Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
On Ultrasonographic Features of Mucinous Carcinoma with Micropapillary Pattern
Received 31 March 2023
Accepted for publication 29 June 2023
Published 17 July 2023 Volume 2023:15 Pages 473—483
DOI https://doi.org/10.2147/BCTT.S415250
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Wei-Sen Yang,1,* Yang Li,2,* Ya Gao3
1Department of Radiology, The Second Affiliated Hospital of Soochow University, Suzhou, People’s Republic of China; 2Department of Radiology, Wuxi No.2 People’s Hospital, Wuxi, People’s Republic of China; 3Center for Medical Ultrasound, Suzhou Municipal Hospital, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ya Gao, Center for Medical Ultrasound, Suzhou Municipal Hospital, The Affiliated Suzhou Hospital of Nanjing Medical University, No. 16 of West Baita Road, Gusu District, Suzhou, 215001, People’s Republic of China, Tel +86-51262364301, Fax +86-51262362596, Email [email protected]
Objective: To describe the sonographic features of pure mucinous carcinoma with micropapillary pattern (MUMPC) and compare with different pathological type of mucinous breast carcinoma.
Methods: Subjects were retrospectively reviewed at Suzhou Municipal Hospital from January 2015 to June 2019. Sonographic features of 49 cases (9 MUMPC, 19cPMBC, and 21 MMBC) pathologically confirmed MBC were recorded according to the Breast Imaging Reporting and Data System (BI-RADS) lexicon. The differences in sonographic features among different type of mucinous breast carcinoma were discussed, including clinical features and sonographic features, shape, lesion boundary, peripheral hyperechoic ring, echo pattern, posterior acoustic feature, thickness peripheral hyperechoic area, and blood flow.
Results: All MUMPC had no lymph node metastasis (88.9%, 8/9), and most of the MUMPC showed no thickness peripheral hyperechoic area (88.9%, 8/9) and blood flow (55.6%, 5/9) within the tumor. Furthermore, MUMPC had mixed cystic and solid components (33.3%, 3/9) and solid echoic (66.7%, 6/9) structures, with regular shape (66.7%, 6/9) and peripheral hyperechoic ring (66.7%, 6/9). Seven cases of the MUMPC showed circumscribed margin (77.8%, 7/9), and there was significant difference among the three groups (p < 0.05). In addition, there were 7 cases (77.8%, 7/9) of MUMPC tumor ≤ 2cm, which was significantly different from cPMBC (26.3%, 5/19) and MMBC (28.6%, 6/21) (p < 0.05). There was no significant difference in ultrasonographic features of MBC with different sizes when stratified by tumor size (p > 0.05).
Conclusion: Most of the MUMPC showed a circumscribed margin, peripheral hyperechoic ring, and without lymph node metastasis and thickness peripheral hyperechoic area. However, it is challenging to distinguish MUMPC from PMBC and MMBC on ultrasound. Future research should focus on developing novel analysis methods for ultrasound imaging, conducting studies with larger sample sizes and diverse population groups.
Keywords: breast, pure mucinous carcinoma, micropapillary, mixed mucinous carcinoma, ultrasonography
Introduction
Mucinous breast carcinoma (MBC) is a comparatively rare specific carcinoma of breast featuring much extracellular mucus and favorable prognosis.1,2 Moreover, MBC takes a proportion of 1–4% in all primary breast cancers approximately. Several factors can influence the prognosis of MBC, including age, stage of the disease, and specific histological features. Although MBC is generally associated with a favorable prognosis, older age at diagnosis can be a negative prognostic factor. Elderly patients may have comorbidities or age-related physiological changes that can impact treatment decisions and overall outcomes. In the existing literature, MBC morbidity is rather high among the elderly, that is about 6–7%.3 Pathologically, there are two subtypes of MBC according to the content of intratumoral mucus. One is pure mucinous breast carcinoma (PMBC); and the other is mixed mucinous breast carcinoma (MMBC). While PMBC is merely constituted by tumor cells producing mucus and the proportion taken by mucus components in the tumor is at least 90%, mucus in MMBC takes a proportion of 50–90% and is also mixed with infiltrating ductal epithelium.4,5 As demonstrated in many studies, PMBC is an indolent tumor characterized by good prognosis and low axillary lymph node metastasis rate;6,7 and by contrast, MMBC exhibits distinct biological behavior from PMBC.8 In recent years, it is clarified in some research that some PMBC is as invasive as MMBC.6,9 For example, Ranade et al point out10 that micropapillary structures can be observed in 60% PMBCs with axillary node positive and 14% PMBCs with axillary node negative. This manifests that the micropapillary structure plays a critical role in progression of axillary node metabasis. As early as 2002, a novel subtype of PMBC was reported by Ng11 for the first time; and it was named mucinous carcinoma with micropapillary pattern, or MUMPC for short. However, MUMPC alignment is believed in some literature to be similar to that of invasive micropapillary carcinoma; and MUMPC is more inclined to be associated with biological behavior of infiltrating tumors, such as lymphatic metastasis and lymphovascular invasion. According to Barbashina et al.12 MUMPC is an invasive subtype in clinics of mucinous breast cancers and should be differentiated from conventional pure mucinous breast carcinoma without micropapillary architecture (cPMBC). Micropapillary patterns consist of small, finger-like projections of tumor cells floating within mucus pools. Micropapillary structures have been associated with increased invasiveness, lymphatic metastasis, and poorer clinical outcomes in various types of breast carcinoma. Understanding the significance of micropapillary patterns is essential for accurate diagnosis and management of these tumors. Therefore, further investigation into the role and implications of micropapillary patterns in mucinous breast carcinoma, particularly in MUMPC, is warranted for better characterization and treatment strategies.
To our knowledge, MUMPC is seldom explored at present, especially relevant literature describing its ultrasound findings. Therefore, the Breast Imaging Reporting and Data System (BI-RADS) was utilized in this paper to carry out ultrasound examinations on MUMPC, analyze ultrasound features of MUMPC, and then compare MUMPC with cPMBC or MMBC.
Methods
Participants
We retrospectively analyzed 872 patients with breast cancer who received relevant therapies at Suzhou Municipal Hospital from January 2015 to June 2019. Based on the classification of breast neoplasms by the World Health Organization (WHO), content of mucus in PMBC exceeds 90%, while that of MMBC is below 90%.13 As for the lower limit of mucus content in MMBC, it remains unsolved. The corresponding inclusion criteria are as follows: (1) the performance status of Eastern Cooperative Oncology Group (ECOG) is 0 or 1; (2) the patients are histopathologically diagnosed with MBC; (3) the patients should experience breast ultrasound and surgical excision; and (4) clinical pathological and ultrasonic data of the patients should be complete. Regarding the exclusion criteria, they are: (1) combined with distant metastasis; (2) being combined with other malignant tumors or a history of tumors; (3) bilateral breast cancers; and (4) male. In strict accordance with the above inclusion and exclusion criteria, 49 patients were incorporated at last, taking a proportion of 5.62% in the total number of patients with breast cancer, showing 49 lesions in total. All of them are females aged 28~84 (mean: 57; and median: 56), including 9 cases with MUMPC and 21 cases with MMBC. This study has been approved by the Ethics Committee of Suzhou Municipal Hospital; and the informed consent has been gained from all patients.
Ultrasound Examinations
Forty-nine patients were all subjected to ultrasonography of breast cancer by using a linear array probe (5–15 MHz). Ultrasound images were judged by a physician-in-charge uninformed of the corresponding pathological outcomes. More particularly, results of ultrasound examinations were also retrospectively analyzed by a sonographer with at least 6 years of experience based on standards specified in BI-RADS categories of image features. Sizes, shapes (regular/irregular), edges (smooth and complete or not), halo signs (yes or no), posterior echo (no alterations, enhancement or attenuation), internal echo (solid and cystic mixed echo, or solid hypoecho/isoecho), malignant halo (yes or no) and lymph node metastasis were all recorded. Besides, vascularity (being present or not) of breast lesions was retrospectively analyzed. By means of Adler’s semi-quantitative grading, blood flow conditions of lesions were classified into 4 grades: (1) Grade 0: No blood flow signals; (2) Grade 1: A few blood flow signals (characterized by 1 or 2 dotted/short rod-like color blood flow signals); (3) Grade 2: Medium blood flows (characterized by 3 or 4 dotted color blood flow signals, or by longer blood flows, probably half of the corresponding lump in length); and (4) Grade 3: Abundant blood flows (characterized by over 4 dotted color blood flow signals, or by two long blood flows).
Tissue Pathology
Breast lesions of all patients were surgically removed. For surgical pathological reports verified by well-experienced pathologists of Suzhou Municipal Hospital, they were all reviewed together with pathological results of ipsilateral lymph node metastasis (LNM).
Statistical Analysis
All outcomes are analyzed by SPSS 20.0 applicable to Windows (Microsoft). Both x2 and Fisher’s precise tests were performed to analyze shapes, edges, halo signs, posterior echo, internal echo, malignant halo, lymph node metastasis and grades of blood flows. For contingency tables larger than 2x2, we use the Chi-squared test for comparison. When the comparison results are statistically significant (P < 0.05), we then use Fisher’s exact test for pairwise comparisons. For 2×2 contingency tables, considering our sample size is not large, we use Fisher’s exact test for comparison. In the event of P < 0.05, their differences are deemed to be statistically significant.
Results
The mean age of 49 patients with MBC is 57, including 9 cases with MUMPC (18.4%) 29–82 years old (Mean: 53), 19 cases with PMBC (38.8%) 28–84 years old (Mean: 59), and 21 cases with MMBC (42.8%) 36–76 years old (Mean: 57). Additional patient data including type of surgery are provided in Supplemental File 1.
Most MUMPC tumors presented with regular shapes (66.7%, 6/9). This was slightly distinct from the presentation of PMBC and MMBC tumors, which had a larger proportion of irregular shapes. However, no statistically significant difference was found in shape among the three subtypes (P > 0.05).
The edges of the MUMPC tumors were generally smooth and complete (77.8%, 7/9). In contrast, PMBC tumors tended to have unsmooth and incomplete edges. A significant difference was observed in the edge smoothness and completeness among the three subtypes (P < 0.05). However, when conducting pairwise comparisons, no significant statistical differences were found between MUMPC and PMBC, or between MUMPC and MMBC. The differences among the three groups are mainly caused by the differences between PMBC and MMBC (P < 0.05).
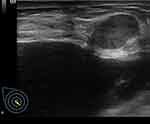
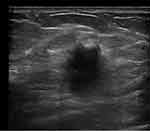
A halo sign was observed in 66.7% (6/9) of MUMPC cases. The presence of the halo sign varied slightly among the three subtypes, but was not significantly different (P > 0.05). Figure 1 shows an example of a halo sign.
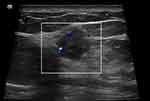
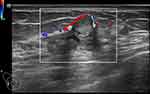
The majority of MUMPC tumors showed posterior echo enhancement (77.8%, 7/9), in contrast to the posterior echo behavior of PMBC and MMBC tumors. However, the difference was not statistically significant (P > 0.05). Figures 2 and 3 show examples of posterior echo enhancement.
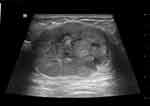
MUMPC tumors were either solid-cystic mixed (33.3%, 3/9) or merely solid (66.7%, 6/9). In comparison, PMBC and MMBC tumors showed variable internal echo patterns, but the difference was not statistically significant among the three groups (P > 0.05). Figure 4 shows an example of solid-cystic mixed echo.
For the blood flows, most MUMPC tumors were characterized by blood flows at grade 0, while PMBC tumors predominantly fell into grades 1 and 2. For MMBC, blood flows were relatively evenly distributed across grades 0 to 3. However, overall, there was no significant difference in the blood flow profiles among the three groups (P > 0.05).
For all three groups, the majority of cases did not exhibit a malignant halo. No significant difference was observed in the presence of a malignant halo among the three subtypes (P > 0.05). Figure 5 shows an example of malignant halo.
Rates of LNM were slightly lower in MUMPC (11.1%) and PMBC (5.3%) than in MMBC (19.1%). Despite this observation, no statistically significant difference was found among the three subtypes (P > 0.05).
Small tumors (≤ 2cm) of MUMPC took a large proportion (77.8%, 7/9), which was statistically significantly different from PMBC (26.3%) and MMBC (28.6%) by pairwise comparison (P < 0.05).
As for the variables of age, ER status, PR status, and HER2 status, there were generally no statistically significant differences among these three groups (P > 0.05). The main comparisons among the three groups for these indicators have been summarized in Table 1.
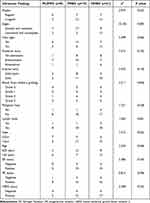 |
Table 1 Ultrasonic Findings and Features of 49 MBC Subtypes |
According to the tumor size, 49 MBCs are stratified to further explore the impacts of tumor sizes on ultrasonic findings. It is thus demonstrated that ultrasonic manifestations of MBCs different in sizes have no obvious differences. For details, please see Table 2.
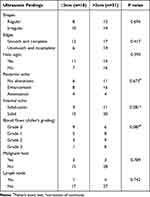 |
Table 2 Ultrasonic Findings of 49 MBCS Different in Sizes |
Discussion
As rare invasive breast carcinoma of histological morphology, MUMPC takes a proportion of less than 1% in all of the breast carcinoma.14,15 Composed of aggregates of micropapillary pattern, MUMPC is characterized by fan-shaped edges and it floats in a mucus pool.7 In 2002, MUMPC was described by Ng as a micropapillary subtype of PMBC.11 Since then, some researchers report that MUMPC incidence rates in PMBC remain at 12–35%.12,14–16 The reasons why MUMPC has a large range of incidence rates may be selection bias and different diagnosis standards. In this research, the incidence rate of MUMPC was proved to be 32.8% (7/22), which falls into the above range.
Regarding most tumors, they can be observed to be irregular in shapes. Generally, such irregular shapes are deemed as imaging features associated with clinical prognosis.17,18 In previous studies, microlobulation as one of the diagnostic features was observed in more MBCs.19,20 Here, 66.7% (14/21) MMBCs and 33.3% (3/9) MUMPCs are irregularly shaped (lobulated or polygonal). In a study made by Kaoku et al,20 90.9% (10/11) PMBCs show irregular shapes. In opinions of Lam et al,19 shape irregularity found in an ultrasound examination is associated with poorly histologically graded MBC.
PMBC and MMBC have diverse edge sonogram features. In this paper, the number of patients with cPMBC, MUMPC or MMBC of unsmooth and incomplete edges takes a proportion of 10.5% (2/17), 22.2% (2/9) and 57.1% (12/21), respectively. Clearly, the proportion occupied by MUMPC lies between that by cPMBC and MMBC. In another study made by WU et al, non-identifying edges in MUMPC and MMBC take a proportion higher than those in cPMBC do.14 Generally, cPMBC and MUMPC are manifested as elliptical lumps with smooth and complete edges, while edges of MMBC show corner angles or are crab-like. A possible reason is that dramatically malignant invasive breast carcinoma components are present in MMBC. As tumor cells infiltrate into surrounding tissues, stromal reactions of different degrees take place, forming angular or spiculated edges.21
Both internal and posterior echo patterns are important ultrasonic imaging features.22,23 The liquefaction ratio of MBC is reported to be 9–35.3%.13,24 Concerning breast carcinoma of three types investigated here, solid hypoechoic/isoechoic masses are dominant despite 28.6% (14/49) tumors showing fluid sonolucent areas. The above proportion is rather close to that reported by Zhang et al13 that is 35.3%. In views of Lam et al,19 solid-cystic components (37.5%, 12/32) and posterior echo enhancement (43.8%, 14/32) in MBC are both critical features obtained by relevant ultrasonic diagnosis. For anechoic regions, they represent mucus with malignant floating cells, but not real cystic features. In this study, most cPMBC and MUMPC show posterior echo enhancement, generating two proportions of 52.6% (10/19) and 77.8% (7/9), respectively; and for posterior echo attenuation, the proportions are calculated to be 5.3% (1/19) and 11.1% (1/9). Moreover, 28.6% (6/21) lesions are manifested as posterior echo attenuation as far as MMBC is concerned. The above phenomena are similar to those reported in literature13,21, Regarding MUMPC, 77.8% (7/9) shows posterior echo enhancement; and this proportion is above that proposed by Lam et al19 but below that raised by Kaoku et al (100%, 11/11).20 It is also pointed out by Kaoku et al that the higher the content of cancer cells and stromal components in breast carcinoma is, the higher the internal echo levels will be.20 This explains why posterior echo enhancement of cPMBC is greater than MMBC (52.6% vs 33.3%). Furthermore, the posterior echo pattern is beneficial for distinguishing PMBC from MMBC.
Color blood flow signals in tumor relate to vascularity. MBC is reported by Zhang et al13 to be blood supply deficient in most cases. In this paper, cPMBC and MUMPC show a main feature of blood supply insufficiency, taking proportions of 26.3% (5/19) and 57.1% (4/9), respectively. Moreover, 71.4% (15/21) patients with MMBC show abundant blood flow signals in their lesions (color blood flows at Grade 1 or 3), which is 55.6% (5/9) for MUMPC. Sufficient blood supply in MMBC may be attributed to the fact that the tumor contains much more malignant invasive components; and the reason why MUMPC has a major manifestation of blood supply insufficiency may be that rich mucus accounts for a large portion of the solid tumor in volume, which further leads to inadequate angiogenesis.
LNM is one of the key factors that influence prognosis of patients with breast carcinoma. Previously, LNM rates of MUMPC range from 20.0% to 42.9%.6,10–12,16,25 LNM rates in MUMPC are also believed to be above those in cPMBC.12,25 According to a study by Liu et al,25 the LNM rate of MUMPC is 9 times greater than that of cPMBC, signifying that MUMPC is more aggressive than cPMBC. In spite of this, results obtained from this study are proved to be inconsistent with them. Here, no LNM is found in 88.9% cases with MUMPC, which may be attributed to a small sample size in this study. However, LNM rate of MMBC is 14.3% (3/21), which is above that of cPMBC [ie, 5.6% (1/18)] or MUMPC [ie, 11.1% (1/9)]. Such a result is similar to that reported in the literature21 26 A possible reason is that lots of mucus are present in cPMBC and MUMPC. Due to the lack of invasive components, their invasiveness is below that of MMBC. In addition, histological grades here are believed to be associated with the state of axillary lymph nodes; and LNM may be an important histological category of prognosis. Due to the existence of micropapillary structures, MUMPC is considered as a subtype of mucinous carcinoma of micropapillary structure. Its biological behavior shows high invasiveness and LNM rates.9,27 In the opinions of Bal et al,28 however, the micropapillary pattern is independent of invasiveness or inert behavior. According to the above results, MUMPC and cPMBC, relative to MMBC, are comparatively inert.
As pointed out by Lin et al, the average tumor size is 3.2 cm (0.8–9.0 cm) when the diagnosis of MUMPC is confirmed.29 In this study, it is found that MUMPC with sizes no more than 2cm occupy a proportion of about 77.8%, which is significantly above that of PMBC or MMBC. Actually, according to our statistical results, we identified edge and size as two factors with significant differences. However, the factor of edge does not seem to serve as a characteristic of MUMPC, as our pairwise comparison shows no significant difference in the edge characteristic between MUMPC and either PMBC or MMBC. We can only assert that there is a significant difference in the edge characteristic between cPMBC and MMBC. The variable of size carries characteristic significance for MUMPC because the majority of MUMPC are small tumors (≤2cm), while most cPMBC and MMBC are larger tumors (>2cm). Moreover, differences in tumor sizes of such three pathological subtypes are of statistical significance (p = 0.014) as proved in a research conducted by Wu Zhou et al.14 Proportions taken by PMBC and MMBC with sizes greater than 2cm are apparently higher than that by MUMPC, which may be attributed to the facts that misdiagnosed rates of PMBC and MMBC are above that of MUMPC and delayed diagnosis of PMBC and MMBC further leads to an increase in tumor sizes. Here, ultrasonic manifestations are further verified to prove that tumor sizes have no influence on MBC.
Shet and Chinoy et al16 prove that MUMPC is generally developed among middle-aged and old women; and most patients are 41–60 years old. As reported by Kim et al,6 the mean age is 53.9, which remains consistent with our results (mean age: 53). Barbashina et al12 find that the median age of patients with MUMPC is 62 and most of them are suffering postmenopausal status. In the present study, the median age is 56; and patients in postmenopausal status take a proportion of 71.4% (5/7). As specified in the 2018 Expert Consensus on Breast Neoplasm Plastic Surgery and Breast Reconstruction,30 age of patients is an important reference factor that affects high incidence rates of breast carcinoma. Under the circumstance that newly developed solid nodes are observed in ultrasound images of the mammary gland among middle-aged and old women, this requires close observation and meticulous diagnosis.
The significance of micropapillary patterns in breast cancer has indeed been studied. Available evidence suggests that the presence of micropapillary patterns may indicate a more aggressive behavior of the tumor and poorer prognosis.31 These findings align with our observations in the current study, where MUMPC was found to demonstrate an ultrasound presentation of smaller tumors with smooth and complete edges, which might correlate to the histological representation of micropapillary structures. In comparing our results with previous findings, some differences arise. For instance, while previous studies have suggested that MUMPC has a higher rate of lymph node metastasis (LNM),32,33 our study found no such correlation. This discrepancy may be attributed to the small sample size in our study or potential differences in the patient population. Nonetheless, these results suggest the complexity of the relationship between ultrasound characteristics and the biological behavior of these tumors. We also note that both MUMPC and PMBC demonstrated a relative insufficiency of blood supply, though not significant, possibly due to the presence of mucus, which takes up a significant volume of the tumor. This blood supply insufficiency could lead to inadequate angiogenesis, contributing to the unique ultrasound features of these tumors.
MMBC was characterized by relatively abundant blood flow signals. This might be attributable to the greater proportion of malignant invasive components in MMBC, suggesting that the ultrasound manifestation of these tumors could reflect their underlying biological behavior. The present study still has some defects. First, all ultrasound images collected from a 2D cut surface are static in such a retrospective study, so that they may fail in presenting some features and cannot be adequately and effectively assessed. Second, this is a retrospective designed, single-center study of a small sample size. While MBC is a rare pathological subtype, MUMPC is even rarer. Although 49 patients are incorporated in this study, there are only 9 cases attacked by MUMPC. However, this fact is an indication of the rarity of MUMPC and reflects real-world clinical settings. Additionally, some patients are excluded because of information loss or surgeries in other medical institutions, and some are excluded due to more advanced stages. This could have introduced some bias and affected our study results to some extent. In the future, relevant studies should be made in a large sample size, especially that of MUMPC. Finally, the interpretation of ultrasound images and the assessment of sonographic features involve some degree of subjectivity. Although efforts were made to minimize bias by having an experienced sonographer analyze the images, there may still be inherent subjectivity in the assessment process.
Conclusions
To sum up, MUMPC in an ultrasound examination is usually manifested as a lump characterized by small tumour, smooth and complete edges, and irregular shapes in the present study. Regarding complicated lesions of MUMPC, they may be in solid or solid-cystic structures, and of posterior echo enhancement and a few internal blood vessels. Although ultrasound findings of MUMPC lie between those of cPMBC and MMBC, they may throw light on the histological classification and prognosis as well. However, no significant statistical differences lie in MUMPC and cPMBC/MMBC. For this reason, it is rather difficult to distinguish MUMPC from another two subtypes in line with BI-RADS. Future research should focus on developing novel analysis methods for ultrasound imaging, including advanced techniques such as contrast-enhanced ultrasound and ultrasound elastography. Additionally, conducting studies with larger sample sizes and diverse population groups would enhance the generalizability of the findings and provide a more comprehensive understanding of MUMPC. These efforts will contribute to improving diagnostic accuracy, treatment planning, and prognostic assessment for patients with MUMPC.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of Suzhou Municipal Hospital.
Written informed consent was obtained from all participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Young Employees (SDFEYQN2025).
Disclosure
All of the authors had no any personal, financial, commercial, or academic conflicts of interest separately in this work.
References
1. Chaudhry AR, El Khoury M, Gotra A, et al. Imaging features of pure and mixed forms of mucinous breast carcinoma with histopathological correlation. Br J Radiol. 2019;92(1095):20180810. PMID: 30632779. doi:10.1259/bjr.20180810
2. Bae SY, Choi MY, Cho DH, Lee JE, Nam SJ, Yang JH. Mucinous carcinoma of the breast in comparison with invasive ductal carcinoma: clinicopathologic characteristics and prognosis. J Breast Cancer. 2011;14(4):308–313. PMID: 22323918; PMCID: PMC3268928. doi:10.4048/jbc.2011.14.4.308
3. Yang WT, Zhu XZ. [The introduction of 2012 WHO classification of tumours of the breast]. Zhonghua Bing Li Xue Za Zhi. 2013. 42(2):78–80. Chinese. PMID: 23710911. doi:10.3760/cma.j.issn.0529-5807.2013.02.002
4. Zhou X, Zheng Z, Li Y, et al. The clinical features and prognosis of patients with mucinous breast carcinoma compared with those with infiltrating ductal carcinoma: a population-based study. BMC Cancer. 2021;21(1):536. PMID: 33975551; PMCID: PMC8111957. doi:10.1186/s12885-021-08262-0
5. Sun Y, Gu W, Wang G, Zhou X. The clinicopathological and prognostic characteristics of mucinous micropapillary carcinoma of the breast. Histol Histopathol. 2022;37(7):691–698. PMID: 35166367. doi:10.14670/HH-18-436
6. Kim HJ, Park K, Kim JY, Kang G, Gwak G, Park I. Prognostic significance of a micropapillary pattern in pure mucinous carcinoma of the breast: comparative analysis with micropapillary carcinoma. J Pathol Transl Med. 2017;51(4):403–409. PMID: 28597867; PMCID: PMC5525037. doi:10.4132/jptm.2017.03.18
7. Lim GH, Yan Z, Gudi M. Diagnostic dilemma of micropapillary variant of mucinous breast cancer. BMJ Case Rep. 2018;2018:bcr2018225775. PMID: 30068578; PMCID: PMC6078245. doi:10.1136/bcr-2018-225775
8. El Amine El Hadj O, Ayadi M, Goucha A, et al. Mucinous breast carcinoma a rare entity to be known: clinico-pathological study of 48 cases. Tunis Med. 2016;94(8–9):525–530. PMID: 28603824.
9. Jang Y, Jung H, Kim HN, et al. Clinicopathologic characteristics of HER2-positive pure mucinous carcinoma of the breast. J Pathol Transl Med. 2020;54(1):95–102. PMID: 31718120; PMCID: PMC6986976. doi:10.4132/jptm.2019.10.24
10. Ranade A, Batra R, Sandhu G, Chitale RA, Balderacchi J. Clinicopathological evaluation of 100 cases of mucinous carcinoma of breast with emphasis on axillary staging and special reference to a micropapillary pattern. J Clin Pathol. 2010;63(12):1043–1047. PMID: 20962055. doi:10.1136/jcp.2010.082495
11. Ng WK. Fine-needle aspiration cytology findings of an uncommon micropapillary variant of pure mucinous carcinoma of the breast: review of patients over an 8-year period. Cancer. 2002;96(5):280–288. PMID: 12378595. doi:10.1002/cncr.10747
12. Barbashina V, Corben AD, Akram M, Vallejo C, Tan LK. Mucinous micropapillary carcinoma of the breast: an aggressive counterpart to conventional pure mucinous tumors. Hum Pathol. 2013;44(8):1577–1585. PMID: 23517923. doi:10.1016/j.humpath.2013.01.003
13. Zhang H, Qiu L, Peng Y. The sonographic findings of micropapillary pattern in pure mucinous carcinoma of the breast. World J Surg Oncol. 2018;16(1):151. PMID: 30041628; PMCID: PMC6058370. doi:10.1186/s12957-018-1449-8
14. Zhou W, Li YZ, Gao LM, Cai DM. Sonographic features of pure mucinous brelast carcinoma with micropapillary pattern. Front Oncol. 2021;11:644180. PMID: 34745931; PMCID: PMC8570766. doi:10.3389/fonc.2021.644180
15. Sun P, Zhong Z, Lu Q, et al. Mucinous carcinoma with micropapillary features is morphologically, clinically and genetically distinct from pure mucinous carcinoma of breast. Mod Pathol. 2020;33(10):1945–1960. PMID: 32358590. doi:10.1038/s41379-020-0554-8
16. Shet T, Chinoy R. Presence of a micropapillary pattern in mucinous carcinomas of the breast and its impact on the clinical behavior. Breast J. 2008;14(5):412–420. PMID: 18673338. doi:10.1111/j.1524-4741.2008.00616.x
17. Budzik MP, Fudalej MM, Badowska-Kozakiewicz AM. Histopathological analysis of mucinous breast cancer subtypes and comparison with invasive carcinoma of no special type. Sci Rep. 2021;11(1):5770. PMID: 33707745; PMCID: PMC7952590. doi:10.1038/s41598-021-85309-z
18. Gök M, Topal U, Öz B, Akgün H, Akcan AC, Sözüer EM. Comparison of clinical features and treatment results of mix mucinous carcinomas and other atypical carcinomas of the breast. Eur J Breast Health. 2019;15(4):222–228. PMID: 31620680; PMCID: PMC6776130. doi:10.5152/ejbh.2019.5032
19. Lam WW, Chu WC, Tse GM, Ma TK. Sonographic appearance of mucinous carcinoma of the breast. AJR Am J Roentgenol. 2004;182(4):1069–1074. PMID: 15039190. doi:10.2214/ajr.182.4.1821069
20. Kaoku S, Konishi E, Fujimoto Y, et al. Sonographic and pathologic image analysis of pure mucinous carcinoma of the breast. Ultrasound Med Biol. 2013;39(7):1158–1167. PMID: 23683410. doi:10.1016/j.ultrasmedbio.2013.02.014
21. Feng GY, Jing XX, Zhong TT. On Ultrasonographic features of different subtypes of MBC. J Med Imaging. 2021;31(03):431–434, 444.
22. Wang PL, Zheng FY, Lu Q, et al. Imaging features of pure mucinous breast carcinoma: correlation with extracellular mucus content. Clin Radiol. 2019;74(7):569.e9–569.e17. PMID: 30967244. doi:10.1016/j.crad.2019.01.031
23. Pintican R, Duma M, Chiorean A, et al. Mucinous versus medullary breast carcinoma: mammography, ultrasound, and MRI findings. Clin Radiol. 2020;75(7):483–496. PMID: 32057415. doi:10.1016/j.crad.2019.12.024
24. Azamjah N, Soltan-Zadeh Y, Zayeri F. Global trend of breast cancer mortality rate: a 25-year study. Asian Pac J Cancer Prev. 2019;20(7):2015–2020. PMID: 31350959; PMCID: PMC6745227. doi:10.31557/APJCP.2019.20.7.2015
25. Liu F, Yang M, Li Z, et al. Invasive micropapillary mucinous carcinoma of the breast is associated with poor prognosis. Breast Cancer Res Treat. 2015;151(2):443–451. PMID: 25953688. doi:10.1007/s10549-015-3413-4
26. Wu PQ, Liu ZY, Liang CH. MRI-based radiomic features in histological grading of breast invasive ductal carcinoma. Postgrad Med J. 2018;31(09):938–942. doi:10.16571/j.cnki.1008-8199.2018.09.008
27. Collins K, Ricci A Jr. Micropapillary variant of mucinous breast carcinoma: a distinct subtype. Breast J. 2018;24(3):339–342. PMID: 29063656. doi:10.1111/tbj.12935
28. Bal A, Joshi K, Sharma SC, Das A, Verma A, Wig JD. Prognostic significance of micropapillary pattern in pure mucinous carcinoma of the breast. Int J Surg Pathol. 2008;16(3):251–256. PMID: 18387988. doi:10.1177/1066896908314784
29. Lin HY, Gao LX, Jin ML, Ding HY. [Clinicopathologic features of micropapillary variant of pure mucinous carcinoma of breast]. Zhonghua Bing Li Xue Za Zhi. 2012. 41(9):613–617. Chinese. PMID: 23157830. doi:10.3760/cma.j.issn.0529-5807.2012.09.009
30. Chinese Anti-Cancer Association, Committee of Breast Cancer Society (CACA-CBCS). 2018 expert consensus on breast neoplasm plastic surgery and breast reconstruction. Chin Clin Oncol. 2018;28(06):439–480. doi:10.19401/j.cnki.1007-3639.2018.06.008
31. Verras G, Tchabashvili L, Mulita F, et al. Micropapillary breast carcinoma: from molecular pathogenesis to prognosis. Breast Cancer. 2022;14:41–61. doi:10.2147/BCTT.S346301
32. Gokce H, Durak MG, Akin MM, et al. Invasive micropapillary carcinoma of the breast: a clinicopathologic study of 103 cases of an unusual and highly aggressive variant of breast carcinoma. Breast J. 2013;19:374–381. doi:10.1111/tbj.12128
33. Lewis GD, Xing Y, Haque W, et al. The impact of molecular status on survival outcomes for invasive micropapillary carcinoma of the breast. Breast J. 2019;25:1171–1176. doi:10.1111/tbj.13432
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.