Back to Journals » Risk Management and Healthcare Policy » Volume 13
Omalizumab for Severe Allergic Asthma Treatment in Italy: A Cost-Effectiveness Analysis from PROXIMA Study
Authors Canonica GW , Colombo GL , Rogliani P , Santus P, Pitotti C, Di Matteo S, Martinotti C , Bruno GM
Received 5 April 2019
Accepted for publication 28 November 2019
Published 22 January 2020 Volume 2020:13 Pages 43—53
DOI https://doi.org/10.2147/RMHP.S211321
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kent Rondeau
Giorgio Walter Canonica, 1 Giorgio Lorenzo Colombo, 2, 3 Paola Rogliani, 4 Pierachille Santus, 5 Claudia Pitotti, 6 Sergio Di Matteo, 3 Chiara Martinotti, 3 Giacomo Matteo Bruno 3
1Asthma & Allergy Clinic, Humanitas University, Milan, Italy; 2S.A.V.E. S.r.l. Studi Analisi Valutazioni Economiche Health Economics & Outcomes Research - Research Center, Milan, Italy; 3Drug Science Department, Pavia University, Pavia, Italy; 4Respiratory Unit, Department of Experimental Medicine and Surgery, University of Rome Tor Vergata, Rome, Italy; 5Department of Biomedical and Clinical Sciences (DIBIC), University of Milan, Division of Pulmonary Diseases, Ospedale L. Sacco, ASST Fatebenefratelli-Sacco, Milan, Italy; 6Novartis Farma S.p.A, Milan, Italy
Correspondence: Giorgio Walter Canonica
Tel +39 0282247752
Email [email protected]
Introduction: Inadequately controlled severe asthma patients require additional therapy accounting for significant clinical and economic burden. Our analysis aims to determine the cost-effectiveness of omalizumab in the management of severe allergic asthma in Italy based on observational data from the PROXIMA study.
Methods: Observational data on efficacy, healthcare resource utilization and changes in quality of life at 12 months after the initiation of omalizumab were examined to estimate the cost-effectiveness compared to pre-omalizumab period and results were expressed with Incremental Cost-Effectiveness Ratio (ICER). The cost–utility analysis estimated the cost per quality-adjusted life-year (QALY) gained. Direct health costs were assessed from the perspective of the Italian National Health Service (NHS).
Results: Omalizumab reduced the incidence of exacerbations, number of hospitalizations, physician visits, and improved quality of life after 12 months of treatment. Omalizumab had a greater effectiveness than pre-omalizumab treatment involving 0.132 QALYs gained and led to a € 3729 per patient reduction in direct healthcare costs, excluding the add-on treatment cost. Nevertheless, the addition of omalizumab cost led to € 7478 increase in total direct costs with respect to pre-omalizumab period. Based on difference in total direct cost and difference in QALY between post and pre-omalizumab period, the ICER was € 56,847. According to sensitivity analysis, omalizumab provided a cost-effective use of NHS resources, already at 20% discounted price.
Conclusion: This study offers a real-world evidence of omalizumab effectiveness in Italy. Despite the high acquisition cost of the innovative drug, omalizumab is a sustainable treatment option for patients with uncontrolled severe allergic asthma.
Keywords: severe allergic asthma, healthcare costs, effectiveness, cost-utility, omalizumab, PROXIMA study
Introduction
Asthma is a common chronic respiratory disorder, with more than 300 million patients worldwide, it is one of the major non-communicable diseases and a global public health problem.1
In Italy, the median prevalence of asthma was reported to be 6.6%, recording a 35% increase in the last two decades.2 The disease is characterized by chronic airway inflammation and it is defined by the history of respiratory symptoms such as wheeze, shortness of breath, chest tightness and cough that vary over time and in intensity, together with variable expiratory airflow limitation.1 Asthma is a heterogeneous disease; it has significant genetic and environmental components. Many phenotypes have been identified and allergic asthma, resulted from the overexpression of immunoglobulin E (IgE) in response to perennial or seasonal allergens, is the most easily recognized asthma phenotype affecting more than 60% of patients with asthma.2–4 Disease severity is defined retrospectively according to the level of treatment required to achieve a good asthma control, both symptom control and future risk of exacerbations. It can be assessed once the patient has been on controller treatment for several months and, if appropriate, treatment step down has been attempted to find the patient’s minimum effective level of treatment. Asthma severity is not a static feature and may change over months or years.5
Treatment options include inhaled and systemic corticosteroids (ICS, OCS), leukotriene receptor antagonist, long-acting inhaled β2 agonist bronchodilator (LABA) and IgE antibody. The goal of the treatment is the achievement and the maintenance of the disease control and the minimization of future risk of exacerbations, following a stepwise approach.1,5 Increasing asthma severity and morbidity are associated with impaired quality of life, work productivity loss, greater mortality risk and higher health care resource utilization and costs.6–9 Asthma-related costs vary from country to country and disease severity. An international review reports a mean cost per patient per year, including all asthmatics (intermittent, mild, moderate and severe asthma) between $USD 1900 in Europe and $USD 3100 in USA.10 As evidence of the high costs associated with severe stages of the disease, a recent Spanish study has estimated a mean annual direct cost per patient equal to €7472, and when indirect costs were considered, the total mean annual cost rose to €8554.11 As regard Italy, severe refractory asthma, occurring in patients not achieving disease control despite high-intensity therapy, good adherence and proper inhalation technique, has been associated with annual costs per patient amounting to €2815, including drug treatment, hospitalization and outpatient services.12 Moreover, findings of a recent online survey completed by employed adults in Brazil, Canada, Germany, Japan, Spain and the UK reported an impact on productivity at work caused by asthma in nearly three-quarters of patients. Overall work productivity loss (both time off and productivity whilst at work) due to asthma was 36%, ranging from 21% (UK) to 59% (Brazil).13 Despite effective treatments and management guidelines, 5–10% of asthmatics suffer from severe asthma (SA) and it accounts for 50% of the global costs of the disease.14–17 Although treatment with daily high-dose inhaled corticosteroids (ICSs) and long-acting inhaled β2 agonists (LABAs), a significant proportion of SA patients do not achieve a good disease control requiring additional therapy.18–20 For those patients, omalizumab, a recombinant DNA-derived humanized monoclonal antibody that selectively binds to human immunoglobulin E (IgE), is an add-on treatment approved in Europe in adults, adolescents and children (6–<12 years of age) with severe persistent allergic asthma inadequately controlled.21 In several clinical studies, omalizumab has been shown to improve asthma control and reduce exacerbations in patients with inadequately controlled severe allergic asthma.22–27 Moreover, add-on omalizumab demonstrated improvements in symptoms and asthma-related quality of life and reduction of medical resource utilization (hospitalizations, emergency room visits and use of corticosteroids and rescue bronchodilators).28–32 The effectiveness and safety of omalizumab in severe allergic asthma management were also shown in different real-world settings.33–44 In Italy, the PROXIMA (Patient Reported Outcomes and Xolair® In the Management of Asthma) study, an observational, multicenter, cross-sectional and prospective cohort study was conducted at 25 centers in outpatients settings (Table S1). The aims were to estimate the prevalence of perennial allergy in adult patients with severe allergic asthma and to evaluate asthma control and treatment adherence up to 12 months in patients treated with omalizumab. The study also aimed to describe patient’s perception toward allergic asthma, incidence of asthma exacerbations, patient’s compliance and persistence with omalizumab, healthcare resources utilization and quality of life during a 12-month period of treatment in longitudinal population. In addition, an ancillary study was carried out to explore asthma protein biomarkers in biologic samples and to assess a correlation with the achievement and maintenance of disease control.45 Focusing on healthcare resource utilization and quality of life results of PROXIMA Study, we carried out an economic evaluation of omalizumab treatment. Economic evaluations are essential for health decision-making, comparison of costs and additional benefits of new health technologies, and calculation of whether their use is an efficient use of resources.46,47 Omalizumab, as biological drug, is more expensive than standard treatments but, due to its efficacy and safety, offers an improvement in the management of the disease allowing an alternative to maintenance therapy with high-dose corticosteroids (ICS/OCS) for uncontrolled patients. This study aimed to evaluate the economic value of omalizumab in the treatment of adult patients with severe asthma in an Italian setting based on real-world data from the PROXIMA Study.
Methods
Study Design
Using the data available from the PROXIMA study, an economic analysis was conducted to evaluate the cost-effectiveness of omalizumab in the management of severe allergic asthma in Italy. The analysis was carried out from the Italian National Health Service (INHS) perspective, including only direct health costs. The time horizon was the 12-month follow-up period, planned in the observational study for the longitudinal phase. Costs and outcomes of treatment were assessed at baseline (pre-omalizumab treatment period) and at 12 months after the initiation of omalizumab (post omalizumab treatment period). The aim of the study has been to compare the cost, clinical and quality of life data referring to the population selected for the longitudinal phase before and after starting treatment with omalizumab, to evaluate the overall effect of the therapy.
Clinical Data Input: PROXIMA Study
The PROXIMA study design and methods have been described in depth previously.45 Briefly, it was an observational, multicenter study structured in 2 phases, cross-sectional and a longitudinal phase. In the cross-sectional phase, study population was composed of patients aged ≥18 years, diagnosed with severe allergic asthma, who were at step 4 as per GINA guidelines and required a therapeutic step-up. Patients who started treatment with omalizumab as per clinician judgement (according to AIFA criteria) at baseline visit were included in the longitudinal phase. Patients started omalizumab treatment not earlier than 15 days before enrolment, and within 90 days after enrolment and were followed-up for 12 months. The sample size was determined based on feasibility criteria. The study lasted from December 2013 until June 2016; the follow-up period consisted of two visits: the first visit after 6 months and the second one after 12 months from the baseline (Figure 1).
 |
Figure 1 PROXIMA study design.Notes: * Patients with diagnosis of severe asthma, GINA 4, and who Need therapeutic step up. |
The study complied with the Declaration of Helsinki, and the AIFA (Agenzia Italiana del Farmaco) guidelines for the classification and management of observational studies on drugs.48 Eligible patients were enrolled only after providing the written informed consent. The ethics committees and institutional review boards of all participating centres approved the study documents, including protocol and informed consent forms.
The two primary outcomes of the study were the proportion of patients with severe allergic asthma having allergy to a perennial form of aeroallergens (in the cross-sectional phase) and the proportion of patients with asthma control at months 6 and 12 with omalizumab therapy (in longitudinal phase). Secondary outcomes in the cross-sectional phase were the level of asthma control, patient disease perception, quality of life and healthcare resource utilization. In the longitudinal phase, secondary variables were: proportion of patients with at least 1 episode of asthma exacerbation during the 12-month study period, patient compliance to omalizumab, persistence with omalizumab treatment, quality of life and healthcare resource utilization.
Asthma disease control was assessed by the Asthma Control Questionnaire (ACQ); Brief Illness Perception Questionnaire (Brief IPQ) and EuroQoL-5 Dimension (EQ-5D) were used to estimate patient disease perception and quality of life, respectively. Patient compliance was assessed as the ratio between the number of injections of omalizumab administered during the observational period over the total number of planned injections. Omalizumab persistence during the 12 months was assessed by Kaplan-Meier survival curve analysis, where the event of interest was the treatment discontinuation over the total number of planned injections. Asthma exacerbations, serum IgE levels and FEV1 percent were measured by using standard clinical and laboratory assessment methods.49 The consumption of healthcare resources was evaluated by using a questionnaire collecting for each patient information on the number of hospitalizations, number of emergency department visits, number of outpatient visits and number of laboratory and diagnostic tests.
Economic Evaluation Assessments
Our analysis focused on healthcare resource use and quality of life data collected in the observational study, in order to assess the variation of these outcomes after the administration of omalizumab compared to baseline treatment and to evaluate the economic value of omalizumab in the Italian healthcare setting from INHS perspective. A cost-utility analysis (CUA) was performed considering total cost related to severe asthmatic patients’ treatment, as cost data and quality-adjusted life-years (QALYs), derived from QoL estimation, as utility measure. Results were expressed using the incremental cost-effectiveness ratio (ICER). When the value of a new therapeutic option needs to be assessed, the ICER provides the additional resources that have to be used to achieve the additional benefit. ICER is the difference in cost (ΔC) divided by the difference in effect (ΔE) between two alternatives.50 The effectiveness indicator used to compare the pre-post omalizumab period was QALYs calculated from the utilities obtained from the EQ-5D questionnaire. ICERs were calculated by dividing the difference in direct costs by the difference in QALYs (effectiveness measure) between post omalizumab and pre-omalizumab phase.

To define a treatment as cost-effective, ICER has to fall below a given cost per QALY threshold.47,51 The ICER per QALY gained accepted differs from country to country. The threshold set by NICE is about £20,000–30,000.52 Although no officially established value is available for Italy, it is worth noting that recent guidelines by the Italian Health Economics Association (AIES)53 recommend that a threshold of €25,000–40,000 be adopted. Other acceptable references of cost-effectiveness for the Italian context are €36,500 and €60,000 and have been calculated by two different authors.54,55
Costs and Quality of Life Utilities
We considered asthma-related healthcare resource utilization and QoL data collected at baseline and at 12 months after the initiation of omalizumab. The outcomes were: number of hospitalizations and/or emergency department visits, outpatient visits and EQ-5D questionnaire results. Pharmacological treatments costs and OCS adverse events-related costs were also included in the healthcare–resource consumption analysis. Healthcare resources consumed were converted into economic values adopting the perspective of the National Health Service and frequency of use from PROXIMA study. Hospitalizations and ER accesses were valued according to the National Diagnosis-Related Group (DRG) system;56 in detail, admission due to moderate/severe bronchitis and asthma without complications (€1832), admission due to moderate/severe bronchitis and asthma with complications (€2537) and admission due to respiratory insufficiency (€3802) were considered (DRG code 97, 96, 87). Physician’s visit cost was derived from National Tariffs (€20.66).57 Drug costs were obtained considering ex-factory prices (with −5–5% mandatory rebates)58 daily dose and duration of administration according to indications reported in package leaflet and clinical practice guidelines for the management of adult asthma. Medications for respiratory disease examined were: inhaled corticosteroids (ICS), long-acting β2 agonist (LABA), short-acting β2 agonists (SABA), inhaled corticosteroids plus β2 agonists fixed combination, leukotriene modifiers, theophylline and oral corticosteroids (OCS).
To calculate omalizumab cost, ex-factory price (−5%, −5%) of a 150 mg subcutaneous vial, €333.56,58 (GU – Farmadati Italia) and the mean number of vials per patient per 4 weeks, estimated at 2.8 (based on sales data) were taken into account.
OCS adverse events-related costs were calculated considering event rates reported in a UK respiratory database (Optimum Patient Care Research Database, OPCRD)59,60 and comorbidities were valued according to the Diagnosis-Related Group (DRG) system.
The analysis conducted is a cost–utility analysis, an economic evaluation that estimates the cost per quality-adjusted life-year (QALY) gained from undertaking one intervention instead of another.61 Utility values were obtained from the PROXIMA study by using the EuroQol-5 Dimension (EQ-5D) health status self-assessment questionnaire and quality-adjusted life years (QALYs) were calculated.
Utilities were used to consider the impact of treatment on the quality of life. Utility values were expressed on a numerical scale where the extreme values were 0 and 1, with 0 representing the worsen status (death) and 1 representing the best status (perfect health). If the quality of life worsens, the utilities and QALYs are reduced. Then, QALYs are overall measures of health outcomes that weight the life expectancy of patients with an estimate of their health-related quality of life score.
Statistical and Sensitivity Analysis
In PROXIMA study the statistical analysis for the longitudinal phase was performed considering all evaluable patients who started the omalizumab therapy as per clinical practice at baseline with the foreseen time window (±15 days). Descriptive statistics of hospitalization number, ER visits, outpatient visits and pharmacological therapies were provided. (Quantitative variables were described using mean and standard deviation (SD), and categorical variables were reported using frequencies and percentages). Moreover, the distribution of patients according to the answers given to the EQ-5D items was given. As regard the pharmacoeconomic analysis, in order to evaluate the uncertainty around the real direct cost of omalizumab paid by hospitals, we tested the effects of four increasing discount rates applied to ex-factory price (20%, 25%, 30%, 35%).
Results
365 patients were enrolled in the PROXIMA study, 130 entered the longitudinal phase, 7 of them were excluded due to a violation of the inclusion criteria. The final sample for longitudinal phase included 123 patients: 76 (61.8%) were female and mean±SD age at enrollment was 52.7 (13.6) years. Further demographic and baseline data are shown in Table 1.
 |
Table 1 Baseline Characteristics of the Longitudinal Population |
Effectiveness and Resource Consumption Outcomes
As an evidence of omalizumab effectiveness in terms of asthma control, a significant decrease in ACQ total score has been observed at 12 months with a mean±SD change of 1.4±1.1 (p< 0.0001) with respect to baseline visit with a mean±SD ACQ score of 2.9±1.1. The number of exacerbations per patients, recorded over 1 year, showed an average decrease of −4.0±4.2 (0.6±1.2 exacerbation at 12 months vs 4.6±4.1 at baseline) which means a reduction of exacerbations of 87% vs baseline. During the 12-month period of observation, the proportion of patients who had experienced at least one asthma exacerbation resulted in 27.27%, 33 patients over 123, [95% CI, 19.57%-36.12%], while the proportion of patient with at least one asthma exacerbation during previous year at baseline was 87.6%. Patient’s compliance with omalizumab treatment in the PROXIMA study was considerably high, since the majority of patients (72.2%) never interrupted omalizumab during the 12-month observational period, and only nine patients discontinued omalizumab permanently. According to the results obtained by means of the EQ-5D questionnaire, there was a consistent increase in patient’s quality of life after 12 months from enrollment visit. Indeed, the proportion of patients reporting no problems on the five dimensions of the EQ-5D (mobility, self-care, usual activities, who did not have pain or discomfort, and anxiety or depression), increased from baseline to 12-month follow-up visit. Omalizumab adoption, as add-on therapy, resulted in a greater effectiveness than pre-omalizumab treatment involving 0.132 QALYs gained [0.630 (0.642–0.618) vs 0.762 (0.768–0.755)], Figure 2. Moreover, a significant decrease in medical resources utilization during the period of treatment with omalizumab was observed. During the 12-month period of observation, reductions in concomitant pharmacological treatments were recorded, in particular, 46.4% and 14.3% of patients treated with oral corticosteroids suspended or reduced the therapy, respectively. From the baseline, the number of hospitalization (58) and physician’s visits (482) diminished at 12-month follow-up (3 and 71, respectively). Total number of hospitalization and/or ER access per patient, due to moderate/severe bronchitis and asthma with/without complications or due to respiratory insufficiency decreased from 0.480 at baseline to 0.029 at 12-month follow-up (only 3 admission due to respiratory insufficiency).
 |
Figure 2 Effects of omalizumab treatment on health-related quality of life: pre-post omalizumab period comparison (EQ-5D). |
Economic Outcomes
The impact of omalizumab treatment on resource consumption involved a significant decrease in healthcare resource costs (Table 2). Omalizumab effectiveness in terms of disease control and risk reduction was reflected also in savings mainly related to a decrease in number of hospitalizations/ER accesses. Indeed, at 12-month follow-up costs per patient related to hospitalization and ER accesses decreased by €2475 compared with pre-omalizumab period. Reduction in pharmacological treatment costs and medical visit costs amounted to about €385 and €150, respectively. Furthermore, by adjusting OCS adverse event rates provided by Sweeney et al60 to PROXIMA data on OCS use, and quantifying adverse events based on DRG system, OCS adverse event-related costs were calculated: after 12 months of omalizumab treatment were about €718 lower than at baseline due to reduction in OCS consumption reported in post-omalizumab period. Total healthcare resource costs per patient amounted to about €779 after 12 months of omalizumab treatment and € 4508 for the pre-omalizumab period. The reduction of direct healthcare costs was €3729 per patient, associated with omalizumab adoption excluding the add-on treatment cost.
 |
Table 2 Effects of Omalizumab Treatment on Healthcare Resource Costs: Pre and Post Omalizumab Treatment Comparison |
The cost of omalizumab per patient-year was €11,207.62, obtained from ex-factory price (€333.56) and mean monthly administration (2.8 vials). By the addition of omalizumab cost, total direct healthcare costs per patient resulted in €11,986 determining a €7478 increase in costs with respect to pre-omalizumab period (€11,986 vs € 4508). Based on the difference in total direct cost (€7478) and difference in QALY (0.132) between post and pre-omalizumab period, the ICER was €56,847. Although omalizumab led to the improvement in effectiveness in terms of quality of life and reduction in healthcare resource consumption, its acquisition cost was only partially offset by the reduction in healthcare resource cost, making a significant impact on cost-effectiveness evaluation in our analysis. It is important to note that we calculated omalizumab cost based on ex-factory price, although it is not the real drug cost paid by health facilities in Italy as a confidential discounted price is usually negotiated between manufacturers and Italian NHS. In order to test the cost-effectiveness of omalizumab by adopting a discounted price closer to the real one, we carried out a sensitivity analysis assuming four feasible scenarios with increasing discounts on price from 20% to 35%. In all scenarios, the ICER resulted below the accepted threshold (Table 3, Figure 3).
 |
Figure 3 Incremental cost-effectiveness results: sensitivity analysis. |
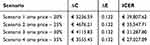 |
Table 3 Incremental Cost-Effectiveness Results: Sensitivity Analysis with Increasing Discounts on Omalizumab Price |
Discussion
This analysis evaluated the economic value of omalizumab in the treatment of adult patients with severe allergic asthma (step 4 GINA) in an Italian setting based on real-world data from the PROXIMA observational study. Since the confirmed therapeutic value of this treatment option, we focused on the impact of omalizumab on healthcare resource consumption and patient’s quality of life. In the observational study omalizumab effectiveness, in terms of asthma control, was supported by significant decrease in ACQ total score and exacerbations reduction of 87% at 12-month follow-up with respect to baseline. Moreover, during the observational period, there was a significant decrease in medical resource utilization, especially hospitalizations. As regard quality of life, EQ-5D results proved significant improvement during the 12-month observational period.
The clinical results are consistent with those published in previous clinical trials and observational studies. The significant reduction in the number of exacerbations supports the results from a recent Italian observational study62 in which more than 50% of patients did not report any exacerbations during the year of treatment. Moreover, PROXIMA findings are in agreement with those provided by large studies, such as eXpeRience observational registry34 and Asterix observational study.36 Data on oral corticosteroids consumption reported in PROXIMA Study (46.4% and 14.3% of patients treated with oral corticosteroids suspended and reduced, respectively, the therapy during the observational period) were also consistent with results of previous studies.31,35,62
In eXpeRience observational registry was observed a similar reduction in maintenance oral corticosteroid use in uncontrolled persistent allergic asthma patients treated with omalizumab for 12 months: 40.2% and 16.4% of patients stopped or reduced the dose of oral corticosteroids, respectively.31,34
Another observational, retrospective study evaluating the real-world effects of omalizumab in UK35 reported analogous results, 48.5% stopped oral corticosteroids within 1 year after omalizumab initiation. Moreover, a systematic review including 24 real-life effectiveness studies highlighted significant dose reductions in OCSs in association with omalizumab treatment: estimates of OCS dose reductions at 1 year were from −12% to −50%, with a decline of −66% at 2 years.63
The PROXIMA results, in line with the literature, thus confirm the OCS-sparing effect of omalizumab.
As shown in the literature, regular use of OCS therapy is linked to the onset of various comorbidities, ranging from minor effects to severe and potentially life-threating diseases such as diabetes, cardiovascular disorders and adrenal suppression.60,64–67 In turn, these corticosteroid-induced morbidities operate a significant healthcare and economic burden, also impacting patient’s quality of life.68,69
Indeed, in our study, the costs associated with the adverse events of corticosteroids, together with those related to hospitalizations, represented the main cost items in the pre-omalizumab period. In view of the high prevalence of corticosteroid related co-morbidities and their economic consequences, the OCS-sparing effect of newer asthma treatments such as omalizumab can find positive implications. By referring to Italy, clinical and economic implications can be investigated more carefully when data from ongoing observatory project, SANI (Severe asthma network in Italy, a web-based registry collecting data on severe asthmatics, recruited by Italian Unit of Allergy and Pulmonology) will be available.14
In our analysis, the decrease in healthcare resources reported in PROXIMA study was converted into economic values from the perspective of the National Health Service (NHS): compared to pre omalizumab period a €3,729.50 per patient reduction in direct healthcare costs was shown after the omalizumab adoption, excluding the add-on treatment cost. Nevertheless, the addition of omalizumab acquisition cost comported considerable increase in healthcare expenses (€11,207), only partially off-set by resource savings mentioned. Therefore, omalizumab improved clinical and health-related quality of life outcomes of SA patients, but with a major cost to the Italian NHS considering the ex-factory price for omalizumab. This performance is typical of innovative therapies and cost-effectiveness analyses are important tools to assess value for money and to position new health technologies by comparing costs and additional benefits. Incremental cost-effectiveness ratio (ICER) was calculated considering total incremental healthcare cost per QALY gained between post- and pre-omalizumab period: the ICER was sensitive to omalizumab cost. Adopting omalizumab ex-factory price (−5%,-5%), we obtained an ICER of €56,847, above the commonly accepted threshold in Italy. In contrast, assuming a discounted price negotiated between manufacturer and National Health Service, detail of which are confidential, we calculated the minimum discount necessary to achieve a favorable cost-effectiveness threshold. Omalizumab represented a cost-effective use of Italian NHS resources already at 20% discounted price reporting an ICER of €39,807 and increasingly favorable with increased discounts assumed in the analysis (25%, 30%, 35%), until ICER of € 27,027 assuming a 35% discounted price. In line with our considerations, other studies highlighted that the cost-effectiveness of omalizumab is highly sensitive to price assumptions and improvement in health-related quality of life.70–72 Several studies on the cost-effectiveness of omalizumab have been published in the international literature, both based on RCTs and observational studies, and a difference in the ICER estimation was observed related to difference in study design, population at baseline and drug unit cost.70–76 One economic evaluation conducted in the UK considered the cost-effectiveness of omalizumab under the list price and Patient Access Scheme (PAS) discounted price for the UK NHS. The incremental cost-effectiveness ratio varied from £30,109 to £57,557 per QALY gained depending on the population considered using the PAS price; incremental cost-effectiveness ratios were over a third higher using the list price. The ICERs were lower in subgroup analyses of patients hospitalized in the year prior to trial entry, and in patients receiving maintenance oral corticosteroids at baseline due to a greater improvement in health-related quality of life.70 This analysis supports our findings, although it was based on a cohort Markov model, while we considered real-world data from an observational study. Real-world evidence is becoming increasingly important in reimbursement decisions, and health care decision makers are developing policies that integrate data from different sources, recognizing the importance of evidence that goes beyond the information collected within the framework of clinical trials.77 Economic evaluation studies that incorporate real-world evidence are of major importance and provide added value to the evidence considered by decision makers, as these reflect the effectiveness of pharmaceutical products in real-life and illustrate how these last translate into the drug’s economic value for patients’ lives.78 Omalizumab improves asthma control in patients with uncontrolled severe allergic asthma, however, due to the high cost of the drug, accurate selection of patients with SA is crucial in the management of the disease.79–81 The identification of biomarkers predictive of response is of major importance for future research. Indeed, it can contribute to minimizing unnecessary drug exposure and health care costs for non-responders.72,73,82 Omalizumab response prediction methods, such as genetic text, may support clinical decisions, in this respect, PROXIMA sub-study results may help in the identification and characterization of super responder patients to this therapy and lead in the future to a tailored-made approach medicine. Our analysis also has assumptions and limitations. PROXIMA observational study provided clinical data, this design has intrinsic limitations inherent in the used data and potential selection bias. However, the inclusion and exclusion criteria of the PROXIMA study were defined in order to represent omalizumab adult patients in participating centers. Patients started a therapy with omalizumab within the time windows between 15 days before enrollment and 90 days after enrollment. The study results can be generalized to the adult population of severe asthmatic Italian patients in GINA step 4 and needed a therapeutic step up. Our analysis considered only direct costs in line with the Italian NHS perspective. This is a limit of the study; indeed the burden of indirect costs associated with severe asthma is significant, as reported in several studies.31,83 Therefore, it would be interesting to plan further analyses taking account of total cost, to define the socioeconomic impact of disease. Furthermore, in our analysis, no adverse events from omalizumab were included, while events related to the long-term use of maintenance OCS were considered given their significant cost and HRQOL implication. Rates of OCS-related events derived from a UK database study;60,69 these rates were used to calculate savings provided by the steroid-sparing effect of omalizumab reported after 12-month follow-up. Although these prevalence data were not related to Italy, pending national results, were generalizable and have been good support for our evaluation.
Conclusion
PROXIMA study demonstrated the effectiveness of omalizumab treatment in severe allergic asthma in step 4 GINA requiring a therapeutic step up. It showed to improve symptoms proving decreased incidence of exacerbations, number of hospitalizations, physician visits and better quality of life after 12 months of treatment. Moreover, it allowed a remarkable reduction in systemic corticosteroids use. Our economic evaluation assessed omalizumab value for money: omalizumab effectiveness was reflected in a reduction of resources for Italian NHS, although higher treatment costs resulted in a cost-effectiveness of €56,847 per QALY that exceeds value thresholds. Nevertheless, the cost-effectiveness of €40,000 per QALY would be already achieved with a 20% price discount, making omalizumab a sustainable innovative treatment option for patients with uncontrolled allergic asthma.
Disclosure
GLC, GMB, SDM, CM are employees of S.A.V.E. S.r.l and consultants for Novartis. CP is an employee of Novartis Pharma, Origgio, Italy. GLC has received research and educational grants from Abbott, Amgen, EISAI, Novo Nordisk, Menarini, Otsuka and Jazzpharma. P.S. has been consultant for Novartis. GWC has been speaker & consultant for Novartis. PR reports grants and/or personal fees from Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, ChiesiFarmaceutici, GlaxoSmithKline, Menarini, Mundipharma, Novartis, and Zambon. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2016. Available from: www.ginasthma.org.
2. De Marco R, Cappa V, Accordini S, et al. Trends in the prevalence of asthma and allergic rhinitis in Italy between 1991 and 2010. Eur Respir J. 2012;39:883–892. doi:10.1183/09031936.00061611
3. Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–1119. doi:10.1016/S0140-6736(08)61452-X
4. Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi:10.1164/rccm.200906-0896OC
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2019. Available from: www.ginaasthma.org.
6. Accordini S, Corsico A, Cerveri I, et al. The socioeconomic burden of asthma is substantial in Europe. Allergy. 2008;63:116–124. doi:10.1111/j.1398-9995.2007.01523.x
7. Chastek B, Korrer S, Nagar SP, et al. Economic burden of illness among patients with severe asthma in a managed care setting. J Manag Care Spec Pharm. 2016;22(7):848–861. doi:10.18553/jmcp.2016.22.7.848
8. Colice G, Wu EQ, Birnbaum H, Daher M, Marynchenko MB, Varghese S. Healthcare and workloss costs associated with patients with persistent asthma in a privately insured population. J Occup Environ Med. 2006;48(8):794–802. doi:10.1097/01.jom.0000229819.26852.0e
9. Custovic A, Johnston SL, Pavord I, et al. EAACI position statement on asthma exacerbations and severe asthma. Allergy. 2013;68(12):1520–1531. doi:10.1111/all.2013.68.issue-12
10. Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3:1. doi:10.1186/s40733-016-0029-3
11. Melero Moreno C, Quirce S, Huerta A, et al. Economic impact of severe asthma in Spain: multicentre observational longitudinal study. J Asthma. 2019;56(8):861–871. doi:10.1080/02770903.2018.1499035
12. Pedrini A, Rossi E, Calabria S, et al. Current management of severe refractory asthma in Italy: analysis of real-world data. Glob Reg Health Technol Assess. 2017;4(1):e216–e220.
13. Gruffydd-Jones K, Thomas M, Roman-Rodríguez M, et al. Asthma impacts on workplace productivity in employed patients who are symptomatic despite background therapy: a multinational survey. J Asthma Allergy. 2019;12:183–194. doi:10.2147/JAA.S204278
14. Senna G, Guerriero M, Paggiaro PL, et al. SANI-Severe Asthma Network in Italy: a way forward to monitor severe asthma. Clin Mol Allergy. 2017;15:9. doi:10.1186/s12948-017-0065-4
15. Godard P, Chanez P, Siraudin L, Nicoloyannis N, Duru G. Costs of asthma are correlated with severity: a 1-yr prospective study. Eur Respir J. 2002;19(1):61–67. doi:10.1183/09031936.02.00232001
16. Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol. 2012;129(5):1229–1235. doi:10.1016/j.jaci.2012.01.039
17. Sullivan SD, Rasouliyan L, Russo PA, Kamath T, Chipps BE, Group TS. Extent, patterns, and burden of uncontrolled disease in severe or difficult to- treat asthma. Allergy. 2007;62(2):126–133. doi:10.1111/j.1398-9995.2006.01254.x
18. Zervas E, Samitas K, Papaioannou AI, et al. An algorithmic approach for the treatment of severe uncontrolled asthma. ERJ Open Res. 2018;4(1):00125–2017. doi:10.1183/23120541.00125-2017
19. Nachef Z, Krishnan A, Mashtare T, et al. Omalizumab versus Mepolizumab as add-on therapy in asthma patients not well controlled on at least an inhaled corticosteroid: a network meta-analysis. J Asthma. 2018;55(1):89–100. doi:10.1080/02770903.2017.1306548
20. Chapman KR, Albers FC, Chipps B, et al. The clinical benefit of mepolizumab replacing omalizumab in uncontrolled severe eosinophilic asthma. Allergy. 2019;74(5):999–1003. doi:10.1111/all.13850
21. European Medicines Agency. Xolair, INN-omalizumab Xolair powder and solvent for solution for injection: summary of product characteristics. 2012.
22. Fahy JV, Fleming HE, Wong HH, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med. 1997;155(6):1828–1834.
23. Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184–190. doi:10.1067/mai.2001.117880
24. Solèr M, Matz J, Townley R, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18(2):254–261. doi:10.1183/09031936.01.00092101
25. Holgate ST, Chuchalin AG, Hebert J, et al. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy. 2004;34(4):632–638. doi:10.1111/cea.2004.34.issue-4
26. Ayres JG, Higgins B, Chilvers ER, et al. Efficacy and tolerability of antiimmunoglobulin E therapy with omalizumab in patients with poorly controlled (moderate-to-severe) allergic asthma. Allergy. 2004;59:701–708. doi:10.1111/all.2004.59.issue-7
27. Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy. (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–316. doi:10.1111/all.2005.60.issue-3
28. Bousquet J, Siergiejko Z, Swiebocka E, et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy. 2011;66:671–678. doi:10.1111/all.2011.66.issue-5
29. McKeage K. Omalizumab: a review of its Use in patients with severe persistent allergic asthma. Drugs. 2013;73:1197–1212. doi:10.1007/s40265-013-0085-4
30. Pelaia G, Vatrella A, Busceti MT, et al. Anti-IgE therapy with omalizumab for severe asthma: current concepts and potential developments. Curr Drug Targets. 2015;16(2):171–178. doi:10.2174/1389450116666141219122157
31. Braunstahl GJ, Canvin J, Peachey G, et al. Healthcare resource utilization in patients receiving omalizumab for allergic asthma in a real-world setting. Biol Ther. 2014;4(1–2):57–67. doi:10.1007/s13554-014-0019-z
32. Katsaounou P, Buhl R, Brusselle G, et al. Omalizumab as alternative to chronic use of oral corticosteroids in severe asthma. Respir Med. 2019;150:51–62. doi:10.1016/j.rmed.2019.02.003
33. Brusselle G, Michils A, Louis R, et al. “Real-life” effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respir Med. 2009;103:1633–1642. doi:10.1016/j.rmed.2009.06.014
34. Braunstahl GJ, Chen CW, Maykut R, et al. The eXpeRience registry: the ‘real-world’ effectiveness of omalizumab in allergic asthma. Respir Med. 2013;107(8):1141–1151. doi:10.1016/j.rmed.2013.04.017
35. Barnes N, Menzies-Gow A, Mansur AH, et al. Effectiveness of omalizumab in severe allergic asthma: a retrospective UK real-world study. J Asthma. 2013;50(5):529–536. doi:10.3109/02770903.2013.790419
36. Bhutani M, Yang WH, Hébert J, et al. The real world effect of omalizumab add on therapy for patients with moderate to severe allergic asthma: the ASTERIX Observational study. PLoS One. 2017;12(8):e0183869. doi:10.1371/journal.pone.0183869
37. Molimard M, de Blay F, Didier A, et al. Effectiveness of omalizumab (Xolair) in the first patients treated in real-life practice in France. Respir Med. 2008;102:71–76. doi:10.1016/j.rmed.2007.08.006
38. Korn S, Thielen A, Seyfried S, et al. Omalizumab in patients with severe persistent allergic asthma in a real-life setting in Germany. Respir Med. 2009;103:1725–1731. doi:10.1016/j.rmed.2009.05.002
39. Tzortzaki EG, Georgiou A, Kampas D, et al. Longterm omalizumab treatment in severe allergic asthma: the South-Eastern Mediterranean “reallife” experience. Pulm Pharmacol Ther. 2012;25:77–82. doi:10.1016/j.pupt.2011.11.004
40. Cazzola M, Camiciottoli G, Bonavia M, et al. Italian real-life experience of omalizumab. Respir Med. 2010;104(10):1410–1416. doi:10.1016/j.rmed.2010.04.013
41. Menzella F, Galeone C, Formisano D, et al. Real-life efficacy of omalizumab after 9 years of follow-up. Allergy Asthma Immunol Res. 2017;9(4):368–372. doi:10.4168/aair.2017.9.4.368
42. Alhossan A, Lee CS, MacDonald K, et al. “Real-life” effectiveness studies of omalizumab in adult patients with severe allergic asthma: meta-analysis. J Allergy Clin Immunol Pract. 2017;5(5):1362–1370. doi:10.1016/j.jaip.2017.02.002
43. Deschildre A, Roussel J, Drumez E, et al. Omalizumab discontinuation in children with severe allergic asthma: an observational real-life study. Allergy. 2019;74(5):999–1003. doi:10.1111/all.2019.74.issue-5
44. Casale TB, Chipps BE, Rosén K, et al. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy. 2018;73(2):490–497. doi:10.1111/all.13302
45. Canonica GW, Bartezaghi M, Marino R, et al. Prevalence of perennial severe allergic asthma in Italy and effectiveness of omalizumab in its management: PROXIMA – an observational, 2 phase, patient reported outcomes study. Clin Mol Allergy. 2015;13(1):10. doi:10.1186/s12948-015-0019-7
46. Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16(2):231–250. doi:10.1016/j.jval.2013.02.002
47. Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the Economic Evaluation of Health Care Programmes.
48. AIFA. Guideline for the classification and management of observational studies on drugs. March 20, 2008. Available from: http://www.agenziafarmaco.com/en.
49. Reddel HK, Taylor DR, Bateman ED, et al. An official american thoracic society/european respiratory society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical v practice. Am J Respir Crit Care Med. 2009;1(180):59–99. doi:10.1164/rccm.200801-060ST
50. Colombo GL, Gaeta GB, Viganò M, et al. A cost-effectiveness analysis of different therapies in patients with chronic hepatitis B in Italy. Clinico Econ Outcomes Res. 2011;3:37–46. doi:10.2147/CEOR.S16655
51. Cleemput I, Neyt M, Thiry N. Using threshold values for cost per quality-adjusted life-year gained in healthcare decisions. Int J Technol Assess Health Care. 2011;27(1):71–76. doi:10.1017/S0266462310001194
52. Raftery J. Review of NICE’s recommendations, 1999–2005. BMJ. 2006;332(7552):1266–1268. doi:10.1136/bmj.332.7552.1266
53. Italian Health Economics Association Associazione Italiana di Economia Sanitaria – AIES. Proposta di linee guida per la valutazione economica degli interventi sanitari in Italia. [Italian guidelines proposal on how to conduct economic evaluation studies of health programs]. Pharmacoecon Ital Res Articles. 2009;11:83–93. doi:10.1007/BF03320660
54. Lucioni C, Ravasio R. Come valutare i risultati di uno studio farmacoeconomico? PharmacoEcon Ital Res Articles. 2004;3:121–130. doi:10.1007/BF03320630
55. Messori A, Santarlasci B, Trippoli S, Vaiani M. Controvalore economico del farmaco e beneficio clinico: statodell’arte della metodologia e applicazione di un algoritmo farmacoeconomico. Pharmacoecon Ital Res Articles. 2003;5:53–67. doi:10.1007/BF03320605
56. Ministero della Salute. (Italian Ministry of Health). Tariffe delle prestazioni di assistenza ospedaliera per acuti [DRG tariffs]. Gazzetta Ufficiale della Repubblica Italiana. Serie N.23; Supplemento N.8 del 28 gennaio. 2013.
57. Ministero della Salute. (Italian Ministry of Health). Tariffe delle prestazioni di assistenza specialistica ambulatoriale. [inpatient tariffs]. Gazzetta Ufficiale della Repubblica Italiana. Serie N.23; Supplemento N.8 del 28 gennaio. 2013.
58. FARMADATI Italia software.
59. Sweeney J, Brightling CE, Menzies-Gow A, et al. Clinical management and outcome of refractory asthma in the UK from the British thoracic society difficult asthma registry. Thorax. 2012;67:754–756. doi:10.1136/thoraxjnl-2012-201869
60. Sweeney J, Patterson CC, Menzies-Gow A, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax. 2016;71:339–346. doi:10.1136/thoraxjnl-2015-207630
61. Drummond MF, Schulpher MJ, Torrance GW, et al. Methods for the Economic Evaluation of Health Care Programmes.
62. Novelli F, Latorre M, Vergura L, et al. Asthma control in severe asthmatics under treatment with omalizumab: a cross-sectional observational study in Italy. Pulm Pharmacol Ther. 2015;31:123–129. doi:10.1016/j.pupt.2014.09.007
63. Abraham I, Alhossan A, Lee CS, et al. Real-life’ effectiveness studies of omalizumab in adult patients with severe allergic asthma: systematic review. Allergy. 2016;71(5):593–610. doi:10.1111/all.12815
64. Sarnes E, Crofford L, Watson M, et al. Incidence and US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33:1413–1432. doi:10.1016/j.clinthera.2011.09.009
65. Poetker DM, Reh DD. A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryngol Clin North Am. 2010;43:753–768. doi:10.1016/j.otc.2010.04.003
66. Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018;141(1):110–116. doi:10.1016/j.jaci.2017.04.009
67. Fardet L, Flahault A, Kettaneh A, et al. Corticosteroid-induced clinical adverse events: frequency, risk factors and patient’s opinion. Br J Dermatol. 2007;157:142–148. doi:10.1111/j.1365-2133.2007.07950.x
68. Manson SC, Brown RE, Cerulli A, et al. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103:975–994. doi:10.1016/j.rmed.2009.01.003
69. Barry LE, Sweeney J, O’Neill C, et al. The cost of systemic corticosteroid-induced morbidity in severe asthma: a health economic analysis. Respir Res. 2017;18:129.
70. Faria R, McKenna C, Palmer S. Optimizing the position and use of omalizumab for severe persistent allergic asthma using cost-effectiveness analysis. Value Health. 2014;17(8):772–782. doi:10.1016/j.jval.2014.07.009
71. Wu AC, Paltiel AD, Kuntz KM, Weiss ST, Fuhlbrigge AL. Cost-effectiveness of omalizumab in adults with severe asthma: results from the Asthma Policy Model. J Allergy Clin Immunol. 2007;120(5):1146–1152. doi:10.1016/j.jaci.2007.07.055
72. Morishima T, Ikai H, Imanaka Y. Cost-effectiveness analysis of omalizumab for the treatment of severe asthma in Japan and the value of responder prediction methods based on a multinational trial. Value Health Reg Issues. 2013;2:29–36. doi:10.1016/j.vhri.2013.01.007
73. Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;13(1). doi:10.1002/14651858.CD003559.pub4/pdf
74. Levy AN, García a Ruiz AJ, García-Agua Soler N, Sanjuan MVH. Cost-effectiveness of omalizumab in severe persistent asthma in Spain: a real-life perspective. J Asthma. 2015;52(2):205–210. doi:10.3109/02770903.2014.941474
75. Sullivan PW, Li Q, Bilir SP, et al. Cost-effectiveness of omalizumab for the treatment of moderate-to-severe uncontrolled allergic asthma in the United States. Curr Med Res Opin. 2019. 1–10. doi:10.1080/03007995.2019.1660539
76. Vennera MC, Valero A, Uría E, Forné C, Picado C. Cost-effectiveness analysis of omalizumab for the treatment of severe persistent asthma in real clinical practice in Spain. Clin Drug Investig. 2016;36(7):567–578. doi:10.1007/s40261-016-0402-2
77. Garrison LP, Neumann PJ, Erickson P, et al. Using real-world data for coverage and payment decisions: the ISPOR Real-World Data Task Force report. Value Health. 2007;10:326–335. doi:10.1111/j.1524-4733.2007.00186.x
78. Ruggeri I, Bragato D, Colombo GL, Valla E, Di Matteo S. Cost and appropriateness of treating asthma with fixed-combination drugs in local health care units in Italy. Clinicoecon Outcomes Res. 2012;4:375–382. doi:10.2147/CEOR.S36499
79. Nygaard L, Henriksen DP, Madsen H, et al. Appropriate selection for omalizumab treatment in patients with severe asthma? Eur Clin Respir J. 2017;4(1):1359477. doi:10.1080/20018525.2017.1359477
80. Braido F, Holgate S, Canonica GWC. From “blockbusters” to “biosimilars”: an opportunity for patients, medical specialists and health care providers. Pulm Pharmacol Ther. 2012;25:483–486. doi:10.1016/j.pupt.2012.09.005
81. Said AA, Cushen B, Costello RW. Targeting patients with asthma for omalizumab therapy: choosing the right patient to get the best value for money. Ther Adv Chronic Dis. 2017;8(2–3):31–45. doi:10.1177/2040622317690494
82. Del Negro RW, Pradelli L, Tognella S, et al. Cost-utility of add-on Omalizumab in difficult-to treat allergic asthma in Italy. Eur Ann Allergy Clin Immunol. 2011;43(2):45–53.
83. Gibbison B, Griggs K, Mukherjee M, Sheikh A. Ten years of asthma admissions to adult critical care units in England and Wales. BMJ Open. 2013;3:e003420. doi:10.1136/bmjopen-2013-003420
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
