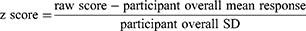Back to Journals » Journal of Pain Research » Volume 17
Opioid Receptor Mu 1 Gene (OPRM1) A118G Polymorphism and Emotional Modulation of Pain
Authors Trimble EA, Kell PA, Avella MA, France CR, Rhudy JL
Received 28 September 2023
Accepted for publication 21 January 2024
Published 1 February 2024 Volume 2024:17 Pages 489—500
DOI https://doi.org/10.2147/JPR.S442431
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Wendy Imlach
Edward A Trimble,1,2 Parker A Kell,2 Matteo A Avella,3,4 Christopher R France,5 Jamie L Rhudy2,6
1Department of Biochemistry, The University of Tulsa, Tulsa, OK, USA; 2Department of Psychology, The University of Tulsa, Tulsa, OK, USA; 3Department of Biology, The University of Tulsa, Tulsa, OK, USA; 4Division of Maternal and Child Health, Sidra Medicine, Education City, Ar-Rayyan, Doha, Qatar; 5Department of Psychology, Ohio University, Athens, OH, USA; 6Department of Health Promotion Sciences, University of Oklahoma Health Sciences Center, Tulsa, OK, USA
Correspondence: Jamie L Rhudy, University of Oklahoma Health Sciences Center, TSET Health Promotion Research Center, Department of Health Promotion Sciences, 4502 E. 41st Street, Tulsa, OK, USA, Tel +1 918-660-3050, Email [email protected]
Purpose: The A118G polymorphism in the opioid receptor mu 1 gene (OPRM1) is associated with decreased opioid receptor availability, altered emotion, and increased pain. Given that emotions modulate pain (positive emotions inhibit pain, negative emotions enhance pain), we predicted that G allele carriers would experience impaired emotional modulation of pain compared to non-G allele carriers.
Patients and Methods: Emotional pictures (ie, erotica, neutral, attack) from the International Affective Picture System were used by permission from the authors to experimentally manipulate emotions in 64 adult participants while painful electrocutaneous stimulations were delivered in a cross-sectional study. Ratings of arousal and valence/pleasure were made in response to pictures, and pain ratings and a physiological measure of spinal nociception (ie, nociceptive flexion reflex, NFR) were collected in response to painful stimulations. Secondary analyses were conducted to examine the relationship between the A118G polymorphism and emotional modulation of pain/NFR.
Results: Exposure to emotional pictures elicited similar changes in valence, but G-carriers rated erotic pictures as more arousing. In non-carriers, pain was facilitated by attack pictures and pain and NFR were inhibited by erotic pictures relative to neutral pictures. Among G-carriers, pain was facilitated by negative emotional pictures but there was no pain or NFR inhibition by positive emotional pictures.
Conclusion: The altered response to pleasant stimuli further supports the role of opioids in appetitive behavior and describes how the A118G polymorphism may prevent carriers from inhibiting pain during pleasure.
Keywords: opioid receptor, genetics, emotion, pain, pain inhibition
Introduction
In healthy individuals, pain modulation allows organisms to respond adaptively to environmental demands. Disrupted pain modulation (eg, excessive facilitation, impaired inhibition) is associated with chronic pain disorders, like fibromyalgia and chronic tension-type headache.1–3 Thus, understanding factors that impair pain modulation could provide insight into chronic pain risk mechanisms.
Emotions are one factor that modulates pain; positive emotions inhibit pain and negative emotions enhance pain (as long as emotional intensity/arousal is not too high).4,5 Emotional modulation of pain can be investigated using the emotional controls of nociception (ECON) paradigm, in which emotionally laden pictures are shown to induce affective responses during which pain is evoked.6–8 Previous research indicates that both pain and the nociceptive flexion reflex (NFR), a spinally mediated withdrawal reflex in the lower limb (assessed using EMG following painful sural nerve stimulations) that assesses spinal nociception, are typically modulated in parallel by ECON.6–9 But they can also diverge from one another, suggesting separate circuits mediate their emotional modulation.7,10
Endogenous opioids play a critical role in descending pain modulation11 and emotional processing;12 therefore, individual differences in opioidergic signaling may be related to altered emotional modulation of pain and NFR.13 The OPRM1 gene (opioid receptor mu 1) codes for mu-opioid receptors14 and affects emotion and pain processing.15–18 Most individuals carry homozygous AA alleles (ie, AA-carriers), yet approximately 10–32% of humans carry a G allele of the OPRM1 exon 1 rs1799971 polymorphism, A118G (ie, G-carriers), in which guanine replaces adenine.19 It has been hypothesized the A118G polymorphism causes a loss of function,15 such that G-carriers have impaired functionality of mu-opioid receptors. Supporting this, G-carriers show altered emotional processing, including heightened sensitivity to social rejection and greater pleasure from social situations.20,21 G-carriers also show impairments in descending pain modulation as evidenced by the need for higher doses of opioid analgesics following Cesarean section22 and greater postoperative pain,23,24 relative to AA-carriers.
To date, no study to our knowledge has investigated opioid receptor polymorphisms and their impact on affective modulation of pain and nociception. Thus, the present secondary analysis of data examines whether G-carriers display altered emotional modulation of pain and NFR relative to AA-carriers during ECON. AA-carriers are hypothesized to show typical emotional modulation of pain and NFR, with pain and NFR inhibition following pleasant pictures as well as pain and NFR facilitation following unpleasant pictures. Given evidence of dysregulated emotion and pain processing in G-carriers, it is hypothesized that G-carriers will show disrupted emotional modulation of pain and NFR, relative to AA-carriers.
Materials and Methods
Participants and Samples
Participants (N=144) were recruited at The University of Tulsa and Ohio University between 2006 and 2009.25 Exclusion criteria were: <18 years, neurological, cardiovascular and/or circulatory problems, recent use of medications affecting pain (ie, analgesic, antidepressant, anxiolytic, and/or antihypertensive), phobia of snakes/spiders (included in imagery), chronic pain, or recent psychological trauma. Seventy-nine (55%) participants were female and 122 (85%) were White, non-Hispanic. Average age was 27.31 years (SD=13.86). Participants were compensated with $100 (Tulsa) or psychology research credit (Ohio). Due to high salt contamination from long-term storage, 64 participants produced usable DNA samples. Table 1 compares those with and without viable samples. Viable samples were from younger participants.
 |
Table 1 DNA Viability Group Age, Sex, and Race |
All participants provided verbal and written informed consent. This study complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as amended in 2013) and was approved by the Institutional Review Boards of The University of Tulsa (IRB#04-67) and Ohio University (IRB# 07F003).
Apparatus
During testing, participants sat in a comfortable chair in a room adjacent to the experimenter. A computer with two monitors controlled the experiment – one displayed emotional pictures and questionnaires to the participant and the other displayed physiological signals to experimenters.
A stimulating electrode (Nicolet, 019-401400, Madison, WI) was applied to the participant’s left ankle over the retromalleolar pathway. Constant-current stimulators were used for painful stimulus delivery (Grass Model S88/S48, West Warwick, RI, at Tulsa; Digitimer DS7A, Hertfordshire, England, at Ohio). Max current=40 mA.
NFR was recorded by electromyogram (EMG) from two Ag-AgCl electrodes filled with conductive gel applied over the left biceps femoris, 10-cm superior to the popliteal fossa (reference electrode on femur lateral epicondyle). Sites were cleaned with alcohol and exfoliated to minimize impedances to below 10 kΩ. Sample rate=1000 Hz. Recording amplifier was Grass Model 15LT at Tulsa and Delsys Bagnoli 2 at Ohio.
Stimulation Intensity Determination
Painful stimuli delivered during ECON were set to 120% of the NFR threshold to ensure that the nociceptive system was activated. Each stimulus was a train of five 1-ms pulses at 250-Hz. The validated and standardized procedure for assessing NFR threshold is described elsewhere.26
Electric Pain Threshold and Tolerance
Subjective thresholds were measured using a single, ascending staircase of electric stimulations. After each stimulation, participants rated their pain using a computerized scale ranging from 0 (no sensation) to 100 (maximum tolerable). Stimulation intensity was increased until participants reported a 100 on the subjective pain scale (or until 40-mA reached). Pain threshold=first stimulus as rated ≥50 on the pain scale. Pain tolerance=stimulus intensity rated 100 (or 40-mA if reached).
Emotional Controls of Nociception (ECON)
Attack (scenes of individuals in danger), neutral (eg, household objects), and erotica (humans in sexual acts) images from the International Affective Picture System were presented in pseudorandom order.27 These contents were chosen because they reliably modulate pain and NFR.7–9 Pictures were displayed for 6-s with 12 to 22-s inter-picture intervals. After each picture, participants rated how the picture made them feel on a valence/pleasure scale (1 to 9; higher scores=more pleasant, lower scores=more unpleasant) and an arousal scale (1 to 9; higher scores=greater arousal).28
Painful stimuli were delivered randomly throughout picture viewing (balanced across contents). Stimulations were delivered 3 to 5-s after picture onset or 11 to 21-s after inter-picture interval onset. Four painful stimuli were delivered during each picture content. Following each painful stimulus, the pain rating scale was presented. NFR magnitude=mean EMG of 90 to 150-ms post-electric stimulus interval minus mean EMG of 60 ms pre-electric stimulus baseline. Consistent with previous studies,7,8,25 NFR magnitudes and pain ratings were converted to z scores to isolate the variance in pain/NFR due to emotional modulation:
Genotyping
Saliva samples were collected from participants at the time of testing using a buccal swab. DNA samples were isolated from the saliva using the BuccalAmp DNA Extraction Kit (Epicentre Biotechnologies, Madison, WI, USA) and stored at −80°C. A nanodrop spectrometer indicated high levels of salt (ie, peaks at 230 nm) within the saliva samples. Thus, a protocol for ethanol precipitation of DNA was followed to remove contaminants from the DNA samples.29 Next, samples were amplified using OPRM1 exon 1 primers (forward primer = AAGGTGGGAGGGGGCTATAC; reverse primer = CCTTAAGCCGCTGAACCCT) and polymerase-chain reaction (PCR). Gel electrophoresis was then conducted on the PCR products to determine their viability and to determine if additional purification was necessary. Viable samples were indicated by band visualization on the gels. Prior to purification of the PCR product, 13% of samples within the entire dataset were accompanied by band visualization, whereas 40% of samples were accompanied by band visualization after PCR purification. Thus, ethanol precipitation, PCR, and gel electrophoresis were conducted on all 144 samples to select usable samples. Of the 144 samples, 68 did not produce a band and were removed from subsequent analyses, leaving 76 included samples. Included samples were then sequenced using Sanger Sequencing services provided by Eurofins Genomics.
Chromatogram files produced from Sanger Sequencing showed all bases in the PCR product, including the locus of interest for this study (rs1799971). Chromatograms (n = 12) that were unreadable at rs1799971 due to interference from other peaks were removed. The remaining chromatograms were then assessed at rs1799971 for A118G polymorphisms to code participants as G-carriers (n = 11) or non-carriers (n = 53). Since the entirety of exon 1 on the OPRM1 gene was amplified with PCR, heterozygotes for other base pairs throughout exon 1 could be identified. Two heterozygotes, both from the non-carrier group, had the same polymorphism. This second variant resulted in an adenine substitution with thymine at the 17th base position of exon 1 of the OPRM1 gene (A17T). This variant also resulted in substitution of a nonpolar amino acid (alanine) with another nonpolar amino acid (valine) on the sixth amino acid residue (A6V) of the OPRM1 receptor gene (UCSC genome browser, chr6:154,360,695–154,360,697). Effects on the OPRM1 receptor’s structure as a result of the missense variant were predicted using a protein modeling program from Imperial College of London.30 Results were verified using the missense variant analyzer PolyPhen-2.31 The polymorphism was not deleterious in both cases, so data provided for these participants were included.
Statistical Approach
Consistent with past research, two groups were formed based on the presence or absence of the G allele.15,23 G carrier and non-carrier groups were not further split by sex due to the small sample size available t-tests or chi-square tests were used to analyze group differences in age, sex, and race/ethnicity.
To analyze valence, arousal, pain, and NFR, 3 (picture content) x 2 (genotype) mixed repeated measures ANOVAs were conducted, with the Picture Content x Genotype interaction being the effect of interest. In the event of a significant interaction, the simple effect of picture content was examined for each group as is typically done in studies of emotional modulation of pain/NFR,32–35 given that this effect best characterizes group differences in emotional modulation. However, a priori planned comparisons of picture content were also conducted even in the absence of a significant interaction to avoid a type II error due to small sample size. One-tailed tests were used given the large body of evidence demonstrating emotional modulation of pain/NFR.6,25
Results
Comparing Participants with and without Viable DNA
Table 2 shows mean (SE) differences between DNA viability groups for valence, arousal, pain, and NFR. Table 3 shows the results of a 2 (viable vs non-viable) x 3 (picture content) mixed repeated measures ANOVAs that found that DNA viability groups did not differ on valence, arousal, pain, and NFR during picture viewing, except that the viable group had generally lower valence/pleasure ratings, p = 0.010.
 |
Table 2 DNA Viability Group Means and SEs for Valence, Arousal, Pain, and NFR |
 |
Table 3 DNA Viability Group Differences ANOVAs |
Viable Participant Characteristics by OPRM1 Genotype
For the samples that were viable, characteristics were evaluated by OPRM1 genotype (Table 4). An independent samples t-test showed that G-carriers and non-carriers did not differ by age under conditions of unequal variance, t(11.24) = −1.61, p = 0.135. Furthermore, results of chi-square tests showed proportion of females [Χ2(1, N = 64) = 2.92, p = 0.088] and proportion of White, non-Hispanics [Χ2(1, N = 64) = 1.90, p = 0.168] did not differ by genotype. Moreover, genotype quality and frequencies did not significantly deviate from Hardy-Weinberg Equilibrium expected values (Χ2 = 0.554, df = 1, p > 0.05).
 |
Table 4 OPRM1 Genotype Group Age, Sex, and Race |
Valence Ratings
For valence ratings (Figure 1), there was a significant main effect of picture content, F(2, 61) = 73.60, p < 0.001, η2 = 0.71, but no main effect of genotype, F(1, 62) = 2.55, p = 0.116, η2 = 0.04. Participants reported greater pleasure (higher valence ratings) during erotica than during neutral or attack contents, ps < 0.001. Furthermore, they reported more displeasure (lower valence ratings) during attack content compared to erotica and neutral contents, ps < 0.001. The picture content by genotype interaction was non-significant, F(2, 61) = 0.45, p = 0.643, η2 = 0.01. Nonetheless, a priori simple effects of picture content were probed for each genotype group. As shown in Figure 1, the simple effect was significant in the non-carrier group, F(2, 61) = 92.65, p < 0.001, η2 = 0.752, as well as the G-carrier group, F(2, 61) = 25.48, p < 0.001, η2 = 0.455. Valence ratings for the three picture contents in each group were significantly different from each other, ps < 0.001. Thus, there were no significant group differences in valence appraisals of the picture stimuli.
Arousal Ratings
For arousal ratings (Figure 2), there was a significant main effect of picture content, F(2, 61) = 66.55, p < 0.001, η2 = 0.69, that was qualified by a significant Picture Content x Genotype interaction, F(2, 61) = 3.51, p = 0.036, η2 = 0.10. The simple effect of picture content was significant for non-carriers, F(2, 61) = 69.94, p < 0.001, η2 = 0.70, and for G-carriers, F(2, 61) = 27.78, p < 0.001, η2 = 0.48. As shown in Figure 2, in the non-carrier group, attack content elicited higher arousal than either neutral or erotica and erotica content elicited higher ratings than neutral content (ps < 0.001). In the G-carrier group, while arousal ratings were significantly higher in response to attack and erotica content than in neutral content (p < 0.001), arousal ratings for attack were not significantly higher than in erotica content (p = 0.390). Thus, erotica elicited relatively greater arousal in the G-carrier group than the non-carrier group. The main effect of genotype was non-significant for arousal ratings, F(1, 62) = 0.057, p = 0.812, η2 = 0.001.
NFR Threshold and Pain Threshold
NFR thresholds for non-carriers (M = 11.52, SD = 7.84) and G-carriers (M = 7.38, SD = 4.34) were not significantly different, t(62) = 1.69, p = 0.096. Similarly, pain threshold did not differ, t(62) = 1.22, p = 0.227, between non-carriers (M = 13.66, SD = 8.61) and G-carriers (M = 10.36, SD = 5.06).
Emotional Modulation of Pain Ratings
Analysis of pain ratings showed a significant main effect of picture content, F(2, 61) = 9.13, p < 0.001, η2 = 0.23, but no significant main effect of genotype, F(1, 62) = 1.67, p = 0.201, η2 = 0.03 or Picture Content x Genotype interaction, F(2, 61) = 1.20, p = 0.308, η2 = 0.04. Nonetheless, a priori simple effects of picture content were probed for each genotype group (Figure 3). The simple effect of picture content was significant in the non-carrier group, F(2, 61) = 20.73, p < 0.001, η2 = 0.41, but not in the G-carrier group, F(2, 61) = 1.94, p = 0.153, η2 = 0.06. For the non-carrier group, pain ratings were greater in the attack content than in the other contents, ps < 0.001, and pain ratings were lower in the erotica content than in the other contents, ps < 0.01. The G-carrier group showed significantly greater pain ratings in attack content than in neutral or erotica content (ps < 0.05), but pain ratings for erotica and neutral content were not significantly differently (p = 0.409). Thus, G-carriers did not demonstrate pain inhibition while viewing erotica.
Emotional Modulation of NFR Magnitudes
For NFR magnitudes, there were no significant effects of picture content, F(2, 61) = 2.15, p = 0.125, η2 = 0.07, genotype, F(1, 62) = 1.11, p = 0.295, η2 = 0.02, or Picture Content x Genotype interaction, F(2, 61) = 0.52, p = 0.598, η2 = 0.02. Even so, a priori simple effects of picture content were explored by genotype group (Figure 4). Analyses of the simple effects of picture content were non-significant for both non-carriers, F(2, 61) = 2.03, p = 0.140, η2 = 0.06, and G-carriers, F(2, 61) = 1.19, p = 0.310, η2 = 0.04. In pairwise comparisons, NFR magnitudes were only significantly different between neutral content and erotica content among non-carriers, p = 0.030. Thus, inhibition of NFR by erotica was not observed in the G-carriers.
Discussion
The present study investigated how emotional modulation of pain (pain inhibition during pleasant stimuli and pain facilitation during unpleasant stimuli) is affected by the A118G polymorphism in the OPRM1 gene. Participants who did not carry the A118G polymorphism displayed pain, arousal, and valence ratings that were in line with previous research in emotional modulation.6,25,36 They reported positive stimuli as being more pleasant (higher valence) and more arousing than neutral stimuli and reported negative stimuli as being more unpleasant and more arousing than neutral. They also reported that negative stimuli were more arousing than positive stimuli. Further, participants reported greater subjective pain during images of attack and less subjective pain during images of erotica, and NFR was inhibited by erotica. Carriers of the A118G polymorphism, while having similar modulation of valence/pleasure ratings as the non-carriers, showed altered responses to pleasant (erotica) stimuli. Specifically, G-carriers reported enhanced arousal to erotica, yet despite this, they showed impaired pain and NFR inhibition in response to erotica.
Appetitive Behavior and Pain Inhibition
In human participants, the A118G polymorphism has been shown to decrease opioid receptor availability in the anterior insula, amygdala, and thalamus.15 These areas of the brain, all of which are associated with affective processing,37,38 are suppressed in those with the A118G polymorphism. With regards to affect and the insular cortex, stimulation to the anterior insula in both non-human primates and rats suppresses appetitive behavior.39,40 It can then be inferred that suppression of the anterior insula, which is seen with A118G carriers, may lead to the opposite effect, ie, enhanced appetitive behavior.
Mu opioid receptor utilization in the amygdala is implicated in analgesic response and suppression of norepinephrine,41,42 and opioid antagonists are associated with increases in norepinephrine.42 With carriers of the A118G polymorphism showing higher sensitivity to social rejection, more affectionate relationships, and more social pleasure,20,21 an increase in norepinephrine could explain the observed characteristics, as well as the higher arousal ratings for pleasurable stimuli noted in this study.
Appetitive motivation is normally associated with a surge of opioids, which stimulates opioid-mediated inhibition of pain.43 This was not seen in G-carriers, suggesting that the typical inhibiting pathway may be disrupted. Given the role of opioids in pleasure and reward,44 changes in participant reactions to pleasant stimuli as a result of reduced opioid receptor functionality are in line with expectations. This disruption of emotional modulation would impact how pain is reported in response to emotional stimuli. Opioid inhibiting pathways, either through opioid antagonists or deleterious polymorphisms of opioid receptors, would effectively make the pleasure-induced inhibition of pain impaired in G-carriers. Having established the role of opioids in pleasure and pain, carriers of the A118G polymorphism may be at risk for blunted inhibition of pain during pleasurable events. Figure 5 illustrates how the A118G polymorphism inhibits opioid receptor availability, thereby altering appetitive behavior and pain inhibition in response to emotional stimuli.
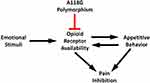 |
Figure 5 Possible pathway in which the A118G polymorphism impairs pleasure-induced pain inhibition. |
Implications
The novelty of the present study stems from the integration of opioid receptor polymorphisms with measures of affect and subjective pain. While previous studies have included a combination of the measures, no study to our knowledge has investigated them together. The present study provides preliminary evidence that pleasure-induced pain inhibition is attenuated in G-carriers. This vital pain regulating mechanism may be impaired in these individuals, potentially resulting in a vulnerability for a chronic pain disorder. It was already suggested that the A118G polymorphism could lead to individuals requiring a higher opioid dosage in order to experience normal analgesic effects.22 With the additional finding that G-carriers exhibit a reduction in the pleasure-induced inhibition of pain, treatments that utilize cognitive-emotional procedures to suppress pain may not be as effective (eg, pleasant imagery). Treatment for individuals with the polymorphism should keep in mind the potentially impaired response to medications and emotion regulation therapies.
However, it is worth noting that the G-carrier group was older and had a higher proportion of men than the non-carrier group (albeit non-significant in both cases). Thus, results should be interpreted with caution. Indeed, given that chronic pain risk is higher in women,45 our findings might underestimate the risk of being a G-carrier on impaired pleasure-induced pain inhibition and chronic pain.
Limitations
There were limitations in this study, one being low sample size. As a result of the low frequency of the A118G polymorphism in the population, there was a small sample of G-carriers in our study. Contamination present in the DNA samples also reduced the number of available samples to be analyzed. This reduced the power of the statistical tests performed and likely contributed to the lack of significant Picture Content x Genotype interactions for pain and NFR. As a result, the carrier group was unable to be split by sex to investigate potential interactions with the A118G polymorphism and pain. For example, female patients with lumbar pain who were carriers for the G allele, compared to male patient G-carriers, reported higher pain over a year.46 Additionally, pain ratings in G-carriers were higher in women than men.47 Thus, confounding effects of sex could be possible. Further, there are limitations in the scale of the genomic analysis performed in the current study, as only exon 1 of the OPRM1 gene was examined. There are other mutations that could result in reduced opioid receptor functionality, above and beyond the reduced functionality of the A118G polymorphism. Variations on other genes could also have an impact on the observed results, as seen with the relationship of serotonin transporter polymorphisms with the emotional modulation of pain.25 Future studies should look to genome-wide analysis to limit the genetic uncertainty that is the nature of single-gene studies.
Summary
The present study found that the OPRM1 A118G polymorphism disrupts typical emotional modulation of pain. Although increased appetitive behavior is followed by pain inhibition in normal genotypes, this was not seen in carriers, despite greater arousal in response to appetitive stimuli. Instead, pain inhibition (and to some degree NFR inhibition) in response to appetitive stimuli was impaired leaving only pain facilitation in response to unpleasant stimuli. This imbalance in pain modulation could place G-carriers at risk for chronic pain.
Acknowledgments
Participant recruitment was partially funded by a Health Research Award (HR06-177, Oklahoma Center for the Advancement of Science and Technology). Genomic analysis was funded by a student research grant from the University of Tulsa Office of Research and Sponsored Programs.
Disclosure
The authors have no conflicts of interest to report for this work.
References
1. Potvin S, Marchand S. Pain facilitation and pain inhibition during conditioned pain modulation in fibromyalgia and in healthy controls. Pain. 2016;157(8):1704–1710. doi:10.1097/j.pain.0000000000000573
2. Pielsticker A, Haag G, Zaudig M, Lautenbacher S. Impairment of pain inhibition in chronic tension-type headache. Pain. 2005;118(1):215–223. doi:10.1016/j.pain.2005.08.019
3. Jensen KB, Kosek E, Petzke F, et al. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain. 2009;144(1):95–100. doi:10.1016/j.pain.2009.03.018
4. Rhudy JL, Meagher MW. The role of emotion in pain modulation. Curr Opin Psychiatry. 2001;14(3):241–245. doi:10.1097/00001504-200105000-00012
5. Rhudy JL. Emotional modulation of pain. In: Neuroscience of Pain, Stress, and Emotion. Elsevier; 2016:51–75.
6. Rhudy JL, Williams AE, McCabe KM, Russell JL, Maynard LJ. Emotional control of nociceptive reactions (ECON): do affective valence and arousal play a role? Pain. 2008;136(3):250–261. doi:10.1016/j.pain.2007.06.031
7. Rhudy JL, Williams AE, McCabe KM, Rambo PL, Russell JL. Emotional modulation of spinal nociception and pain: the impact of predictable noxious stimulation. Pain. 2006;126(1–3):221–233. doi:10.1016/j.pain.2006.06.027
8. Rhudy JL, Williams AE, McCabe KM, Nguyê˜n MATV, Rambo P. Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology. 2005;42(5):579–587. doi:10.1111/j.1469-8986.2005.00313.x
9. Rhudy JL, Bartley EJ, Williams AE, et al. Are there sex differences in affective modulation of spinal nociception and pain? J Pain. 2010;11(12):1429–1441. doi:10.1016/j.jpain.2010.04.003
10. Roy M, Piché M, Chen J-I, Peretz I, Rainville P. Cerebral and spinal modulation of pain by emotions. Nat Precedings. 2009;2009;1.
11. Bagley EE, Ingram SL. Endogenous opioid peptides in the descending pain modulatory circuit. Neuropharmacology. 2020;173:108131. doi:10.1016/j.neuropharm.2020.108131
12. Van Ree JM, Niesink RJM, Van Wolfswinkel L, et al. Endogenous opioids and reward. Eur J Pharmacol. 2000;405(1):89–101. doi:10.1016/S0014-2999(00)00544-6
13. Masih J, Verbeke W. Exploring association of opioid receptor genes polymorphism with positive and negative moods using Positive and Negative Affective States Scale (PANAS). Clin Exp Psychol. 2019;5(1):1–6.
14. Kasai S, Ikeda K. Pharmacogenomics of the human µ-opioid receptor. Pharmacogenomics. 2011;12(9):1305–1320. doi:10.2217/pgs.11.68
15. Peciña M, Love T, Stohler CS, Goldman D, Zubieta J-K. Effects of the mu opioid receptor polymorphism (OPRM1 A118G) on pain regulation, placebo effects and associated personality trait measures. Neuropsychopharmacology. 2015;40(4):957–965. doi:10.1038/npp.2014.272
16. Zubieta JK, Smith YR, Bueller JA, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293(5528):311–315. doi:10.1126/science.1060952
17. Perry M, Baumbauer K, Young EE, Dorsey SG, Taylor JY, Starkweather AR. The influence of race, ethnicity and genetic variants on postoperative pain intensity: an integrative literature review. Pain Manage Nurs. 2019;20(3):198–206. doi:10.1016/j.pmn.2018.11.002
18. Hawn SE, Overstreet C, Stewart KE, Amstadter AB. Recent advances in the genetics of emotion regulation: a review. Curr Opin Psychol. 2015;3:108–116. doi:10.1016/j.copsyc.2014.12.014
19. Daniel AM, Rushing BG, Tapia Menchaca KY. Variation of the human mu-opioid receptor (OPRM1) gene predicts vulnerability to frustration. Sci Rep. 2020;10(1):1–8. doi:10.1038/s41598-020-78783-4
20. Troisi A, Frazzetto G, Carola V, et al. Social hedonic capacity is associated with the A118G polymorphism of the mu-opioid receptor gene (OPRM1) in adult healthy volunteers and psychiatric patients. Soc Neurosci. 2011;6(1):88–97. doi:10.1080/17470919.2010.482786
21. Way BM, Taylor SE, Eisenberger NI. Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proc Natl Acad Sci USA. 2009;106(35):15079–15084. doi:10.1073/pnas.0812612106
22. Tan EC, Lim EC, Teo YY, Lim Y, Law HY, Sia AT. Ethnicity and OPRM variant independently predict pain perception and patient-controlled analgesia usage for post-operative pain. Molecular Pain. 2009;5:32. doi:10.1186/1744-8069-5-32
23. Kolesnikov Y, Gabovits B, Levin A, et al. Chronic pain after lower abdominal surgery: do catechol-O-methyl transferase/opioid receptor μ-1 polymorphisms contribute? Molecular Pain. 2013;9(1):19. doi:10.1186/1744-8069-9-19
24. Frangakis SG, MacEachern M, Akbar TA, et al. Association of genetic variants with postsurgical pain: a systematic review and meta-analyses. Anesthesiology. 2023;139(6):827–839. doi:10.1097/ALN.0000000000004677
25. Palit S, Sheaff RJ, France CR, et al. Serotonin transporter gene (5-HTTLPR) polymorphisms are associated with emotional modulation of pain but not emotional modulation of spinal nociception. Biol Psychol. 2011;86(3):360–369. doi:10.1016/j.biopsycho.2011.01.008
26. Rhudy JL, France CR. Defining the nociceptive flexion reflex (NFR) threshold in human participants: a comparison of different scoring criteria. Pain. 2007;128(3):244–253. doi:10.1016/j.pain.2006.09.024
27. Lang PJ, Bradley MM, Cuthbert BN International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical Report A-6. University of Florida, Gainesville, FL; 2005.
28. Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry. 1994;25(1):49–59. doi:10.1016/0005-7916(94)90063-9
29. Harvard. Ethanol precipitation of RNA/DNA; n.d. Available from: https://projects.iq.harvard.edu/files/hlalab/files/ethanol-precipitation-of-rna_hla.pdf.
30. Ittisoponpisan S, Islam SA, Khanna T, Alhuzimi E, David A, Sternberg MJE. Can predicted protein 3D structures provide reliable insights into whether missense variants are disease associated? J Mol Biol. 2019;431(11):2197–2212. doi:10.1016/j.jmb.2019.04.009
31. Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nature Methods. 2010;7(4):248–249. doi:10.1038/nmeth0410-248
32. DelVentura JL, Terry EL, Bartley EJ, Rhudy JL. Emotional modulation of pain and spinal nociception in persons with severe insomnia symptoms. Ann Behav Med. 2014;47(3):303–315. doi:10.1007/s12160-013-9551-1
33. Palit S, Kerr KL, Kuhn BL, et al. Examining emotional modulation of pain and spinal nociception in native Americans: a preliminary investigation. Int J Psychophysiol. 2013;90(2):272–281. doi:10.1016/j.ijpsycho.2013.08.009
34. Rhudy JL, DelVentura JL, Terry EL, et al. Emotional modulation of pain and spinal nociception in fibromyalgia. PAIN®. 2013;154(7):1045–1056. doi:10.1016/j.pain.2013.03.025
35. Terry EL, DelVentura JL, Bartley EJ, Vincent AL, Rhudy JL. Emotional modulation of pain and spinal nociception in persons with major depressive disorder (MDD). PAIN®. 2013;154(12):2759–2768. doi:10.1016/j.pain.2013.08.009
36. Palit S, Williams A, Rhudy J, et al. Further validation of the Emotional Controls (ECON) paradigm: what types of emotional picture contents best modulate pain and nociception? J Pain. 2009;10(4):S26. doi:10.1016/j.jpain.2009.01.114
37. Seara-Cardoso A, Sebastian C, Viding E, Roiser J. Affective resonance in response to others’ emotional faces varies with affective ratings and psychopathic traits in amygdala and anterior insula. Soc Neurosci. 2015;11:1–13. doi:10.1080/17470919.2015.1029593
38. Lapate RC, Lee H, Salomons TV, van Reekum CM, Greischar LL, Davidson RJ. Amygdalar function reflects common individual differences in emotion and pain regulation success. J Cognitive Neurosci. 2012;24(1):148–158. doi:10.1162/jocn_a_00125
39. Saga Y, Ruff CC, Tremblay L. Disturbance of approach-avoidance behaviors in non-human primates by stimulation of the limbic territories of basal ganglia and anterior insula. Eur J Neurosci. 2019;49(5):687–700. doi:10.1111/ejn.14201
40. Haaranen M, Scuppa G, Tambalo S, et al. Anterior insula stimulation suppresses appetitive behavior while inducing forebrain activation in alcohol-preferring rats. Transl Psychiatry. 2020;10(1):150. doi:10.1038/s41398-020-0833-7
41. Veinante P, Yalcin I, Barrot M. The amygdala between sensation and affect: a role in pain. J Mol Psychiatry. 2013;1(1):9. doi:10.1186/2049-9256-1-9
42. Quirarte GL, Galvez R, Roozendaal B, McGaugh JL. Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Res. 1998;808(2):134–140. doi:10.1016/S0006-8993(98)00795-1
43. Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9(4):314–320. doi:10.1038/nrn2333
44. Coolen LM, Fitzgerald ME, Yu L, Lehman MN. Activation of μ opioid receptors in the medial preoptic area following copulation in male rats. Neuroscience. 2004;124(1):11–21. doi:10.1016/j.neuroscience.2003.10.045
45. Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111(1):52–58. doi:10.1093/bja/aet127
46. Hasvik E, Iordanova Schistad E, Grøvle L, Julsrud Haugen A, Røe C, Gjerstad J. Subjective health complaints in patients with lumbar radicular pain and disc herniation are associated with a sex - OPRM1 A118G polymorphism interaction: a prospective 1-year observational study. BMC Musculoskelet Disord. 2014;15(1):161. doi:10.1186/1471-2474-15-161
47. Fillingim RB, Kaplan L, Staud R, et al. The A118G single nucleotide polymorphism of the mu-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. J Pain. 2005;6(3):159–167. doi:10.1016/j.jpain.2004.11.008
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.