endpoint. Notably, 22% of these patients had a diagnosis of SLL and in this subgroup the ORR was 68% by independent review committee (IRC) and 86% by investigator assessment. All of these responses were classified as partial by IRC. In the overall treatment cohort, the median PFS was 9.5 months with a median OS of 29 months.54 In the phase 3 DUO trial, patients with R/R CLL/SLL were randomized to receive duvelisib 25mg BID or ofatumumab. Patients had to have progressed after 1 prior line of therapy and could not have been previously treated with BTK or PI3Kδ inhibitor. With a median follow up of 22.4 months, treatment with duvelisib resulted in a superior PFS of 13.3 months compared with 9.9 months in the ofatumumab arm with this benefit extending to patients with del (17p) and TP53 mutations. By investigator assessment, median PFS in the duvelisib arm was 17.6 months vs 9.7 months in the ofatumumab. The ORR was also more favorable in the duvelisib arm (74% vs 45%). Similar to other B-cell receptor inhibitors, the majority were partial responses, with 85% of patients treated with duvelisib having a lymph node response (defined at >50% decrease in the sum of the products of target lymph nodes). The median OS was not reached in either arm with 86% of patients alive at 12-months.36 The efficacy of duvelisib in these clinical trials is summarized in Table 2.
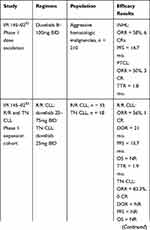 |
Table 2 Efficacy of Duvelisib in Clinical Trials |
Safety and Tolerability of Duvelisib in CLL
In the phase 1, dose-escalation trial, IPI 145–02, serious AEs (≥ grade 3) occurred in 80% and 87% of patients who received duvelisib 25mg BID and 75mg BID, respectively. Hematologic toxicities including neutropenia, anemia and thrombocytopenia were common (≥ grade 3, 32%), however, treatment was rarely discontinued due to these events. The most common non-hematologic toxicities of any grade were diarrhea (42%; ≥ grade 3, 11%), fatigue (41%; ≥ grade 3, 9%), elevated ALT (38%; ≥ grade 3, 16%), elevated AST (38%; ≥ grade 3, 14%), and pyrexia (35.2%; ≥ grade 3, 1.4%). The median duration of transaminase elevation was 2 weeks, with a median time of onset of 1.2 months. Elevated transaminases led to discontinuation of therapy in 7% of patients. The median time to onset of any grade diarrhea was 2 months with a median time to severe diarrhea (≥ grade 3) measured at ~5 months. Infectious complications occurred in 61% of patients, including 3 patients with Pneumocystis jirovecii pneumonia (PJP) and 2 patients with cytomegalovirus (CMV) infection. A protocol amendment in March 2013 required mandatory PJP prophylaxis in all study patients. In total, 27% of patients discontinued therapy at a dose of 25mg BID due to treatment-related AEs.52
In the treatment expansion cohort of study 145–02 which included 73 patients with R/R or TN CLL, the incidence of AEs was similar to other advanced hematologic malignancy subtypes. The most frequent non-hematologic toxicity, diarrhea (47%; ≥ grade 3, 9%), led to discontinuation in two patients. Although transaminase elevation occurred in 31% (≥ grade 3, 9%) of patients, therapy was ultimately able to be restarted, often with dose-reductions. Infection occurred in 73% of R/R patients, mostly presenting in the form of pneumonia (38%) or upper respiratory tract infection (27%). A total of 20 R/R patients (36%) discontinued duvelisib due to AEs including the following which occurred in 2 or more patients: pneumonitis (4), pneumonia (2), stomatitis (2), diarrhea (2) and colitis (2). Two patients with R/R CLL died due to treatment-related infectious complications. Toxicities were overall similar in the TN cohort, with a higher incidence of grade 3 or higher diarrhea (22%). In both the R/R and TN cohorts, the most frequent reason for treatment discontinuation was AEs (36% and 33%, respectively).53
In the phase 2 DYNAMO trial, which enrolled patients with iNHL, the most common AEs (all grades) were diarrhea (49%), nausea (29.5%), neutropenia (29%), fatigue (28%), and cough (27%). The most common grade 3 or higher AEs included neutropenia (24.8%), diarrhea (15%), anemia (15%), and thrombocytopenia (11%). Dose interruptions or dose-reductions occurred in 70% of study patients. In this study, approximately one fourth (24%) of patients discontinued therapy due to AEs and 5 deaths were considered treatment-related including pancolitis (1), fatal viral infection (1), septic shock (1), and severe skin toxicity (2).54
In patients who received duvelisib in the phase 3 DUO trial, adverse effects were similar to earlier studies with few notable differences. The rate of grade 3 or higher elevated AST or ALT was lower (3% each) than previously reported in the CLL expansion cohort of IPI 145–02 (14–16%), but similar to DYNAMO (3–5%). Additionally, colitis emerged as a significant toxicity and was the most common immunomodulatory AEs of grade 3 or higher (12%), resulting in treatment discontinuation in 5% of patients. In total, 4 deaths occurred due to AEs including staphylococcal pneumonia (2), sepsis (1) and general health deterioration (1). Similar to the expansion cohort of IPI-145-02 of R/R or TN CLL patients, the most common cause for treatment discontinuation were AEs (35%), followed by progressive disease (22%).36
Taken together, these studies highlight the major toxicities of duvelisib that require careful monitoring. These seem to be related to a class effect of PI3Ki and include immunomodulatory AEs, infectious complications, and hematologic toxicities. Appropriate careful monitoring allows for dose interruption or dose-reduction to minimize the risk of severe complications. Interestingly, the most frequent reason for treatment discontinuation remained AEs rather than disease progression in the CLL cohort. The findings in patients with a CLL diagnosis differs from those reported in DYNAMO where, in a study population of iNHL of which only a minority of patients had SLL (21%), only one quarter of treatment discontinuations were due to AEs.36,52–54 This prompts to question whether there may be something unique to patients with a CLL/SLL diagnosis that leads to higher rates of AEs requiring discontinuation. Investigations are ongoing to evaluate the role of intermittent dosing of duvelisib to reduce toxicity in clinical trial NCT03961672. Preliminary data examining intermittent dosing after 2 cycles of continuous therapy with a selective PI3Kδ inhibitor in development, ME-401 (Zandelisib), suggest this strategy may be effective in reducing rates of delayed immune mediated AEs without sacrificing efficacy.55
As a result of rare, but fatal, events seen in clinical trials to date, duvelisib carries black-box warnings for serious infections, severe diarrhea or colitis, serious cutaneous reactions and pneumonitis. These toxicities need to be monitored for and the manufacturer has provided guidance regarding the management of these AEs depending on their severity. All patients on duvelisib should receive infection prophylaxis for PJP and CMV when initiating duvelisib; along with monthly CMV viral load monitoring. It is recommended to withhold duvelisib until any new infection resolves and to discontinue its use if PJP is confirmed. For immunologic toxicities such as diarrhea/colitis, pneumonitis, or rash, we recommend holding duvelisib and prompt initiation of steroid therapy along with supportive care when infectious causes have been ruled out, with permanent discontinuation in cases of grade 3 or higher AEs. Regarding transaminitis, patients are usually asymptomatic, hence the need to monitor liver enzymes during routine medical care and to hold further dosing until the transaminitis resolves or decreases to grade 1 toxicity. For hematological toxicities (including neutropenia and thrombocytopenia), duvelisib should be withheld when the absolute neutrophil count falls below 500/mm3 (grade 4) or the platelet count drops below 25,000/mm3 (grade 4).
It is important to evaluate potential drug-drug interactions to minimize the risk of under- or over-dosing. Co-administration of duvelisib with strong CYP3A4 inducers may decrease the efficacy of the drug and should be avoided. When co-administered with strong or moderate CYP3A inhibitors, duvelisib should be dosed at 15 mg twice daily and clinicians should monitor carefully for potential toxicities.
Comparing Duvelisib to Idelalisib for the Treatment of Relapsed/Refractory CLL/SLL
As discussed earlier, idelalisib (GS-1101 or CAL-101) is an oral, highly selective inhibitor of PI3Kδ and is the first PI3K inhibitor to be FDA approved for the treatment of CLL/SLL. Idelalisib’s approval was based on the results from Study 116, a phase 3 randomized control trial comparing idelalisib with rituximab vs rituximab monotherapy in patients unfit to receive cytotoxic therapy. The median number of prior therapies in this trial was 3 and prior therapy had to include a CD20 antibody or at least two prior cytotoxic regimens. PFS at 24 weeks was 93% in the idelalisib group vs 46% in the rituximab group. OS at 12 months was also superior in the idelalisib-treated arm (92% vs 80%).38 With longer follow up the median PFS in patients randomized to receive idelalisib was 20.3 months.56
There have been no clinical trials directly comparing duvelisib and idelalisib to date. Given that there are differences in the clinical trials leading to each drug’s approval, cross-trial comparisons are not possible. Table 3 summarizes patient characteristics, outcomes and specific AEs (those of interest that were reported comparably) in DUO and Study 116.36,56 At present, due to lack of direct comparison, and differences in trial population and follow-up, we are unable to recommend one PI3K inhibitor over another and believe both to be reasonable options if PI3K inhibition is the selected therapeutic approach.
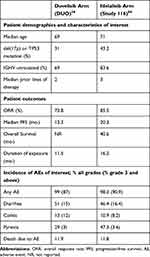 |
Table 3 Patient Characteristics and Outcomes in DUO and Study 116 |
Future Considerations
There are currently 3 registered, ongoing, clinical trials utilizing duvelisib in CLL/SLL (Table 4; clinicaltrials.gov). As discussed previously, the number one reason for discontinuation of therapy in patients with CLL is AEs rather than progressive disease.36,53 While prophylaxis for PJP and CMV with careful monitoring for infection may mitigate some of the potentially fatal toxicities of duvelisib, further study is needed to mitigate the immunomodulatory side effects including diarrhea, colitis, pneumonitis and rash. One potential solution may be to develop new dosing schedules. In the phase 2 trial NCT03961672, investigators are evaluating the role of a 12-week (3-cycle) induction phase where patients receive continuous therapy with duvelisib 25m BID, followed by a maintenance phase involving duvelisib dosing on days 1–2, 8–9, 15–16 and 22–23 of each 28-day cycle or the first 2 days of each week. As the median time to severe diarrhea in IPI-145-02 was 5 months, by altering dosing after 12 weeks, one may be able to mitigate the development of this AE while still effectively targeting PI3K-δ,γ.53
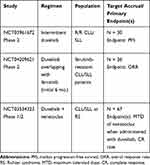 |
Table 4 Ongoing Trials of Duvelisib in CLL/SLL |
Pre-clinical studies have shown that duvelisib can overcome ibrutinib resistance mediated by the in BTK C481S mutation, however, there is very limited data regarding the efficacy of duvelisib after BTKi therapy in human subjects.50 Very few patients (6) had received prior BTKi therapy in IPI-145-02 and in the phase 3 DUO trial patients with prior BTKi therapy were excluded, therefore clinical data regarding the efficacy of duvelisib in ibrutinib-resistant and ibrutinib-intolerant patients are lacking.36,53 In the ongoing trial, NCT04209621, investigators are evaluating the use of duvelisib after development of ibrutinib resistance and hopefully more data will be forthcoming. As patients may have rapid progression upon abrupt discontinuation of ibrutinib, BTKi therapy in this trial is overlapped for 6 months while initiating duvelisib to prevent a potential “rebound” effect. Recruitment status was suspended, however, on September 24, 2020 due to an unexpected sudden death on study.
Lastly, in NCT03534323, duvelisib is being studied in combination with venetoclax for the treatment of patients with CLL who had progressed after 1 prior therapy and those with Richter’s transformation. Preclinical studies in CLL/SLL have shown that duvelisib therapy results in upregulation of the anti-apoptotic protein BCL2. Furthermore, combination duvelisib and venetoclax therapy in vitro, resulted in more apoptosis than venetoclax therapy alone.57 These findings provide the rationale for this combination in early clinical trials. If efficacious, tolerable and safe, a follow up study will be prudent to assess whether this therapy can be given for a fixed duration similar to venetoclax plus obinutuzumab.
An important, practical question is when to employ PI3K inhibitor therapy in the treatment of CLL/SLL. Expert consensus is generally to utilize BTKi and venetoclax therapy in first and second lines of therapy, in no specific order, as these are felt to be the most active agents.58 Patients exposed to both these agents were generally excluded, however, from trials involving duvelisib, therefore efficacy is largely extrapolated in this population. Retrospective data from Mato et al. suggest that patients who are refractory to both BTKi and venetoclax appear to have a short duration of response with PI3K inhibitor therapy.59 For these high-risk patients we recommend enrollment in a clinical trial or evaluation for cellular therapies (e.g chimeric antigen receptor T-cell therapy or allogeneic stem cell transplant) when possible. In the absence of these options, most would offer PI3K inhibitor therapy given the lack of other more effective, commercially available, targeted agents. At the moment, patients with del(17p) or double refractory to BTKi and BCL2i remain an unmet need and may benefit from duvelisib salvage therapy in the absence of a clinical trial.
Conclusions
In recent years, several novel agents have been developed for the treatment of CLL/SLL. Unfortunately, due to the relapsing nature of the disease, treatment options targeting different signaling pathways are still needed in order to prolong survival. By inhibiting the γ and δ isoforms of PI3K, duvelisib targets key oncogenic pathways resulting in direct cytotoxic effects on tumor cells and abrogation of pro-survival cytokine and chemokine signaling in the tumor microenvironment.41 Duvelisib has demonstrated efficacy in CLL/SLL based on the Phase 1 IPI 145–02 and phase 3 DUO trials and has received FDA approval in patients with R/R CLL/SLL after 2 prior therapies. One of the obstacles to duvelisib therapy, however, is its safety profile, which leads to treatment discontinuation in the majority of cases.36,53 Consequently, several clinical trials are investigating alternative dosing schedules to reduce toxicities while maintaining efficacy. The efficacy of duvelisib in CLL is also being studied in ibrutinib-resistant patients and in combination with other targeted agents such as venetoclax. The results of these clinical trials will help to better define the role of duvelisib in the growing CLL/SLL armamentarium. Overall, duvelisib represents a promising targeted agent with an evolving role in the treatment of relapsed/refractory CLL/SLL.
Acknowledgments
Jacqueline C. Barrientos’ work is supported in part by the 2015 American Society of Hematology-Harold Amos Medical Faculty Development Program fellowship and the 2019 Paul Foundation Innovation Award.
Disclosure
JCB has received honoraria for consulting for Gilead, AstraZeneca, Janssen, Genentech, Abbvie and research support from Pharmacyclics/Abbvie, AstraZeneca, and Gilead. The authors report no other conflicts of interest in this work.
References
1. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi:10.1182/blood-2016-01-643569
2. World Health Organization. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC; 2001.
3. Chiorazzi N, Chen SS, Rai KR. Chronic lymphocytic leukemia - cancer stat facts. Cold Spring Harb Perspect Med. 2021;11(2):a035220. doi:10.1101/cshperspect.a035220
4. Weide R, Feiten S, Chakupurakal G, et al. Survival improvement of patients with chronic lymphocytic leukemia (CLL) in routine care 1995–2017. Leuk Lymphoma. 2019;61(3):557–566. doi:10.1080/10428194.2019.1680840
5. Patel K, Danilov AV, Pagel JM. Duvelisib for CLL/SLL and follicular non-Hodgkin lymphoma. Blood. 2019;134(19):1573–1577. doi:10.1182/blood.2019001795
6. Pflug N, Bahlo J, Shanafelt TD, et al. Development of a comprehensive prognostic index for patients with chronic lymphocytic leukemia. Blood. 2014;124(1):49–62. doi:10.1182/blood-2014-02-556399
7. Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910–1916. doi:10.1056/NEJM200012283432602
8. Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840–1847. doi:10.1182/blood.V94.6.1840
9. Kröber A, Seiler T, Benner A, et al. VH mutation status, CD38 expression level, genomic aberrations, and survival in chronic lymphocytic leukemia. Blood. 2002;100(4):1410–1416. doi:10.1182/blood.V100.4.1410.h81602001410_1410_1416
10. Rassenti LZ, Jain S, Keating MJ, et al. Relative value of ZAP-70, CD38, and immunoglobulin mutation status in predicting aggressive disease in chronic lymphocytic leukemia. Blood. 2008;112(5):1923–1930. doi:10.1182/blood-2007-05-092882
11. Zenz T, Eichhorst B, Busch R, et al. TP53 mutation and survival in chronic lymphocytic leukemia. J Clin Oncol. 2010;28(29):4473–4479. doi:10.1200/JCO.2009.27.8762
12. Rossi D, Cerri M, Deambrogi C, et al. The prognostic value of TP53 mutations in chronic lymphocytic leukemia is independent of Del17p13: implications for overall survival and chemorefractoriness. Clin Cancer Res. 2009;15(3):995–1004. doi:10.1158/1078-0432.CCR-08-1630
13. Rossi D, Rasi S, Spina V, et al. Integrated mutational and cytogenetic analysis identifies new prognostic subgroups in chronic lymphocytic leukemia. Blood. 2013;121(8):1403–1412. doi:10.1182/blood-2012-09-458265
14. Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. doi:10.1182/blood-2017-09-806398
15. Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437. doi:10.1056/NEJMoa1509388
16. Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. doi:10.1056/NEJMoa1400376
17. Farooqui MZH, Valdez J, Martyr S, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol. 2015;16(2):169–176. doi:10.1016/S1470-2045(14)71182-9
18. Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(1):43–56. doi:10.1016/S1470-2045(18)30788-5
19. O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–1418. doi:10.1016/S1470-2045(16)30212-1
20. Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib–rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432–443. doi:10.1056/NEJMoa1817073
21. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi:10.1056/NEJMoa1215637
22. Munir T, Brown JR, O’Brien S, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353–1363. doi:10.1002/ajh.25638
23. Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102(10):1796–1805. doi:10.3324/haematol.2017.171041
24. Sharman JP, Banerji V, Fogliatto LM, et al. ELEVATE TN: phase 3 study of acalabrutinib combined with obinutuzumab (O) or alone vs O plus chlorambucil (Clb) in patients (Pts) with treatment-naive chronic lymphocytic leukemia (CLL). Blood. 2019;134(Supplement_1):31. doi:10.1182/blood-2019-128404
25. Ghia P, Pluta A, Wach M, et al. Acalabrutinib versus investigator’s choice of idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory CLL: the randomised, controlled, phase 3 ASCEND; 2020. Available from: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3463285.
26. Calquence met primary efficacy endpoint in head-to-head trial against ibrutinib in chronic lymphocytic leukaemia; 2021. Available from: https://www.astrazeneca.com/media-centre/press-releases/2021/calquence-met-primary-endpoint-against-ibrutinib.html.
27. Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225–2236. doi:10.1056/NEJMoa1815281
28. Al-Sawaf O, Zhang C, Tandon M, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21(9):1188–1200. doi:10.1016/S1470-2045(20)30443-5
29. AbbVie Inc. VENCLEXTA™ (venetoclax) [package insert]. U.S. Food and Drug Administration website. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208573s000lbl.pdf. Accessed February 20, 2021.
30. Tam CS, O’Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112(4):975–980. doi:10.1182/blood-2008-02-140582
31. Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–4088. doi:10.1200/JCO.2005.12.051
32. Thompson PA, Tam CS, O’Brien SM, et al. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood. 2016;127(3):303–309. doi:10.1182/blood-2015-09-667675
33. Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208–215. doi:10.1182/blood-2015-06-651125
34. Woyach JA. FCR holds up to the test of time: CLL8 follow-up. Blood. 2016;127(2):172–173. doi:10.1182/blood-2015-11-678557
35. Benjamini O, Jain P, Trinh L, et al. Second cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: distribution and clinical outcomes. Leuk Lymphoma. 2015;56(6):1643–1650. doi:10.3109/10428194.2014.957203
36. Flinn IW, Hillmen P, Montillo M, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132(23):2446–2455. doi:10.1182/blood-2018-05-850461
37. Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax–rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107–1120. doi:10.1056/NEJMoa1713976
38. Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. doi:10.1056/NEJMoa1315226
39. Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17(6):768–778. doi:10.1016/S1470-2045(16)30019-5
40. Barrientos JC. Sequencing of chronic lymphocytic leukemia therapies. Hematol Am Soc Hematol Educ Program. 2016;2016(1):128–136. doi:10.1182/asheducation-2016.1.128
41. Fruman DA. Targeting PI3K-gamma in non-hodgkin lymphoma. J Clin Oncol. 2019;37(11):932–934. doi:10.1200/JCO.19.00215
42. Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9(8):550–562. doi:10.1038/nrc2664
43. Mollon L, Aguilar A, Anderson E, et al. Abstract 1207: a systematic literature review of the prevalence of PIK3CA mutations and mutation hotspots in HR+/HER2- metastatic breast cancer. Cancer Res. 2018;78(13Supplement):1207. doi:10.1158/1538-7445.AM2018-1207
44. Vangapandu HV, Jain N, Gandhi V. Duvelisib: a phosphoinositide-3 kinase δ/γ inhibitor for chronic lymphocytic leukemia. Expert Opin Investig Drugs. 2017;26(5):625–632. doi:10.1080/13543784.2017.1312338
45. Barrientos JC. Idelalisib for the treatment of indolent non-Hodgkin lymphoma: a review of its clinical potential. OncoTargets Ther. 2016;9:2945–2953. doi:10.2147/OTT.S102573
46. Frustaci AM, Tedeschi A, Deodato M, Zamprogna G, Cairoli R, Montillo M. Duvelisib for the treatment of chronic lymphocytic leukemia. Expert Opin Pharmacother. 2020;21(11):1299–1309. doi:10.1080/14656566.2020.1751123
47. Winkler DG, Faia KL, DiNitto JP, et al. PI3K-δ and PI3K-γ inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem Biol. 2013;20(11):1364–1374. doi:10.1016/j.chembiol.2013.09.017
48. Rodrigues DA, Sagrillo FS, Fraga CAM. Duvelisib: a 2018 novel FDA-approved small molecule inhibiting phosphoinositide 3-kinases. Pharmaceuticals. 2019;12(2):69. doi:10.3390/ph12020069
49. Balakrishnan K, Peluso M, Fu M, et al. The phosphoinositide-3-kinase (PI3K)-delta and gamma inhibitor, IPI-145 (Duvelisib), overcomes signals from the PI3K/AKT/S6 pathway and promotes apoptosis in CLL. Leukemia. 2015;29(9):1811–1822. doi:10.1038/leu.2015.105
50. Dong S, Guinn D, Dubovsky JA, et al. IPI-145 antagonizes intrinsic and extrinsic survival signals in chronic lymphocytic leukemia cells. Blood. 2014;124(24):3583–3586. doi:10.1182/blood-2014-07-587279
51. Chen -S-S, Kutok JL, Ferrer G, et al. Dual inhibition of PI3K-δ and PI3K-γ by duvelisib eliminates CLL B cells, impairs CLL-supporting cells, and overcomes ibrutinib resistance in a patient-derived xenograft model. Blood. 2018;132(Supplement 1):4420. doi:10.1182/blood-2018-99-109853
52. Flinn IW, O’Brien S, Kahl B, et al. Duvelisib, a novel oral dual inhibitor of PI3K-δ,γ, is clinically active in advanced hematologic malignancies. Blood. 2018;131(8):877–887. doi:10.1182/blood-2017-05-786566
53. O’Brien S, Patel M, Kahl BS, et al. Duvelisib, an oral dual PI3K-δ,γ inhibitor, shows clinical and pharmacodynamic activity in chronic lymphocytic leukemia and small lymphocytic lymphoma in a phase 1 study. Am J Hematol. 2018;93(11):1318–1326. doi:10.1002/ajh.25243
54. Flinn IW, Miller CB, Ardeshna KM, et al. DYNAMO: a phase II study of duvelisib (IPI-145) in patients with refractory indolent non-hodgkin lymphoma. J Clin Oncol. 2020.
55. Zelenetz AD, Soumerai JD, Jagadeesh D, et al. Preliminary safety and efficacy results with an intermittent schedule of the PI3kδ inhibitor ME-401 alone or in combination with rituximab for B-cell malignancies. Blood. 2018;132(Supplement1):2893. doi:10.1182/blood-2018-99-115670
56. Sharman JP, Coutre SE, Furman RR, et al. Final results of a randomized, phase III study of rituximab with or without idelalisib followed by open-label idelalisib in patients with relapsed chronic lymphocytic leukemia. J Clin Oncol. 2019;37(16):1391–1402. doi:10.1200/JCO.18.01460
57. Patel VM, Balakrishnan K, Douglas M, et al. Duvelisib treatment is associated with altered expression of apoptotic regulators that helps in sensitization of chronic lymphocytic leukemia cells to venetoclax (ABT-199). Leukemia. 2017;31(9):1872–1881. doi:10.1038/leu.2016.382
58. Roeker LE, Mato AR. Approaches for relapsed CLL after chemotherapy-free frontline regimens. Hematology. 2020;2020(1):10–17. doi:10.1182/hematology.2020000168
59. Mato AR, Roeker LE, Jacobs R, et al. Assessment of the efficacy of therapies following venetoclax discontinuation in CLL reveals BTK inhibition as an effective strategy. Clin Cancer Res. 2020;26(14):3589–3596. doi:10.1158/1078-0432.CCR-19-3815
60. Liu N, Rowley BR, Bull CO, et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110α and p110δ activities in tumor cell lines and xenograft models. Mol Cancer Ther. 2013;12(11):2319–2330. doi:10.1158/1535-7163.MCT-12-0993-T
61. Lannutti BJ, Meadows SA, Herman SEM, et al. CAL-101, a p110δ selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117(2):591–594. doi:10.1182/blood-2010-03-275305
62. Burris HA, Patel MR, Lanasa MC, et al. Activity of TGR-1202, a novel once-daily PI3Kδ inhibitor, in patients with relapsed or refractory hematologic malignancies. J Clin Oncol. 2014;32(15_suppl):2513. doi:10.1200/jco.2014.32.15_suppl.2513
63. Lampson BL, Brown JR. PI3Kδ-selective and PI3Kα/δ-combinatorial inhibitors in clinical development for B-cell non-hodgkin lymphoma. Expert Opin Investig Drugs. 2017;26(11):1267–1279. doi:10.1080/13543784.2017.1384815
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.