Back to Journals » Drug Design, Development and Therapy » Volume 16
Open-Label, Phase I, Pharmacokinetic Studies in Healthy Chinese Subjects to Evaluate the Bioequivalence and Food Effect of a Novel Formulation of Abiraterone Acetate Tablets
Authors Feng Z , Liu Y, Kuang Y, Yang S, Li J, Ye L, Huang J, Pei Q , Huang Y, Yang G
Received 20 September 2021
Accepted for publication 14 December 2021
Published 3 January 2022 Volume 2022:16 Pages 3—12
DOI https://doi.org/10.2147/DDDT.S339305
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Zeying Feng,1,2,* Yaxin Liu,1,2,* Yun Kuang,2 Shuang Yang,2 Jinlei Li,2 Ling Ye,2 Jie Huang,2 Qi Pei,3 Yuanyuan Huang,1,4 Guoping Yang1– 3
1XiangYa School of Pharmaceutical Sciences, Central South University, Changsha, Hunan, 410013, People’s Republic of China; 2Center of Clinical Pharmacology, The Third Xiangya Hospital, Central South University, Changsha, Hunan, 410013, People’s Republic of China; 3Department of Pharmacy, The Third Xiangya Hospital, Central South University, Changsha, Hunan, 410013, People’s Republic of China; 4Clinical Research and Develpment Division II, Jiangsu Hengrui Medicine Co., Ltd., Shanghai, 201200, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuanyuan Huang
Clinical Research and Develpment Division II, Jiangsu Hengrui Medicine Co., Ltd., 1288 Haike Road, Pudong District, Shanghai, 201200, People’s Republic of China
Email [email protected]
Guoping Yang
The Third Xiangya Hospital, Central South University, Yinpenling Street, Yuelu District, Changsha, Hunan, 410013, People’s Republic of China
Tel +86 731-89918665
Fax +86 731-88618326
Email [email protected]
Purpose: Abiraterone acetate tablets (I)(N-AbA) is a novel tablet co-formulated with the absorption enhancer sodium N-(8-[2-hydroxybenzoyl] amino) caprylate (SNAC). This study aimed to compare the pharmacokinetics, bioequivalence, safety, and food effects of N-AbA with the reference ZYTIGA® (R-AbA) in healthy Chinese male subjects.
Patients and Methods: This study was conducted in three parts. Part I was an open, dose-escalation trial conducted in 16 Chinese healthy males; Part II was a randomized, open-label, 2 × 4 crossover, single-dose bioequivalence trial conducted in 36 subjects; Part III was a randomized, 3 × 3 crossover trial conducted on 24 volunteers to investigate the effect of food on the pharmacokinetics of N-AbA.
Results: The exposure (AUC0-∞) and maximum concentration (Cmax) of abiraterone and excipient SNAC were linear in the range of 75– 450 mg dose. The bioavailability of N-AbA 300 mg was equivalent to that of R-AbA 1000 mg. The drug exposure of prednisone and prednisolone was not affected by SNAC co-administration. The Cmax of orally administered abiraterone as R-AbA in a modified fed state was 5.9 times and AUC0-∞ was 4.3 times, respectively, higher than those in of orally administered abiraterone as N-AbA in a high-fat diet. The Cmax and AUC0-∞ of orally administered abiraterone as N-AbA on a high-fat diet were 2.2 times and 2 times, respectively, higher than those on a fasting state. All adverse events reported in the three parts of the study were grade 1 or 2, and no serious adverse events were reported.
Conclusion: These three Phase I trials showed that N-AbA and excipient SNAC had excellent linear pharmacokinetic characteristics. A single dose of N-AbA 300 mg was bioequivalent to R-AbA 1000 mg in healthy subjects under fasting conditions. Meanwhile, SNAC had no effect on the pharmacokinetics of prednisone and prednisolone. The effect of food on N-AbA was significantly lower than that on R-AbA.
Keywords: food effect, pharmacokinetics, abiraterone acetate, bioequivalence
Introduction
Prostate cancer is one of the most common cancers among men and is the second leading cause of cancer-related death in men in the United States.1 In Asians too, the age-adjusted incidence of prostate cancer has increased gradually over time. Androgen deprivation therapy is considered the primary treatment option for metastatic prostate cancer.2 Abiraterone is a potent and selective irreversible inhibitor of the enzyme CYP17A1, which is required for androgen biosynthesis. It slows the progression of prostate cancer by reducing the production of androgens in the testes, adrenal glands, and prostate tumor tissue.3,4 Abiraterone acetate, a prodrug of abiraterone and marketed under the original brand name R-AbA, is approved as a medication co-administered with prednisone for the treatment of metastatic castration-resistant prostate cancer (mCRPC) and metastatic high-risk castration-sensitive prostate cancer.5
Abiraterone acetate is a Biopharmaceutics Classification System class IV compound, with an extremely low oral bioavailability when administered in the fasted state.6 However, the administration of abiraterone acetate with high-fat food results in up to a 10-fold increased area under the concentration–time curve (AUC) and up to a 17-fold increase in the maximum plasma concentration (Cmax) of abiraterone.7 Because of the low oral bioavailability and significant food effect of abiraterone acetate, its administration carries a risk of toxicity and inaccurate dosing.8 The specification for ZYTIGA® is that abiraterone acetate should be taken on an empty stomach, at least 1 h before or at least 2 h after a meal. Its large typical daily dose (1000 mg) under the fasting condition is a significant pill burden for patients.
Abiraterone acetate tablets (I)(N-AbA), developed by Jiangsu Hengrui Medicine Co., Ltd., are prepared using nanocrystalline technology in which abiraterone acetate is co-formulated with the absorption enhancer sodium N-(8-[2-hydroxybenzoyl] amino) caprylate (SNAC). Despite not being included in the FDA list of excipients, SNAC has been widely used to enhance the oral absorption of different types of drugs. The glucagon-like peptide-1 receptor agonist semaglutide co-formulated with SNAC exhibited improved drug absorption.9 This new co-formulation has been approved by the FDA.10 The ability of SNAC to improve drug absorption has been demonstrated in many studies.11,12
N-AbA was developed to promote the gastrointestinal absorption, low oral bioavailability, high pharmacokinetic variability, and food effects of abiraterone. Here, we conducted this study aimed at comparing the pharmacokinetic characteristics, safety, and food effects of N-AbA and R-AbA.
Materials and Methods
Ethics Statement
This series of clinical trials is registered with the Chinese Clinical Trial Registry (http://www.chictr.org.cn; ChiCTR2000032273, ChiCTR1900025101, ChiCTR1900025027; date of registration: April 13, 2020; August 11, 2019; April 8, 2019) and has been approved by the Institutional Review Board of The Third Xiangya Hospital of Central South University (2019070, 2019091, 2019195). The study execution dates for these trials were April 8, 2020 to May 2, 2020; July 15, 2020 to August 1, 2020; and August 29, 2020 to September 22, 2020. The study was conducted in accordance with the Declaration of Helsinki (1989), the Guidelines of Good Clinical Practice, and local applicable laws and regulations. All subjects have provided written informed consent forms prior to their participation in the study.
Study Design
This study was conducted in three parts. Part I was a single-center, open, and sequential trial conducted in 16 healthy male Chinese subjects under fasting condition. All subjects were randomized to receive a single dose of 75 mg, 150 mg, 300 mg, and 450 mg N-AbA. Each of the four doses was administered with a 7-day washout period in between each dose.
Part II was a randomized, open-label, two-sequence, four-period, single-dose bioequivalence trial conducted in 36 healthy male Chinese subjects under fasting condition. All subjects were assigned with randomized numbers into two treatment sequence groups: TRTR and RTRT (where T refers to 300 mg N-AbA with 5 mg prednisone, and R refers to R-AbA with 5 mg prednisone). The subjects received T or R products according to the randomization schedule. The washout period was 7 days.
Part III was a randomized, open-label, three-sequence, three-period, self-crossover study conducted in 24 healthy male Chinese subjects to compare the food effects on pharmacokinetic and safety profiles between the two formulations. All subjects were randomized into one of the three cohorts: 1) subjects were administered 300 mg N-AbA under fasting state; 2) subjects were administered 300 mg N-AbA on a high-fat diet; 3) subjects were administered 1000 mg R-AbA on modified fed conditions. The washout period was 7 days.
For the fasting condition, the subjects were asked to fast overnight (10 h) prior to dosing. The high-fat diet contained about 800–1000 kcal in total with about 50% of the calories coming from fat. The high-fat meal was provided 30 min before administration and finished within 30 min. Under the modified fed condition, subjects were asked to eat a medium-fat meal (~450 kcal with 30% of the calories coming from fat) 2 h before and 1 h after administration. No water was consumed between 1 h before and 1 h after drug administration, except the 240 mL water to swallow the study drugs.
Study Drugs
The test drug (N-AbA) was manufactured by Chengdu Suncadia Medicine Co., Ltd. (subsidiary of Jiangsu Hengrui Medicine Co., Ltd.); specifications: 150 mg per tablet; batch number: P20060411. The reference drug (R-AbA) was manufactured by Patheon Inc.; specifications: 250 mg per tablet; batch number: CCTSV. The combination drug prednisone acetate tablets were manufactured by Shanghai Pharmaceuticals Sine Co., Ltd.; specifications: 5 mg per tablet; batch number: 018190903.
Pharmacokinetic Evaluation, Blood Sample Collection, and Bioanalytical Assay
Plasma concentrations of abiraterone and SNAC were measured in all three trials in this study. In Part II, prednisone and prednisolone concentrations were also tested. Blood samples were collected prior to dosing (0 h) and at 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 4, 5, 6, 8, 12, 24, 36 and 48 h post-dose. For Part II, blood samples were also collected at 3.5 and 4.5 h post-dose. Blood samples were centrifuged at 1300 × g for 10 min at 4°C. The obtained plasma samples were stored in a refrigerator at −20±5°C for pre-freezing, and the samples were transferred to the refrigerator at ≤-60°C on the last day in each period for subsequent analyses. The time from the end of centrifugation to freezer storage was less than 1 h.
The serum concentration of abiraterone, SNAC, prednisone, and prednisolone was detected by HPLC-MS/MS. The limit of quantification of abiraterone, SNAC, prednisone, and prednisolone was 0.100–400, 0.250–1000, 0.500–50.0, and 2.00–200 ng/mL. Of all analytical methods of four compounds, the percent co-efficient of variation of inter-assay precision was ≤7.4%, and the assay accuracy was in the range of −8.0% to 1.7%.
Safety Assessments
Safety was evaluated by observing vital signs, physical examination, electrocardiography, laboratory examination (blood routine, blood biochemistry, blood coagulation function, urine routine, fecal routine, fecal occult blood, and blood transfusion), adverse event (AE) reporting. AEs were recorded and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0). Subjects with AEs were followed up until the symptoms or the corresponding physical and clinical examinations returned to normal.
Statistical Analysis
Pharmacokinetic parameters were calculated using the noncompartmental model (NCA module) on WinNonlin 8.2 (Pharsight Corporation, Mountain View, CA, USA). Statistical analysis was performed with SAS 9.4 Statistical Package (SAS Institute Inc., Cary, NC, USA). In Part I, the confidence interval method was used to analyze the linear relationship of AUC and Cmax with the dose. The natural logarithm of AUC and Cmax was used to perform linear regression analysis with the dose. The slope of the linear regression equation and 90% confidence interval were observed. In Part II, natural log-transformed AUC0-t, AUC0-∞, and Cmax were analyzed by analysis of variance with a mixed-effects model. Treatment sequence, formulation, and period were the fixed effect variables, and participant was the random effect variable. The relative bioavailability of the test drug and reference drug was evaluated based on average bioequivalence (ABE) or reference-scaled average bioequivalence (RSABE). In Part III, the least-squares mean ratios and 90% confidence intervals of Cmax, AUC0-t, and AUC0-∞ of abiraterone in the plasma were calculated after oral administration under different feeding conditions. The adjusted mean differences and 90% CIs for the differences were exponentiated to provide estimates of the ratio of adjusted geometric means (test/reference) and 90% CIs for the ratios.
Results
Participant Demographics
A total of 76 healthy male subjects were enrolled in this study (Part I: n = 16; Part II: n = 36; Part III: n = 24). One subject dropped out in each of the three parts of the study due to AEs. The flowchart of the subject distribution is shown in Figure 1. The median age of the subjects was 28.25, 27.81, and 25.08 years in the three trials. The median body mass index was 21.61, 22.24, and 21.44 kg/m2. The participant characteristics are shown in Table 1.
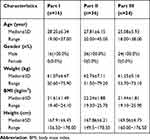 |
Table 1 Demographics and Characteristics of Subjects |
Pharmacokinetics
Part I
The plasma concentration profiles and pharmacokinetic parameters of abiraterone and SNAC are shown in Table 2. The median Tmax of abiraterone and SNAC was approximately 1 h and 0.5 h, respectively. Following the oral administration of 75–450 mg AbA, the Cmax and AUC0-∞ of abiraterone and SNAC were proportional to the dose (Figure 2).
 |
Table 2 PK Parameters of Abiraterone and SNAC After Dosing Abiraterone Acetate with Different Doses |
 |
Figure 2 Linear scale of mean plasma concentration–time plot of Abiraterone (A) and SNAC (B). |
Part II
After the administration of 300 mg N-AbA with 5 mg prednisone tablet or 1000 mg R-AbA with 5 mg prednisone tablet, the mean plasma concentration profiles under fasting condition are shown in Figure 3. For the test group, compared to the reference group, the geometric mean ratios of Cmax, AUC0-∞, and AUC0-t were 106.62%, 92.38%, and 94.01%, respectively. All 90% confidence intervals (CIs) were within the range of 80–125%. To evaluate the effect of SNAC on the pharmacokinetics of prednisone, we compared the Cmax, AUC0-∞, and AUC0-t of prednisone and prednisolone between the two groups and found that these pharmacokinetic parameters of prednisone and prednisolone were similar between the test and reference groups (Table 3).
 |
Table 3 Summary of Bioequivalence Analysis Under Fasting Conditions |
 |
Figure 3 Mean plasma concentration–time plot of Abiraterone (A), prednisone (B) and prednisolone (C) for R and T groups. |
Part III
The pharmacokinetic profiles of abiraterone under fasting conditions (300 mg N-AbA), high-fat diet conditions (300 mg N-AbA), and modified fed conditions (1000 mg R-AbA) are shown in Figure 4. The Cmax of abiraterone as orally administered R-AbA on a modified fed state was 5.89 times and AUC0-∞ was 4.3 times, respectively, higher than that of abiraterone as orally administered N-AbA on a high-fat diet. Under the fasting state, the Cmax of abiraterone as orally administered N-AbA on a high-fat diet was 2.2 times and AUC0-∞ was 2 times, respectively, higher. For SNAC, the geometric mean ratios of Cmax, AUC0-t, and AUC0-∞ were 71.64%, 126.17%, and 125.92%, respectively, when comparing the high-fat diet condition (300 mg N-AbA) with fasting condition (300 mg N-AbA). The details are shown in Table 4.
 |
Table 4 Mean Pharmacokinetic Parameters of Abiraterone and SNAC |
 |
Figure 4 Mean plasma concentration–time plot of Abiraterone (A) and SNAC (B) under fasting conditions, postprandial conditions and modified fed conditions. |
Safety
In Part I, 16 AEs were reported in 9 subjects (56.3%, 9/16). All AEs were of grade 1. Of these, 14 AEs were related to the study drug. There was no significant trend between dose level and incidence of AEs. One subject discontinued Part I because of increased uric acid levels. In Part II, 24 AEs occurred in 9 subjects (25%, 9/36). The frequency of AE occurrence was similar between the test group and the reference group (11 vs 13). Of those, 9 subjects reported 21 drug-related AEs (50%, 8/16). One subject terminated the study due to elevated blood triglyceride levels. In Part III, 28 AEs occurred in 12 subjects (50%, 12/24), of which 19 AEs were related to the study drug. One subject terminated the study because of elevated levels of blood bilirubin. In all three trials, no severe AEs or AEs that caused death were reported.
The most frequent treatment-related AEs were hemobilirubin and hypertriglyceridemia. The incidence of elevated hemobilirubin and hypertriglyceridemia among patients treated with N-AbA and R-AbA did not differ. Other AEs included urinary tract infection, cough, hypertension, arrhythmia, and diarrhea. The details are shown in Table 5. All treatment-related AEs in this study are reported in the specification for ZYTIGA®.
 |
Table 5 Summary of Treatment-Related AEs Occurred in the Study |
Discussion
The main results of Part I showed linear pharmacokinetic characteristics of abiraterone and SNAC in the range of 75–450 mg after a single oral administration of N-AbA on an empty stomach. N-AbA was safe and well tolerated during the trial. In the 300 mg cohort, the Cmax and AUC0-∞ of abiraterone were 81.4 ng/mL and 250.27 ng∙h/mL, respectively, which were similar to the values reported in previous studies on R-AbA.13 Based on these data, we further evaluated and compared the pharmacokinetics of 300 mg N-AbA and 1000 mg R-AbA.
The results of Part II showed that under fasting condition, the test/reference geometric mean ratios are all within the range of 80% to 125% indicating that the exposure of N-AbA 300 mg was bioequivalent with that of R-AbA 1000 mg, and the intra-individual variation in AUC0-t and AUC0-∞ for N-AbA was lower than that for R-AbA. As the CYP17 inhibitor, abiraterone acetate had some side effects such as fluid retention, hypertension, and hypokalemia. Low-dose prednisone decreases steroid build-up upstream of CYP17 and prevents mineralocorticoid excess.14 In 2012, AbA plus prednisone was approved by FDA for the treatment of patients with mCRPC. European Association of Urology guidelines on the treatment of CRPC mention this combination as one of the first-line treatments for CRPC.15 Thus, in Part II, we also studied whether SNAC would affect prednisone pharmacokinetics. The pharmacokinetic parameters of prednisone and prednisolone were similar after the combined use of N-AbA or R-AbA, indicating that SNAC had no effect on the pharmacokinetics of prednisone and prednisolone.
The specifications for ZYTIGA® state that the tablets must be taken on an empty stomach and should not be taken at least 2 h before and at least 1 h after administration because food effects can result in fluctuations in drug exposure. Therefore, in Part III, we set up a modified fed group based on these two time nodes. The results showed that after oral administration of the test drug after a high-fat meal, the Cmax of abiraterone increased only by about 2.2 times, and the AUC0-∞ increased by about 2 times, indicating that N-AbA has a significantly lower food effect than R-AbA. Thus, even if patients do not follow regular diet management before and after taking the drug, drug exposure will not be drastically altered, which is an important safety advantage.
YONSA®, the improved version of R-AbA, was approved for the treatment of patients with mCRPC in 2018. The proprietary YONSA® formulation using SoluMatrix Fine Particle TechnologyTM allows for the same systemic exposure to be achieved with 500 mg relative to the recommended dose of R-AbA 1000 mg.16 The Cmax of abiraterone was approximately 6.5-fold higher, and the AUC0-∞ was 4.4-fold higher when a single dose of YONSA 500 mg was administered with a high-fat meal (56–60% fat, 900–1000 calories) than when administered after overnight fasting in healthy subjects.17 Nanocrystalline technology and the addition of SNAC further improve the bioavailability of N-AbA and reduce the impact of food.
SNAC is a new type of absorption enhancer originally developed by Emisphere Technology Inc. Despite it not being listed in the FDA excipient database at present, SNAC is widely used in improving the oral absorption of different types of drugs, including proteins and macromolecules, such as insulin, calcitonin, and heparin.18,19 SNAC has been reported to enhance the oral bioavailability of the long-acting GLP-1 analog semaglutide, which is used to treat type 2 diabetes and was approved by the FDA on September 20, 2019.20,21 A vitamin B12 product that exhibits enhanced oral absorption due to SNAC is already marketed.22 This study confirms that the use of SNAC not only improves the bioavailability of abiraterone but also greatly reduces the effect of food on the absorption of abiraterone. SNAC may be used to enhance the oral bioavailability of several other drugs.
Conclusion
N-AbA and the excipient SNAC showed linear pharmacokinetic characteristics within the dose range of 75 mg-450 mg. A single 300 mg dose of N-AbA was bioequivalent to 1000 mg of R-AbA in healthy subjects under fasting conditions. Meanwhile, no pharmacokinetic interactions were observed between SNAC and prednisone after combined administration of prednisone. N-AbA shows lower intra-individual variation and food effects than R-AbA.
Data Sharing Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Acknowledgments
This study was funded by Jiangsu Hengrui Medicine Co., Ltd. and the Key Research and Development Project of Hunan Province (2020SK2010) as well as the Hunan Provincial Natural Science Foundation of China (No. 2020JJ5852). Zeying Feng and Yaxin Liu are co-first authors for this study.
Disclosure
YH was employed by Jiangsu Hengrui Medicine Co., Ltd. The authors report no other conflicts of interest in this work.
References
1. Cancer.Net. Prostate cancer: statistics; 2021. Available from: https://www.cancer.net/cancer-types/prostate-cancer/statistics.
2. Teoh JYC, Hirai HW, Ho JMW, Chan FCH, Tsoi KKF, Ng CF. Global incidence of prostate cancer in developing and developed countries with changing age structures. PLoS One. 2019;14(10):e0221775. doi:10.1371/journal.pone.0221775
3. Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor Abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28(9):1481–1488. doi:10.1200/jco.2009.24.1281
4. Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled Phase 3 study. Lancet Oncol. 2015;16(2):152–160. doi:10.1016/s1470-2045(14)71205-7
5. Caffo O, Veccia A, Kinspergher S, Maines F. Abiraterone acetate and its use in the treatment of metastatic prostate cancer: a review. Fut Oncol. 2018;14(5):431–442. doi:10.2217/fon-2017-0430
6. Boleslavská T, Rychecký O, Krov M, et al. Bioavailability enhancement and food effect elimination of Abiraterone acetate by encapsulation in surfactant-enriched oil marbles. AAPS J. 2020;22(6):122. doi:10.1208/s12248-020-00505-5
7. FDA Center for Drug Evaluation and Research (CDER). Clinical pharmacology and biopharmaceutics review(s): application number:202379Orig1s000. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202379Orig1s000ClinPharmR.pdf.
8. Schultz HB, Meola TR, Thomas N, Prestidge CA. Oral formulation strategies to improve the bioavailability and mitigate the food effect of Abiraterone acetate. Int J Pharm. 2020;577:119069. doi:10.1016/j.ijpharm.2020.119069
9. Buckley ST, Bækdal TA, Vegge A, et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med. 2018;10(467). doi:10.1126/scitranslmed.aar7047
10. Bucheit JD, Pamulapati LG, Carter N, Malloy K, Dixon DL, Sisson EM. Oral semaglutide: a review of the first oral glucagon-like peptide 1 receptor agonist. Diabetes Technol Ther. 2020;22(1):10–18. doi:10.1089/dia.2019.0185
11. Baekdal TA, Donsmark M, Hartoft-Nielsen ML, Søndergaard FL, Connor A. Relationship between oral semaglutide tablet erosion and pharmacokinetics: a pharmacoscintigraphic study. Clin Pharmacol Drug Dev. 2021;10(5):453–462. doi:10.1002/cpdd.938
12. Li Y, Yang D, Zhu C. Impact of sodium N-[8-(2-Hydroxybenzoyl)amino]-caprylate on intestinal permeability for notoginsenoside R1 and salvianolic acids in Caco-2 cells transport and rat pharmacokinetics. Molecules. 2018;23(11):2990. doi:10.3390/molecules23112990
13. Wang C, Hu C, Gao D, et al. Pharmacokinetics and bioequivalence of generic and branded Abiraterone acetate tablet: a single-dose, open-label, and replicate designed study in healthy Chinese male volunteers. Cancer Chemother Pharmacol. 2019;83(3):509–517. doi:10.1007/s00280-018-3754-x
14. Crawford ED, Shore ND, Petrylak DP, et al. Abiraterone acetate and prednisone in chemotherapy-naïve prostate cancer patients: rationale, evidence and clinical utility. Ther Adv Med Oncol. 2017;9(5):319–333. doi:10.1177/1758834017698644
15. Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–479. doi:10.1016/j.eururo.2013.11.002
16. Goldwater R, Hussaini A, Bosch B, Nemeth P. Comparison of a novel formulation of Abiraterone acetate vs. the originator formulation in healthy male subjects: two randomized, open-label, crossover studies. Clin Pharmacokinet. 2017;56(7):803–813. doi:10.1007/s40262-017-0536-2
17. FDA Center for Drug Evaluation and Research (CDER). Clinical pharmacology and biopharmaceutics review(s): application number:210308Orig1s000. Avaliable from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210308Orig1s000ClinPharmR.pdf.
18. Bandeira L, Lewiecki EM, Bilezikian JP. Pharmacodynamics and pharmacokinetics of oral salmon calcitonin in the treatment of osteoporosis. Expert Opin Drug Metab Toxicol. 2016;12(6):681–689. doi:10.1080/17425255.2016.1175436
19. Welt FG, Woods TC, Edelman ER. Oral heparin prevents neointimal hyperplasia after arterial injury: inhibitory potential depends on type of vascular injury. Circulation. 2001;104(25):3121–3124. doi:10.1161/hc5001.100837
20. Andersen A, Knop FK, Vilsbøll T. A pharmacological and clinical overview of oral semaglutide for the treatment of type 2 diabetes. Drugs. 2021;81(9):1003–1030. doi:10.1007/s40265-021-01499-w
21. Newsome PN, Buchholtz K, Cusi K, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384(12):1113–1124. doi:10.1056/NEJMoa2028395
22. Castelli MC, Wong DF, Friedman K, Riley MG. Pharmacokinetics of oral cyanocobalamin formulated with sodium N-[8-(2-hydroxybenzoyl)amino]caprylate (SNAC): an open-label, randomized, single-dose, parallel-group study in healthy male subjects. Clin Ther. 2011;33(7):934–945. doi:10.1016/j.clinthera.2011.05.088
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

