Back to Journals » Psoriasis: Targets and Therapy » Volume 11
Off-Label Treatments for Pediatric Psoriasis: Lessons for the Clinic
Authors Haulrig MB, Zachariae C , Skov L
Received 8 December 2020
Accepted for publication 14 January 2021
Published 11 February 2021 Volume 2021:11 Pages 1—20
DOI https://doi.org/10.2147/PTT.S268462
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Uwe Wollina
Morten B Haulrig,1 Claus Zachariae,1,2 Lone Skov1,2
1Department of Dermatology and Allergy, Herlev and Gentofte Hospital, University of Copenhagen, Hellerup, DK-2900, Denmark; 2Copenhagen Research Group for Inflammatory Skin (CORGIS), Hellerup, Denmark
Correspondence: Morten B Haulrig
Department of Dermatology and Allergy, Herlev and Gentofte Hospital, Gentofte Hospitalsvej 15, DK-2900, Denmark
Tel +45 38 67 31 32
Fax +45 38 67 71 18
Email [email protected]
Abstract: Psoriasis is a chronic inflammatory skin disease that affects up to 1.2% of children and adolescents. The treatment options for childhood psoriasis are often based on the same principles as in adults. However, most data on safety and efficacy derive from adult studies, and only a few of the frequently used treatments have achieved approval for use in children. The aim of this study was to review the current literature on off-label treatments for psoriasis in children and adolescents. We searched PubMed and identified 50 studies on off-label treatments. Of these, 23 studies were clinical trials (four randomized). There are only a small number of available studies on off-label treatments for children and adolescents with psoriasis, and many of these are retrospective reviews with few participants. Despite the current lack of studies, we still recommend the use of unapproved treatments since we have clinical experience with treatments such as topical corticosteroids, vitamin D analogs, and methotrexate that have shown promising effects. Regular clinical trials are needed to investigate the safety and efficacy of unapproved treatments. Due to The Pediatric Investigation Plans issued by The European Union, new drugs developed by pharmaceutical companies are required to undergo clinical trials in a pediatric population to get their application for marketing authorization processed. This will hopefully lead to much more data on the efficacy and safety of the new treatments, including treatments for children and adolescents with psoriasis.
Keywords: psoriasis, unapproved treatment, childhood, adolescents
Introduction
Psoriasis is a chronic inflammatory skin disease affecting between 0.5% and 1.2% of all children and adolescents.1–3 The treatment of pediatric psoriasis is in many ways based on the same principles as in adults. While the treatments are registered for adults, this may not be the case for their pediatric counterparts. Limited by lack of data and standardized approaches, clinicians usually base treatment decisions on guidelines for psoriasis in adults, case series, expert opinions, or experience of drug safety and efficacy acquired in clinical trials investigating other pediatric disorders.
Off-label treatments are therefore widely used, and the treatment of psoriasis in children and adolescents remains a challenge. The purpose of this study is to review the current literature for all conducted studies regarding off-label treatments used for pediatric psoriasis.
Psoriasis in Children and Adolescents
Psoriasis is a chronic inflammatory skin disease affecting 2–3% of the Western adult population with an equal distribution between men and women.4 Disease onset is bimodal with peaks around 16–22 years and 57–60 years. Approximately one third of patients have already had disease onset before turning age 16 with a mean age of 8–11.5–7 Though occurring less frequently than in adults, psoriasis in children and adolescents affects approximately 0.5–1.2%, and the prevalence linearly increases from 0.1% at age 1 to 1.2% at age 18.1–3,8
The pathogenesis of psoriasis is complex and is caused by an interaction between the skin and the immune system, as well as genetic and environmental factors. On a cellular level, it is a T-cell mediated inflammatory disease characterized by keratinocyte hyperproliferation, vascular endothelial proliferation, as well as inflammatory cell infiltration of the dermis and epidermis,9 where Th17 cells and the cytokines interleukin (IL) −17 and IL-23 are especially involved.10 According to twin studies, the risk of developing psoriasis is caused by genetic factors in up to 70% of the cases.11 There is an increased occurrence of psoriasis in first- and second-degree relatives to patients with psoriasis compared to the background population. The rate of concordance is increased eight-fold in monozygotic twins and four-fold in dizygotic twins.12 Currently, at least 80 chromosomal loci that are significantly tied to psoriasis have been discovered, and some loci of these are particularly linked to the onset of psoriasis in childhood.13–15
Several environmental factors are known for triggering de novo psoriasis and escalate already existing disease. The most important factors in children are mental stress, physical trauma (known as Köbner phenomenon), and upper respiratory tract infection caused by streptococci.10,16
In many cases, psoriasis in children is a straightforward clinical diagnosis. Compared to adults, however, pediatric psoriasis may be more difficult to diagnose due to a milder disease course with smaller and thinner plaques that are less scaling. The manifestations in children and adolescents may be atypical, and thus, distinguishing psoriasis from other diseases such as atopic dermatitis, seborrheic dermatitis, and fungal infections can be complicated.17–19 No validated consensus-based diagnostic criteria for psoriasis have been approved, which makes the diagnosis difficult.20 The Psoriasis Area and Severity Index (PASI) is a clinical tool for appraising disease severity in plaque psoriasis and is commonly used in clinical trials with children also. This system is developed for adults, and thus, has its limitations in young children, particularly since body proportions in this patient group are quite different compared to adults.21
Psoriasis in children and adolescents can be divided into several different manifestations depending on the type of lesion and sites involved. Psoriasis vulgaris (plaque psoriasis) is the most common manifestation, affecting 34–71% of children and adolescents with psoriasis.22 Guttate psoriasis affects 6–26% of children, which is more frequent compared to adults.15,22 Guttate psoriasis is often triggered by an infection in the upper respiratory tract caused by streptococci, and approximately one third of these patients are at higher risk of developing plaque psoriasis later in life.10,15 Other clinical manifestations include diaper psoriasis, which affects up to 13%, whereas inverse psoriasis, pustular psoriasis, and nail psoriasis each affect roughly 0.5–3.6% of children and adolescents with psoriasis.15,16,22 Psoriasis affecting the entire body surface is potentially life-threatening and is termed erythroderma, which can be caused by any form of psoriasis.10 In children, the face and scalp are the most frequently involved areas, followed by the groin, trunk, and extensor areas on knees and elbows.2,5,16,22
The risk of developing comorbidities in pediatric psoriasis is up to twice as great when compared to healthy children.2 These comorbidities include somatic diseases such as hyperlipidemia, overweight, hypertension, diabetes mellitus, polycystic ovary syndrome, nonalcoholic fatty liver disease, rheumatoid arthritis, and Crohn’s disease.23,24
There is also an increased risk of developing mental diseases such as depression and anxiety.25,26 Juvenile psoriatic arthritis appears in approximately 3% of children with psoriasis, which is less frequent compared to 22% of European adults.23 Some studies have found an association between pediatric psoriasis and prenatal tobacco exposure, which suggests smoking during pregnancy might play a role in the development of psoriasis.27
Psoriasis has a negative effect on the quality of life in both children and adults, comparable to that of cancer, heart disease, and depression. Pediatric psoriasis is particularly associated with increased social stigma in the form of bullying and shaming. Observation of life quality is difficult since no specific measuring tool for quality of life in patients with psoriasis and especially children exist at this stage.28–30
Treatment of Psoriasis in Children and Adolescents
Currently, topical treatments such as topical corticosteroids, vitamin D analogs and a fixed combination of these treatments are considered first-line treatments for mild to moderate localized psoriasis.10,17,31 These treatments are followed by systemic nonbiological treatment based on disease severity, lack of response to topical treatment, speed of disease progression, and presence of comorbidities such as psoriasis arthritis as part of a regular treatment strategy.31,32 The most commonly used systemic nonbiological treatments are methotrexate (MTX) followed by cyclosporine. MTX is considered the first-line option for moderate to severe plaque psoriasis in children.33,34 Only if the treatment response to nonbiological systemic therapy is absent, contraindicated, or not tolerated, may approved biological medicine be considered.32
The latest guidelines from The Joint American Academy of Dermatology and The National Psoriasis Foundation (AAD-NPF) and The German Society of Dermatology (DDG) for psoriasis in children and adolescents provide largely similar treatment strategies, based on the type of psoriasis and disease severity.21,31,32 Certain treatments differ in approval status, which are provided by The US Food and Drug Administration (FDA) and The European Medicines Agency (EMA), and as a result, some treatments are considered off-label in North America while they are approved in Europe. Table 1 shows the current treatment strategies for pediatric psoriasis and their approval status according to the FDA and EMA. Clear information regarding approval status is not provided for all treatments, which includes treatments such as phototherapy and coal tar. These treatments have traditionally been used for psoriasis but only have limited supporting evidence concerning safety and efficacy in children.31 Other treatments such as topical calcineurin inhibitors are approved for atopic dermatitis in children, whereas MTX is approved for polyarticular juvenile idiopathic arthritis. Since the efficacy and safety of these treatments have not been investigated specifically for psoriasis in children and adolescents, neither topical calcineurin inhibitors or MTX are approved by FDA or EMA for use in this patient group.21 Off-label treatments are generally used in situations where studies conclude a greater clinical advantage or higher chance of achieving a treatment goal compared to approved treatments. Intentional use of treatments outside their approved indication, age group, or dosage is generally acceptable among clinicians and is performed due to cases of severe disease where no other approved treatments are considered effective, or no evidence is otherwise available. At all times the clinician is required to inform the patient and the parents if an initiated treatment is off-label.
 |
Table 1 Treatment Approval Status for Psoriasis in Children and Adolescents According to Medical Agency |
According to The Pediatric Investigation Plans issued by The European Union (EU), new drugs developed by pharmaceutical companies are required to undergo clinical trials in a pediatric population to get their application for marketing authorization processed.35 Subsequently, there has been an increase in treatments that are being tested and approved for pediatric psoriasis, predominantly biological treatments. Even so, the EU regulations do not require the traditionally used treatments to undergo clinical trials in children, and the pharmaceutical companies have limited financial gain in testing the traditional treatments.
All treatments should ideally be used for their indicated purpose based on research investigating the efficacy and safety. However, we often use off-label treatments with success, as the following two cases demonstrate. After presenting the cases, we will go through the available data on off-label treatments for psoriasis in children and adolescents.
Cases from a Clinical Practice
We present two cases from our clinic to emphasize that off-label treatments can be useful in the treatment of children and adolescents with psoriasis.
Case 1
A 9-year-old boy presented with multiple small, hyperkeratotic elements primarily located on the trunk and scalp. The elements measured approximately 2 cm in diameter and were scaly and the patient complained of itching. The elements had started four months earlier and there was no family history of psoriasis. The patient was overweight but otherwise healthy. Prior to the visit to our clinic, the patient had been treated with a combination of topical betamethasone dipropionate and salicylic acid for the scalp once daily for three weeks without effect. Upon clinical examination, a positive Köbner phenomenon was observed when scratching on the patient’s back. On suspicion of a mixed plaque and guttate psoriasis, treatment with coal tar ointment three times weekly for the scalp and topical corticosteroid creme (group III according to The World Health Organization (WHO) classification) for the body was initiated. Since there was no effect after three weeks, the treatment was stopped, and instead, Narrow-band (NB)-UVB (NB-UVB) phototherapy was started three times weekly. After 15 treatments, there was no effect. Consequently, treatment was changed to MTX 0.2 mg/kg and folic acid 5mg once weekly. Two months after MTX treatment was started, there was no effect on the disease severity of psoriasis (see Figure 1) and dosage was increased to 0.26 mg/kg. The patient had no adverse events and liver enzymes were normal. After another three months, the psoriasis had completely cleared, and the patient still had clearance after two years. At 12 years, the MTX dose was increased since the patient had grown and gained more weight. At the latest follow-up in our clinic, the patient had almost clearance except for mild scalp psoriasis.
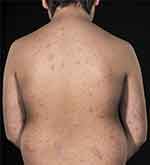 |
Figure 1 Mixed plaque and guttate psoriasis on the back of a 9-year-old boy. |
Case 2
A 4-year-old boy presented with thick, scaly plaques on the trunk, and to a lesser degree, on the upper and lower extremities (see Figure 2). The plaques were itching and had debuted four months earlier after an infection with varicella virus. The patient was otherwise healthy and had two aunts with a history of psoriasis. A throat swab had revealed group A Streptococcus for which the patient had been treated with penicillin. Topical corticosteroid cream (group II, WHO classification) had previously been applied once daily for five months with no effect. We suspected plaque psoriasis and initiated treatment with a fixed combination of vitamin D analogs and betamethasone propionate foam once daily for the trunk and extremities, while the patient continued group II topical corticosteroid for the face and neck. The patient initially had complete clearance of psoriatic plaques after one month, but relapse was seen after three months. Treatment with topical corticosteroid was replaced with basic skincare containing 10% coal tar once daily for the entire body, but had no effect. Six months after the first visit, treatment with oral MTX 0.3 mg/kg and folic acid 5 mg once weekly was initiated. Due to a lack of response two months after, the MTX dosage was increased to 0.4 mg/kg weekly. No adverse events were reported, and blood analysis revealed normal liver enzymes. The patient gradually responded to this treatment at follow-up after four months but still had residual plaques. The patient is still being treated with MTX, and a combination of vitamin D analogs, while betamethasone dipropionate gel is used for flares.
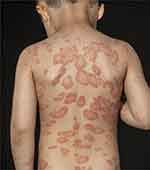 |
Figure 2 Plaque psoriasis on the back of a 4-year-old boy. |
Literature Search on off-Label Treatments for Children and Adolescents with Psoriasis
We conducted a literature search in PubMed up to 25 November, 2020, to identify all retrospective and prospective studies on treatments used for psoriasis that were either traditionally used or approved for use in adults but not approved by either FDA or EMA for use in children and adolescents. Studies on combinations of therapies were not included except for topical corticosteroids in a fixed combination with vitamin D analogs. We searched on the following treatments: “topical corticosteroid”, “tacrolimus OR pimecrolimus OR TCI OR topical calcineurin inhibitors”, “tazarotene OR topical retinoid”, “anthralin OR dithranol OR Ingram regimen”, “vitamin D analogues”, “coal tar OR Goeckerman therapy”, “phototherapy”, “methotrexate OR MTX”, “acitretin OR etretinate OR systemic retinoids”, “ciclosporin OR cyclosporin”, “fumaric acid esters”, “apremilast OR phosphodiesterase 4 inhibitor OR otezla” and “adalimumab OR humira”. We also searched on several other treatments that did not give any results. For a full list of the treatments we searched on, see Table 2.
 |  |  |  |
Table 2 Characteristics of the Included Studies (n = 50) |
The following terms were used for our search on PubMed: (Psoriasis) AND (Clinical Trial OR Prospective Study OR Case Series) AND (Children OR Pediatric OR Infants OR Childhood) AND (Name of treatment). We also included all available guidelines published after 2010 using the free-text term “Guideline” followed by the following keywords: (Psoriasis) AND (Children OR Pediatric OR Infants OR Childhood). Review articles and articles cited in original papers allowed us to identify additional studies. Articles that did not report on the efficacy of treatments were excluded. We excluded case reports but included case series. We excluded articles written in languages other than English and articles that were not fully available to read. An inadequate study design was not an exclusion criterion.
A total of 50 studies were included in this review. Nine studies were case series, 17 were retrospective reviews, 1 was a retrospective cohort study, and 23 were clinical trials, of which four were randomized. The studies were sorted into groups as follows: (1) Topical treatments, (2) coal tar/Goeckerman therapy, (3) phototherapy, and (4) systemic therapy. Four studies referred to more than one off-label treatment and were thus included in more groups. See Figure 3 for the amount of included studies in each group.
Topical Treatments
Topical Corticosteroids
Two studies (20 patients aged 5 to 18 years) reported on the efficacy of topical corticosteroids in plaque psoriasis.36,37 Both studies were clinical trials in which the patients applied a group IV corticosteroid (WHO classification) two times daily for two weeks. Kimball et al36 randomized patients with both atopic dermatitis and psoriasis (a total of nine patients with psoriasis) into two groups. Group A applied a total of 50 g clobetasol weekly, while group B applied vehicle foam. Efficacy was measured with the Investigator’s Static Global Assessment (ISGA). Twenty-five percent of the patients in the clobetasol group had clear or almost clear psoriasis compared to 0% in the vehicle group. The authors also found a reversible hypothalamic–pituitary–adrenal axis suppression in 47% of the patients with atopic dermatitis aged 6 to 12 years and in 0% of the patients aged 12 and 18 years. Since younger patients aged 0 to 6 years have a relatively higher body surface area to weight ratio compared to adolescents and adults, they have an increased risk of developing systemic side effects when using topical corticosteroids.31 In the clinical trial by Herz et al37 (Table 2), a total of 40 g halobetasol was applied weekly. 10/11 (90.9%) patients had either a marked improvement or clearing of disease when assessed with the Physician’s Global Assessment Scale (PGA). Though both studies were clinical trials, they each only had few participants. The randomized clinical trial by Kimball et al36 included a total of 497 patients. However, only a fraction of the included patients were children and adolescents with psoriasis, and only one child with psoriasis was randomized to apply vehicle foam. Overall, there were very few studies on topical corticosteroids. This scarcity does not correspond with the frequent use of topical corticosteroids experienced in clinical practice.31
Topical Calcineurin Inhibitors
Two studies (24 patients aged 22 months to 16 years) examined the effect of the topical calcineurin inhibitor tacrolimus.38,39 In both studies, tacrolimus 0.1% was applied two times daily for psoriasis in the face and the intertriginous areas. In the clinical trial by Brune et al38 only patients who had discontinued all psoriasis treatment at least two weeks prior to study start were eligible for inclusion. The clinical evaluation was assessed with a modified version of the PGA. The authors reported a significant decrease in the severity of erythema, infiltration, and desquamation, as well as the overall severity score. By day 30, 12% of patients had completely cleared and the remaining 88% achieved excellent improvement. One patient discontinued due to treatment-related pruritus. Steele et al39 performed a retrospective review in which treatment response was assessed by clinicians on follow-up visits with a nonspecified assessment score; 92% of the patients had clearance after two weeks. One patient applied tacrolimus 0.03% and had no response. Concurrent application of other topical treatments was allowed. Overall, only two studies using tacrolimus were available and both included few patients; nevertheless, tacrolimus demonstrated efficacy, and few adverse events were reported.
Anthralin
The effect of anthralin was investigated in two studies (94 patients aged 3 to 17 years), in which patients with plaque psoriasis were treated with dithranol cream once daily in increasing doses and for longer periods.40,41 In the clinical trial by Oostveen et al40 PASI was reduced by 69%, and concurrent treatment with any psoriasis treatment was an exclusion criterion. The patients could choose between follow-up with regular day care or day care with telemedicine. There was no significant difference in PASI reduction when comparing the two groups. In the retrospective review by De Jager et al41 73% of the patients achieved “almost clearance” or “clearance” of their psoriatic plaques. Skin irritation was reported in 63% of the patients. Efficacy was not measured objectively with a severity baseline and a specific rating score. However, the authors claimed efficacy was standardized since all the included patients had moderate to severe psoriasis. A treatment episode lasted up to nine days and was stopped if clearance or near clearance was achieved or if there was no effect of treatment. After an episode of anthralin was finished, patients were allowed to apply topical corticosteroids or vitamin D analogs for the remaining plaques. When summarizing treatment efficacy in both studies, anthralin had predominantly moderate to good effect. Both studies had only a few patients and many experienced skin irritation.
Vitamin D Analogs
Four studies (165 patients aged 2 to 17 years) examined the efficacy of vitamin D analogs in patients with primarily plaque psoriasis.42–45 Oranje et al42 performed a double-blind, randomized clinical trial in which 77 patients were divided into two groups receiving either calcipotriol or vehicle ointment two times daily. Concurrent treatment was an exclusion criterion. Though PASI reduction was higher in the group treated with calcipotriol (52% compared to 37.1% in the vehicle group), it was not significant (p <0.14). There was an insignificant decrease in the thickness of scales (p <0.075); however, there was a significant decrease in redness (p <0.037) and scaliness (p <0.018). Treatment compliance was low and only seen in 42% of the patients who applied calcipotriol and in 52.9% of the patients who applied vehicle ointment. Laboratory analysis of blood samples including calcium, 25-OH-D, and other indicators of bone metabolism from both groups revealed no significant differences. In the randomized clinical trial by Saggese et al43 ten patients with plaque psoriasis applied calcitriol followed by occlusive dressing on one side of the body, and petrolatum (vehicle) on the other side of the body once daily. All patients had cleared psoriasis on the calcitriol-treated side after four weeks whereas no appreciable changes were observed on the vehicle side. The authors used an unspecified score for scaling, erythema, and thickness.
In the clinical trial by Park et al44 12 patients received calcipotriol between 12 and 103 weeks. 64.7% of the patients achieved PASI75. Blood analysis of the indicators of bone metabolism was also performed and found decreasing, yet insignificant, mean values of 1,25-dihydroxyvitamin D3 levels below the normal range in half of the patients at the end of treatment; no change in calcium metabolism was found. An analysis with similar results was also performed in the clinical trial by Darley et al45 for patients that received calcipotriol twice daily for up to eight weeks. In this study, 65% of the patients achieved PASI75 after eight weeks, and no significant differences in biochemical measurements compared to baseline status were reported. Overall, the studies on vitamin D analogs were all clinical trials, of which two were randomized. Two of the studies only included a small number of participants however, and the patient age varied from small children to adolescents.
Vitamin D Analogs + Betamethasone Dipropionate
Four studies (255 patients aged 3 to 18 years) examined the efficacy of a fixed combination of vitamin D analogs and betamethasone dipropionate (group III, WHO classification).46–49 All studies were clinical trials and investigated the efficacy on psoriasis primarily located on the scalp. In the study by Gooderham et al46 calcipotriol plus betamethasone dipropionate gel was applied daily for eight weeks in adolescents with moderate to severe scalp psoriasis. Clearance or almost clearance was achieved in 47% at week two and 85% at the end of treatment, according to the Investigator’s Global Assessment (IGA). Fifty-nine percent of the patients were fully compliant or missed less than 10% of the applications. Thirty-five percent had adverse events, but there were no reports of hypercalcemia.
In the clinical trial by van Geel et al47 patients with plaque psoriasis were divided into two groups using calcipotriol plus betamethasone dipropionate ointment. One group applied ointment intensively on a daily basis for four weeks, followed by four times weekly, while the other group applied ointment four times weekly during the whole study period. The group intensively applying ointment had significantly higher baseline PASI compared to the other group and achieved PASI reduction of 34.6% at week 48. The group applying ointment four times weekly had no change in PASI score. In the clinical trial by Oostveen et al48 a reported reduction of psoriasis scalp severity index (PSSI) of 32% was observed at week 12 with the maintenance of this effect until follow-up at week 48 (p <0.001). Eichenfeld et al49 reported on the efficacy of calcipotriol plus betamethasone for moderate to severe scalp psoriasis in adolescents. Fifty-five percent achieved clear or almost clear psoriasis according to the Investigator’s Global Assessment (IGA) after eight weeks; 90% reported mild or no itching at the end of study. Blood analysis revealed signs of mild adrenal suppression in one patient at week four. The patient used 94 g of product during the first two weeks of the study period compared to 51 g in the rest of the participants; the treatment was stopped, and adrenal function was normal after four weeks. No studies allowed concurrent systemic treatment or other topical treatment of the scalp. Blood analysis was performed in two of the studies and no hypercalcemia was reported.46,49 Overall, the included studies reported moderate to good efficacy but used different severity scoring systems.
Coal Tar/Goeckerman Therapy
Our search on coal tar as a single treatment for psoriasis in children and adolescents did not produce any results. When we searched on coal tar combined with UV-radiation, which is called Goeckerman therapy, we found four studies (126 patients aged 3 months to 18 years).50–53 Three of the included papers were published by the same first author and generally used the same treatment methods. Here, efficacy, as well as different safety aspects of Goeckerman therapy were examined. In one study, Borska et al50 reported significantly increased markers of oxidative stress and chromosomal aberrations in peripheral lymphocytes, whereas the authors reported urinary mutagenicity and a temporary genotoxic effect during treatment in another study.52 All three studies reported a significant decrease in PASI score. The studies had few participants, however, and adverse events were not reported even though Goeckerman therapy increases the risk of burning.
In the retrospective review by Kortuem et al53 the participants were treated with Goeckerman therapy and NB-UVB. At least 80% clearance of plaques was seen in 85% of the patients. The most frequent adverse event was folliculitis, which was reported in 42% of the patients. However, the used severity scoring system was not specified, and the phototherapy device was not comparable to newer devices. Furthermore, efficacy was not reported for patients with guttate psoriasis or erythroderma, and the age of the participants varied from young children to adolescents. Most of the included studies had few participants, and three out of four did not report adverse events.
Phototherapy
Eleven studies reported on the efficacy of phototherapy in children with plaque and guttate psoriasis (364 patients aged 3 to 17).54–64 NB-UVB treatment was used in all studies, whereas the retrospective review by Ersoy-Evans et al62 also included psoralen-UVA (PUVA) and regular UVB. In this study, the authors concluded that the efficacy of NB-UVB and UVB treatment were equally more effective with 75% less clearance achieved in 93% of the patients compared to PUVA treatment with <75% clearance achieved in 83%; the assessment score was not specified. In a clinical trial by Jain et al55 the patients applied mineral oil on one side of their body before irradiation with NB-UVB treatment. A significant improvement in scaling, induration, and modified PASI score was observed on the pretreated side after three weeks, while the cumulative dose for clearance was lower compared to the side without mineral oil. In a retrospective review by Eustace et al60 86% achieved PASI75 at the end of treatment. At follow-up after 12 months, 43% remained clear while 28.6% had initiated systemic therapy. The remaining participants were lost to follow-up.
Phototherapy was effective overall in a large number of participants, although erythema was a common adverse event observed in 8/11 studies.56–58,60–64 Two patients discontinued treatment in one study due to the development of erythroderma following NB-UVB treatment.54 It is notable that topical treatment was used or allowed in 6/11 studies,58,60,62–64 while the clinical trial by Tan et al56 allowed both topical and systemic treatment. We noticed that studies allowing topical treatments such as topical corticosteroids reported higher clearance rates. This was also observed in a recent systematic review and meta-analysis on NB-UVB treatment for psoriasis in children.65 In their analysis, the authors did not find any significant association between the amount of treatment sessions, cumulative irradiation dose, or maximum irradiation dose and efficacy. The authors did, however, find a positive association between the number of sessions and efficacy. Phototherapy was the treatment with the most studies in this review. Still, 9/11 studies included less than 40 patients each, and most studies were retrospective reviews; further, many of the studies did not report the type of psoriasis or specified the used assessment score.
Systemic Therapy
Methotrexate
The efficacy of MTX was examined in nine studies in children and adolescents who mainly had moderate to severe plaque psoriasis (409 patients aged 2 to 18 years), with MTX used in doses of 0.03 to 0.7 mg/kg week.66–73 In the clinical trial by Papp et al66 children and adolescents with severe plaque psoriasis were randomized into three groups. One group received oral MTX 0.1 mg/kg once at week 0, which was increased to up to 0.4 mg/kg at week 1 if blood analysis was normal (maximum 25 mg weekly). The other groups received adalimumab in a low dose (0.4 mg/kg) or high dose (0.8 mg/kg) every other week. Each group received either placebo tablets or placebo injections, depending on their group. Early response to high dose adalimumab was seen after four weeks with PASI75 response achieved in 23.7% compared to 13.5% of the patients in the MTX group at week six. Of the 37 patients that received MTX, 32% achieved PASI75 at the end of treatment at week 16. In comparison, PASI75 was achieved in 43.6% and 57.9% of the patients treated with low dose adalimumab and high dose adalimumab, respectively. The difference in PASI scores between MTX and high dose adalimumab was significant (p <0.027), whereas the difference in PGA was not significant. The safety profiles were similar.
Nonresponders (PASI75 not achieved) at week 16 entered a long-term extension treatment period and continued with open-label adalimumab at a high dose every other week for 52 weeks. Responders (PASI75 achieved) at week 16 were withdrawn from treatment for up to 36 weeks but could enter retreatment if they had a loss of disease control. The patients were then retreated with the same treatment they received in the first 16 weeks, with either adalimumab in low or high doses, or adalimumab in high dose, if the initial treatment was MTX. After 16 weeks of treatment, these patients also entered the long-term extension period of 52 weeks. The results from the long-term extension period were reported by Thaci et al74 where 108 of the 114 patients who had been randomized in the initial study period entered the long-term extension period. The group that initially received MTX and then high dose adalimumab during the long-term extension period achieved PASI75 improvement of 31–86% from entry to the end of the study period. The group that initially received a low dose of adalimumab followed by either a low or high dose adalimumab achieved PASI75 improvement of 28–47% from entry to the end, whereas it was 50–72% in the group that only received high dose adalimumab.
Bronckers et al70 compared the efficacy and safety of MTX and biological treatment in a retrospective review. The patients that received biological treatment were treated with etanercept (83.3%), adalimumab (12.5%), or ustekinumab (4.2%). The analysis of the biological treatment was performed for all drugs as one group. The specific dosage of MTX was not reported. When evaluated after six months, 40% of the patients in the MTX group had achieved PASI75 compared to 71.1% in the group treated with biologics. Patients who were treated with biological treatment were more likely to achieve PASI75 compared to MTX (odds ratio for PASI75 4.95%, p <0.01), and the overall drug survival rate at five years was lower for MTX (35.9%) compared to biological treatment (57.1%).
In a clinical trial by Ergun et al68 the effect of MTX, acitretin, and cyclosporin was examined (85, 61, and 80 patients, respectively). Of the patients that received acitretin 0.3 mg/kg daily, 47.5% achieved PASI75. This was the highest efficacy, followed by 40% of patients that received cyclosporin 3 mg/kg daily, whereas PASI75 was achieved in 34.1% of patients that received MTX 0.3–0.7 mg/kg weekly (route of administration not reported). The one-year drug survival rate was 36.3% (acitretin), 21.1% (MTX), and 15.1% (cyclosporin). The main reasons for treatment termination were primary lack of efficacy or secondary loss efficacy seen in 22.9% (MTX), 28.4% (acitretin), and 31.7% (cyclosporin). The study was limited by not reporting baseline disease severity, and no comparison between the drugs was thus performed.
Several studies reported adverse events that included nausea, vomiting, infection, and loss of appetite, but no studies changed the treatment to subcutaneous MTX injections.66–68,71 Adverse events leading to discontinuation were reported in four patients due to increased liver enzymes.67,68,73 In the retrospective review by Charbit et al69 four patients discontinued due to hypereosinophilia, digestive disorder, and rheumatism. Overall, the included studies reported varying efficacy of MTX. Follow-up periods of up to one year were performed in several studies. For more information, see Table 2.
Retinoids
Four studies reported on the efficacy of systemic retinoids in children with plaque and pustular psoriasis (236 patients aged 5 months to 14 years) at doses of 0.2 to 1.0 mg/kg daily.68,69,75,76 The efficacy of acitretin in the clinical trial by Ergun et al68 was described earlier. Charbit et al69 performed a retrospective review in which they examined systemic treatments for children and adolescents with moderate to severe psoriasis of different types. Acitretin was the most frequently used systemic treatment in 55% of the included patients, followed by MTX, biological treatments, and ciclosporin in the order listed. Thirty-three percent of the patients that received acitretin achieved PASI75 at week 12, which was surpassed by all other examined treatments. However, the study was mostly descriptive, and no comparison analysis of treatment efficacy was performed. 73.9% of the patients used concurrent topical treatments in all groups, which could affect the overall efficacy. Regarding the safety of acitretin, Di Lernia et al75 reported a lack of efficacy as the reason for discontinuation in 50% of the patients in their retrospective review. Discontinuation due to adverse events was observed in eight patients who experienced alopecia, headache, itching, view disorder, digestive disorder, and arthralgia.68,69,75 Acitretin was reported as the first-line systemic therapy in two studies.69,75 The overall efficacy of acitretin in the studies was varied, and between 33% and 93.3% of the patients achieved PASI75. However, only a small number of studies were included, and most of these were retrospective reviews.
Cyclosporin
Cyclosporin was examined in nine studies (191 patients aged 11 months to 17 years) in doses 1 to 7.5 mg/kg daily.68,69,77–83 The patients had primarily plaque psoriasis and pustular type psoriasis, and most of the included studies were case series. The only clinical trial was by Ergun et al68 and was described earlier. In the retrospective review by Charbit et al69 44% of the patients that received cyclosporin achieved PASI75 at week 12. When compared to the other systemic treatments examined in the study, cyclosporin was the treatment with the highest rate of severe adverse events, requiring treatment termination in 20% of the patients. Topical treatment and treatment with coal tar were allowed in the study. In the retrospective cohort study by Di Lernia et al77 39.4% achieved PASI75 at week 12, and 39.5% of the patients were nonresponders. All patients experienced mucocutaneous adverse effects. Increased serum creatinine was observed in one patient in the study by Ergun et al.68 The included case series generally had few participants, and some of the studies did not use objective psoriasis severity measurements. Overall, the included studies reported varying efficacy (Table 2).
Fumaric Acid Esters
The effect of fumaric acid esters was examined in three small studies (34 patients aged 6 to 17) in doses 180 to 1200 mg daily.84–86 In the retrospective review by Steinz et al84 PASI75 was achieved in all six patients after 12 weeks while 50% achieved PASI100. Topical treatment with corticosteroids or vitamin D analogs was allowed during the treatment period. Eighty percent had a temporary reduction of lymphocytes, which reversed after the treatment was stopped. In the case series by van Geel et al85 14 patients were treated with dimethyl fumarate. 64.3% achieved PASI75 while 45.5% had reversible lymphocytopenia, whereas one patient discontinued due to persistent low lymphocyte counts. Five of the children included in the case series by van Geel et al85 were also included in the case series by Balak et al.86 In this study, five patients discontinued treatment due to insufficient clinical response. All studies reported several adverse events though only one led to discontinuation. All three studies included a small number of participants.
Apremilast
The only study that reported on the efficacy of apremilast was a clinical trial by Paller et al (42 patients aged 6 to 17).87 The participants were divided into three study groups consisting of children and adolescents with moderate to severe plaque psoriasis. The groups were based on age and weight-based doses. The best efficacy was reported in the group that consisted of children aged 6 to 11 years who were dosed 0.4 mg/kg twice daily for 2 weeks, followed by a 48-week extension. In this group, the patients achieved a 79% PASI reduction. PASI reduction in the two other groups was 69.6% and 66.5% in adolescents aged 12 to 17 years receiving 0.4 and 0.8 mg/kg twice daily, respectively. A comparison analysis of the PASI results was not performed. Ninety-five percent of all the participants experienced treatment-related adverse events, and two patients discontinued due to eosinophilia and headache. According to clinicaltrials.gov, apremilast is currently going through a Phase 3 trial for use in children and adolescents.
Adalimumab
The tumor necrosis factor (TNF) inhibitor adalimumab was examined in three studies (119 patients aged 4 to 18 years).66,72,74 Results from the randomized clinical trial by Papp et al66 and the follow-up study by Thaci et al74 on MTX and adalimumab, as well as the retrospective study by Bronckers et al70 on MTX and biological treatments, were described earlier. In the case series by Klufas et al72 patients were treated with adalimumab every other week. The patients achieved temporary PGA reduction from 2.4 to 0.7 after five to seven months, which increased to 2.0 after one year. Three patients did not respond to treatment. We did not search on etanercept, ustekinumab, and ixekizumab, since these studies are both approved by EMA and FDA (Table 1). We did not find any relevant studies when searching on the unapproved biological treatments secukinumab (unapproved by FDA), risankizumab, guselkumab, certolizumab pegol, tildrakizumab, and brodalumab. In our search, we only found case reports that examined the safety and efficacy of these unapproved biological treatments. However, all of the treatments are currently being tested for use in children and adolescents with psoriasis, according to clinicaltrials.gov.88–93 Our search did not include treatment with Janus kinase (JAK) inhibitors such as tofacitinib, since these treatments are not yet registered for use in adults with psoriasis, and they are not suggested as treatment options for pediatric psoriasis in any of the available guidelines.31,32
Discussion
Patients with psoriasis require treatment regardless of their age. Only a few of the current treatment options are approved for use in children and adolescents. For the most part, data on treatment efficacy and safety derive from studies in adults, and off-label treatment is therefore normally used in pediatric populations. Despite this, there is a general shortage of data concerning off-label treatments for psoriasis in children and adolescents. More than half of the included studies were case series or retrospective reviews, and most of these had few participants. Of the 23 clinical trials included, only four were randomized. Regarding treatments such as topical corticosteroids and topical calcineurin inhibitors, the literature was limited to clinical trials with few participants and a retrospective review with no supplementary supporting evidence (Table 2). In contrast, there is plenty of data available on the safety and efficacy of topical corticosteroids and topical calcineurin inhibitors for atopic dermatitis in children and adolescents.94
Data related to pediatric use of unapproved treatments derive from studies that include patients with other diseases or, which applies to the majority, from studies in adults with psoriasis. Therefore, the choice of treatment for psoriasis is generally based on the consideration that children and adolescents with psoriasis are small adults and only to a lesser extent on evidence-based literature.
Topical treatments are widely considered for localized disease and play a key role in the treatment of psoriasis in children and adolescents.21,31 Of these, a combination therapy consisting of vitamin D analogs and betamethasone dipropionate is increasingly being preferred as first-line therapy due to the synergistic, complementary effectiveness.95 While this combination therapy is approved by FDA in adolescents aged 12 years for use on the body and scalp, it is unapproved by the EMA. In this review, a total of ten studies were available on the treatment with vitamin D analogs, topical corticosteroids, and fixed combination therapy. None of these studies examined long-term efficacy and safety except for one clinical trial with 12 included patients.44 Despite lacking approval, there are currently data showing a good effect of a fixed combination of vitamin D analogs and betamethasone dipropionate in adult populations.96
In regard to nonbiological systemic therapy, there is a paucity of EMA- and FDA-approved systemic therapies. MTX is the most commonly used systemic medicine as first-line therapy for moderate to severe psoriasis in children and adolescents.32,97 Still, only one randomized clinical trial investigated the efficacy and safety of MTX as a control to adalimumab in the pediatric population.66
At this point, the EMA has approved five biological therapies while FDA has approved three for pediatric psoriasis (Table 1). In this review of off-label treatments, the only literature on a specific biological treatment was on the use of adalimumab, which is currently considered off-label by FDA. Papp et al66 compared the safety and efficacy of adalimumab and MTX, which was the only randomized clinical trial that compared a TNF inhibitor versus MTX. Adalimumab had greater efficacy compared to MTX, while the safety profiles were similar.
Bronckers et al70 also compared MTX to biological treatment, but in this study, the patients were treated with different biologics, and the study was a retrospective review with data lacking data on specific doses and routes of administration for MTX. A multicenter review that was also performed by Bronckers et al97 reported more frequent medication-related adverse events with MTX compared to TNF inhibitors. Currently, more biological treatments are being tested for approved use in children and adolescents with psoriasis, which may result in more treatment options with greater efficacy and safety compared to the current options. Even so, children and adolescents with psoriasis have a long median switching time from topical to systemic treatment.96 The clinician should not be too reluctant to change from topical to systemic treatment when the treatment effect is absent, even though there is a lack of data on systemic treatments in children and adolescents. Studies in adults have indicated that TNF inhibitors and MTX may carry the potential to inhibit the development of cardiovascular comorbidities.98,99 Whether early intensive treatment in new-onset psoriasis can modify the long-term natural course of the disease is still under debate.100
There is a need for future studies to investigate the efficacy and safety of unapproved treatments since they constitute the foundation of current treatment options. In clinical practice, topical corticosteroids alone or in combination with vitamin D analogs have shown efficacy. Since the main group of children and adults with psoriasis have mild disease, more data, especially on the efficacy and longtime safety in children, is needed for the topical treatments. MTX administration with either tablets or subcutaneous injections is also well tolerated in children and has shown good efficacy in adults. Still, there are cases where the effect of systemic treatment is absent, and regular clinical trials may be necessary to examine the actual effect of current traditionally used systemic treatments.
Conclusion
The current treatment strategy for children and adults with psoriasis is almost the same. Treatment options for psoriasis in children and adolescents are mostly off-label, with little available data on efficacy and safety. In clinical practice, off-label treatment with topical vitamin D analogs combined with betamethasone dipropionate for mild located psoriasis and systemic therapy with MTX for moderate to severe psoriasis show good effects. New drugs, particularly biological treatments developed by pharmaceutical companies, are required to undergo clinical trials in a pediatric population, and consequently, more approved treatments will be tested for pediatric psoriasis. Still, most children and adolescents have mild psoriasis, and there is a vast need for clinical trials investigating the efficacy and long-term safety of topical treatments as well as currently used systemic treatments such as MTX.
Acknowledgments
We thank the two patients and their respective parents for allowing us to present their cases. Written informed consent to share and publish the case details and photographs in this paper was granted by the parents.
Disclosure
Morten B. Haulrig has received an honorarium as a consultant for Novartis. Lone Skov has been an advisor, investigator, and speaker for Abbvie, Eli Lilly, Novartis, Sanofi, Celgene, Leo pharma, BMS, UCB, and Almirall, outside the submitted work. Lone Skov reports nonfinancial support from Abbvie, Sanofi, Janssen, and grants from Novartis, Janssen, BMS, and Sanofi. Claus Zachariae has been a scientific consultant, advisor, investigator, and speaker for Eli Lilly, Jansen Cilag, Novartis, Abbvie, Takeda, Amgen, Almirall, CSL, UCB, Regeneron, MSD, and Leo Pharma.
References
1. Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol. 2017;31(2):205–212. doi:10.1111/jdv.13854
2. Augustin M, Glaeske G, Radtke MA, Christophers E, Reich K, Schäfer I. Epidemiology and comorbidity of psoriasis in children. Br J Dermatol. 2010;162(3):633–636. doi:10.1111/j.1365-2133.2009.09593.x
3. Parisi R, Symmons DPM, Griffiths CEM, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385. doi:10.1038/jid.2012.339
4. Christophers E. Psoriasis - epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26(4):314–320. doi:10.1046/j.1365-2230.2001.00832.x
5. Raychaudhuri SP, Gross J. A comparative study of pediatric onset psoriasis with adult onset psoriasis. Pediatr Dermatol. 2000;17(3):174–178. doi:10.1046/j.1525-1470.2000.01746.x
6. Tollefson MM, Crowson CS, McEvoy MT, Maradit Kremers H. Incidence of psoriasis in children: a population-based study. J Am Acad Dermatol. 2010;62(6):979–987. doi:10.1016/j.jaad.2009.07.029
7. Henseler T, Christophers E. Psoriasis of early and late onset: characterization of two types of psoriasis vulgaris. J Am Acad Dermatol. 1985;13(3):450–456. doi:10.1016/S0190-9622(85)70188-0
8. Fotiadou C, Lazaridou E, Ioannides D. Management of psoriasis in adolescence. Adolesc Health Med Ther. 2014;5:25–34. doi:10.2147/AHMT.S36672
9. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509. doi:10.1056/NEJMra0804595
10. Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386(9997):983–994. doi:10.1016/S0140-6736(14)61909-7
11. Farber EM, Nall ML. The natural history of psoriasis in 5,600 patients. Dermatologica. 1974;148(1):1–18. doi:10.1159/000251595
12. Lønnberg AS, Skov L, Skytthe A, Kyvik KO, Pedersen OB, Thomsen SF. Heritability of psoriasis in a large twin sample. Br J Dermatol. 2013;169(2):412–416. doi:10.1111/bjd.12375
13. Strange A, Capon F, Spencer CCA, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42(11):985–990. doi:10.1038/ng.694
14. Nikamo P, Cheuk S, Lysell J, et al. Genetic variants of the IL22 promoter associate to onset of psoriasis before puberty and increased IL-22 production in T cells. J Invest Dermatol. 2014;134(6):1535–1541. doi:10.1038/jid.2014.5
15. Lysell J, Tessma M, Nikamo P, Wahlgren C-F, Ståhle M. Clinical characterisation at onset of childhood psoriasis - a cross sectional study in Sweden. Acta Derm Venereol. 2015;95(4):457–461. doi:10.2340/00015555-1986
16. Blegvad C, Nybo Andersen A-M, Groot J, Zachariae C, Barker J, Skov L. Clinical characteristics including cardiovascular and metabolic risk factors in adolescents with psoriasis. J Eur Acad Dermatol. 2020;34(7):1516–1523. doi:10.1111/jdv.16229
17. Mahé E. Childhood psoriasis. Eur J Dermatol. 2016;26(6):537–548. doi:10.1684/ejd.2016.2932
18. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583):263–271. doi:10.1016/S0140-6736(07)61128-3
19. Kouwenhoven TA, Bronckers IMGJ, van de Kerkhof PCM, Kamsteeg M, Seyger MMB. Psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children. J Eur Acad Dermatol. 2019;33(2):e74–e76. doi:10.1111/jdv.15213
20. Burden-Teh E, Thomas KS, Gran S, Murphy R. Development of clinical diagnostic criteria for plaque psoriasis in children: an electronic Delphi consensus study with the international psoriasis council. Br J Dermatol. 2019;181(4):856–857. doi:10.1111/bjd.17994
21. Eisert L, Augustin M, Bach S, et al. S2k guidelines for the treatment of psoriasis in children and adolescents – short version part 1. J Dtsch Dermatol Ges. 2019;17(8):856–870. doi:10.1111/ddg.13907
22. Morris A, Rogers M, Fischer G, Williams K. Childhood psoriasis: a clinical review of 1262 cases. Pediatr Dermatol. 2001;18(3):188–198. doi:10.1046/j.1525-1470.2001.018003188.x
23. Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251–265.e19. doi:10.1016/j.jaad.2018.06.027
24. Blegvad C, Egeberg A, Tind Nielsen TE, et al. Autoimmune disease in children and adolescents with psoriasis: a cross-sectional study in Denmark. Acta Derm Venereol. 2017;97(10):1225–1229. doi:10.2340/00015555-2743
25. Kimball AB, Wu EQ, Guérin A, et al. Risks of developing psychiatric disorders in pediatric patients with psoriasis. J Am Acad Dermatol. 2012;67(4):651–657.e2. doi:10.1016/j.jaad.2011.11.948
26. Todberg T, Egeberg A, Jensen P, Gislason G, Skov L. Psychiatric comorbidities in children and adolescents with psoriasis: a population-based cohort study. Br J Dermatol. 2017;177(2):551–553. doi:10.1111/bjd.15095
27. Groot J, Nybo Andersen AM, Blegvad C, Pinot de Moira A, Skov L. Prenatal, infantile, and childhood tobacco exposure and risk of pediatric psoriasis in the Danish National Birth Cohort offspring. J Am Acad Dermatol. 2020;1–8. doi:10.1016/j.jaad.2019.09.038
28. Randa H, Todberg T, Skov L, Larsen LS, Zachariae R. Health-related quality of life in children and adolescents with psoriasis: a systematic review and meta-analysis. Acta Derm Venereol. 2017;97(5):555–563. doi:10.2340/00015555-2600
29. Rapp SR, Feldman SR, Exum ML, Fleischer AB, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3):401–407. doi:10.1016/S0190-9622(99)70112-X
30. De Jager MEA, De Jong EMGJ, Evers AWM, Van De Kerkhof PCM, Seyger MMB. The burden of childhood psoriasis. Pediatr Dermatol. 2011;28(6):736–737. doi:10.1111/j.1525-1470.2011.01489.x
31. Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National psoriasis foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82(1):161–201. doi:10.1016/j.jaad.2019.08.049
32. Eisert L, Augustin M, Bach S, et al. S2k guidelines for the treatment of psoriasis in children and adolescents – short version part 2. J Dtsch Dermatol Ges. 2019;17(8):856–870. doi:10.1111/ddg.13907
33. Van Geel MJ, Mul K, De Jager MEA, Van De Kerkhof PCM, De Jong EMGJ, Seyger MMB. Systemic treatments in paediatric psoriasis: a systematic evidence-based update. J Eur Acad Dermatol Venereol. 2015;29(3):425–437. doi:10.1111/jdv.12749
34. Napolitano M, Megna M, Balato A, et al. Systemic treatment of pediatric psoriasis: a review. Dermatol Ther (Heidelb). 2016;6(2):125–142. doi:10.1007/s13555-016-0117-6
35. Europa EMA. Paediatric requirements marketing authorisation applications. Availabe from: https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/paediatric-medicines/paediatric-requirements-marketing-authorisation-applications.
36. Kimball AB, Gold MH, Zib B, Davis MW. Clobetasol propionate emulsion formulation foam 0.05%: review of Phase II open-label and Phase III randomized controlled trials in steroid-responsive dermatoses in adults and adolescents. J Am Acad Dermatol. 2008;59(3):448–455. doi:10.1016/j.jaad.2008.04.020
37. Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized plaque psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25(6):1166–1169. doi:10.1016/0190-9622(91)70319-W
38. Brune A, Miller DW, Lin P, Cotrim-Russi D, Paller AS. Tacrolimus ointment is effective for psoriasis on the face and intertriginous areas in pediatric patients. Pediatr Dermatol. 2007;24(1):76–80. doi:10.1111/j.1525-1470.2007.00341.x
39. Steele JA, Choi C, Kwong PC. Topical tacrolimus in the treatment of inverse psoriasis in children. J Am Acad Dermatol. 2005;53(4):713–716. doi:10.1016/j.jaad.2005.05.036
40. Oostveen AM, Beulens CA, Van De Kerkhof PCM, De Jong EMGJ, Seyger MMB. The effectiveness and safety of short-contact dithranol therapy in paediatric psoriasis: a prospective comparison of regular day care and day care with telemedicine. Br J Dermatol. 2014;170(2):454–457. doi:10.1111/bjd.12621
41. De Jager MEA, Van De Kerkhof PCM, De Jong EMGJ, Seyger MMB. Dithranol therapy in childhood psoriasis: unjustifiably on the verge of falling into oblivion. Dermatology. 2010;220(4):329–332. doi:10.1159/000278241
42. Oranje AP, Marcoux D, Svensson A, et al. Topical calcipotriol in childhood psoriasis. J Am Acad Dermatol. 1997;36(2):203–208. doi:10.1016/S0190-9622(97)70281-0
43. Saggese G, Federico G, Battini R. Topical application of 1,25-dihydroxyvitamin D3 (calcitriol) is an effective and reliable therapy to cure skin lesions in psoriatic children. Eur J Pediatr. 1993;152(5):389–392. doi:10.1007/BF01955893
44. Park SB, Suh DH, Youn JI. A pilot study to assess the safety and efficacy of topical calcipotriol treatment in childhood psoriasis. Pediatr Dermatol. 1999;16(4):321–325. doi:10.1046/j.1525-1470.1999.00084.x
45. Darley CR, Cunliffe WJ, Green CM, Hutchinson PE, Klaber MR, Downes N. Safety and efficacy of calcipotriol ointment (Dovonex) in treating children with psoriasis vulgaris. Br J Dermatol. 1996;135(3):390–393. doi:10.1046/j.1365-2133.1996.d01-1010.x
46. Gooderham M, Debarre JM, Keddy-Grant J, Xu Z, Kurvits M, Goodfield M. Safety and efficacy of calcipotriol plus betamethasone dipropionate gel in the treatment of scalp psoriasis in adolescents 12–17 years of age. Br J Dermatol. 2014;171(6):1470–1477. doi:10.1111/bjd.13235
47. van Geel MJ, Mul K, Oostveen AM, van de Kerkhof PCM, de Jong EMGJ, Seyger MMB. Calcipotriol/betamethasone dipropionate ointment in mild-to-moderate paediatric psoriasis: long-term daily clinical practice data in a prospective cohort. Br J Dermatol. 2014;171(2):363–369. doi:10.1111/bjd.12895
48. Oostveen AM, De Jong EMGJ, Donders ART, Van De Kerkhof PCM, Seyger MMB. Treatment of paediatric scalp psoriasis with calcipotriene/betamethasone dipropionate scalp formulation: effectiveness, safety and influence on children’s quality of life in daily practice. J Eur Acad Dermatol Venereol. 2015;29(6):1193–1197. doi:10.1111/jdv.12789
49. Eichenfield LF, Ganslandt C, Kurvits M, Schlessinger J. Safety and efficacy of calcipotriene plus betamethasone dipropionate topical suspension in the treatment of extensive scalp psoriasis in adolescents ages 12 to 17 years. Pediatr Dermatol. 2015;32(1):28–35. doi:10.1111/pde.12429
50. Borska L, Andrys C, Krejsek J, et al. Oxidative damage to nucleic acids and benzo(a)pyrene-7,8-diol-9,10-epoxide-DNA adducts and chromosomal aberration in children with psoriasis repeatedly exposed to crude coal tar ointment and UV radiation. Oxid Med Cell Longev. 2014;2014:1–10. doi:10.1155/2014/302528
51. Borska L, Fiala Z, Krejsek J, et al. Immunologic changes in TNF-alpha, sE-selectin, sP-selectin, sICAM-1, and IL-8 in pediatric patients treated for psoriasis with the Goeckerman regimen. Pediatr Dermatol. 2007;24(6):607–612. doi:10.1111/j.1525-1470.2007.00548.x
52. Borska L, Smejkalova J, Cerna M, et al. Urinary mutagenicity and genotoxic risk in children with psoriasis after therapeutic exposure to polycyclic aromatic hydrocarbons and ultraviolet radiation. Mutat Res Genet Toxicol Environ Mutagen. 2010;696(2):144–147. doi:10.1016/j.mrgentox.2010.01.003
53. Kortuem KR, Davis MDP, Witman PM, McEvoy MT, Farmer SA. Results of Goeckerman treatment for psoriasis in children: a 21-year retrospective review. Pediatr Dermatol. 2010;27(5):518–524. doi:10.1111/j.1525-1470.2010.01124.x
54. Jain VK, Aggarwal K, Jain K, Bansal A. Narrow-band UV-B phototherapy in childhood psoriasis. Int J Dermatol. 2007;46(3):320–322. doi:10.1111/j.1365-4632.2007.03148.x
55. Jain VK, Bansal A, Aggarwal K, Jain K. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25(5):559–564. doi:10.1111/j.1525-1470.2008.00729.x
56. Tan E, Lim D, Rademaker M. Narrowband UVB phototherapy in children: a New Zealand experience. Australas J Dermatol. 2010;51(4):268–273. doi:10.1111/j.1440-0960.2010.00701.x
57. Zamberk P, Velázquez D, Campos M, Hernanz JM, Lázaro P. Paediatric psoriasis - narrowband UVB treatment. J Eur Acad Dermatol Venereol. 2010;24(4):415–419. doi:10.1111/j.1468-3083.2009.03425.x
58. Pašić A, Čeović R, Lipozenčić J, et al. Phototherapy in pediatric patients. Pediatr Dermatol. 2003;20(1):71–77. doi:10.1046/j.1525-1470.2003.03016.x
59. Pavlovsky M, Baum S, Shpiro D, Pavlovsky L, Pavlotsky F. Narrow band UVB: is it effective and safe for paediatric psoriasis and atopic dermatitis? J Eur Acad Dermatol Venereol. 2011;25(6):727–729. doi:10.1111/j.1468-3083.2010.03832.x
60. Eustace K, Dolman S, Alsharqi A, Sharpe G, Parslew R. Use of phototherapy in children. Pediatr Dermatol. 2017;34(2):150–155. doi:10.1111/pde.13072
61. Sen BB, Rifaioglu EN, Ekiz O, Sen T, Celik E, Dogramaci AC. Narrow-band ultraviolet B phototherapy in childhood. Cutan Ocul Toxicol. 2014;33(3):189–191. doi:10.3109/15569527.2013.832281
62. Ersoy-Evans S, Altaykan A, Şahin S, Kölemen F. Phototherapy in childhood. Pediatr Dermatol. 2008;25(6):599–605. doi:10.1111/j.1525-1470.2008.00773.x
63. Wong Y, Koh MJA, Chong WS. Role of narrowband ultraviolet B phototherapy in the treatment of childhood psoriasis in asian children. Pediatr Dermatol. 2015;32(5):e221–e223. doi:10.1111/pde.12632
64. Jury CS, McHenry P, Burden AD, Lever R, Bilsland D. Narrowband ultraviolet B (UVB) phototherapy in children. Clin Exp Dermatol. 2006;31(2):196–199. doi:10.1111/j.1365-2230.2006.02061.x
65. Kim E, Lee G, Fischer G. Use of narrowband ultraviolet B (NBUVB) in paediatric psoriasis: a systematic literature review and meta-analysis. Australas J Dermatol. 2020. doi:10.1111/ajd.13471
66. Papp K, Thaçi D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017;390(10089):40–49. doi:10.1016/S0140-6736(17)31189-3
67. Van Geel MJ, Oostveen AM, Hoppenreijs EPAH, et al. Methotrexate in pediatric plaque-type psoriasis: long-term daily clinical practice results from the Child-CAPTURE registry. J Dermatolog Treat. 2015;26(5):406–412. doi:10.3109/09546634.2014.996515
68. Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44(6):630–634. doi:10.1111/1346-8138.13713
69. Charbit L, Mahé E, Phan A, et al. Systemic treatments in childhood psoriasis: a French multicentre study on 154 children. Br J Dermatol. 2016;174(5):1118–1121. doi:10.1111/bjd.14326
70. Bronckers IMGJ, Paller AS, West DP, et al. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol. 2020;156(4):384–392. doi:10.1001/jamadermatol.2019.4835
71. Kaur I, Dogra S, De D, Kanwar AJ. Systemic methotrexate treatment in childhood psoriasis: further experience in 24 children from India. Pediatr Dermatol. 2008;25(2):184–188. doi:10.1111/j.1525-1470.2008.00629.x
72. Klufas DM, Wald JM, Strober BE. Treatment of moderate to severe pediatric psoriasis: a retrospective case series. Pediatr Dermatol. 2016;33(2):142–149. doi:10.1111/pde.12782
73. Collin B, Vani A, Ogboli M, Moss C. Methotrexate treatment in 13 children with severe plaque psoriasis. Clin Exp Dermatol. 2009;34(3):295–298. doi:10.1111/j.1365-2230.2008.02907.x
74. Thaçi D, Papp K, Marcoux D, et al. Sustained long-term efficacy and safety of adalimumab in paediatric patients with severe chronic plaque psoriasis from a randomized, double-blind, phase III study. Br J Dermatol. 2019;181(6):1177–1189. doi:10.1111/bjd.18029
75. Di Lernia V, Bonamonte D, Lasagni C, et al. Effectiveness and safety of acitretin in children with plaque psoriasis: a multicenter retrospective analysis. Pediatr Dermatol. 2016;33(5):530–535. doi:10.1111/pde.12940
76. Chen P, Li C, Xue R, et al. Efficacy and safety of acitretin monotherapy in children with pustular psoriasis: results from 15 cases and a literature review. J Dermatolog Treat. 2018;29(4):353–363. doi:10.1080/09546634.2017.1395798
77. Di Lernia V, Stingeni L, Boccaletti V, et al. Effectiveness and safety of cyclosporine in pediatric plaque psoriasis: a multicentric retrospective analysis. J Dermatolog Treat. 2016;27(5):395–398. doi:10.3109/09546634.2015.1120852
78. Bulbul Baskan E, Yazici S, Tunali S, Saricaoglu H. Clinical experience with systemic cyclosporine A treatment in severe childhood psoriasis. J Dermatolog Treat. 2016;27(4):328–331. doi:10.3109/09546634.2015.1115813
79. Dogra S, Mahajan R, Narang T, Handa S. Systemic cyclosporine treatment in severe childhood psoriasis: a retrospective chart review. J Dermatolog Treat. 2017;28(1):18–20. doi:10.3109/09546634.2015.1034072
80. Šebnem Kiliç S, Hacimustafaoglu M, Çelebi S, Karadeniz A, Ildirim I. Low dose cyclosporin A treatment in generalized pustular psoriasis. Pediatr Dermatol. 2001;18(3):246–248. doi:10.1046/j.1525-1470.2001.018003246.x
81. Pereira TM, Vieira AP, Fernandes JC, Sousa-Basto A. Cyclosporin A treatment in severe childhood psoriasis. J Eur Acad Dermatol. 2006;20(6):651–656. doi:10.1111/j.1468-3083.2006.01562.x
82. Mahé EB. Cyclosporine in childhood psoriasis. Arch Dermatol. 2002;138(January2001):2001–2002.
83. Perrett CM, Ilchyshyn A, Berth-Jones J. Cyclosporin in childhood psoriasis. J Dermatolog Treat. 2003;14(2):113–118. doi:10.1080/09546630310012136
84. Steinz K, Gerdes S, Domm S, Mrowietz U. Systemic treatment with fumaric acid esters in six paediatric patients with psoriasis in a psoriasis centre. Dermatology. 2014;229(3):199–204. doi:10.1159/000363103
85. Van Geel MJ, Van De Kerkhof PCM, Oostveen AM, De Jong EMGJ, Seyger MMB. Fumaric acid esters in recalcitrant pediatric psoriasis: a prospective, daily clinical practice case series. J Dermatolog Treat. 2016;27(3):214–220. doi:10.3109/09546634.2015.1088131
86. Balak DMW, Oostveen AM, Bousema MT, et al. Effectiveness and safety of fumaric acid esters in children with psoriasis: a retrospective analysis of 14 patients from the Netherlands. Br J Dermatol. 2013;168(6):1343–1347. doi:10.1111/bjd.12231
87. Paller AS, Hong Y, Becker EM, et al. Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a Phase 2 open-label study. J Am Acad Dermatol. 2020;82(2):389–397. doi:10.1016/j.jaad.2019.08.019
88. Pharmaceuticals N. Study to assess the long-term safety, tolerability, efficacy of Secukinumab in pediatric patients of age 6 to <18 years, with moderate to severe plaque psoriasis. Available from: Https://clinicaltrials.gov/ct2/show/NCT03668613. NLM identifier: NCT03668613.
89. AbbVie. A study of subcutaneous risankizumab injection for pediatric participants with moderate to severe plaque psoriasis to assess change in disease symptoms. Available from: Https://clinicaltrials.gov/ct2/show/NCT04435600. NLM identifier: NCT04435600.
90. Janssen Research & Development, LLC. A study to evaluate the efficacy, safety, and pharmacokinetics of subcutaneously administered guselkumab for the treatment of chronic plaque psoriasis in pediatric participants (PROTOSTAR). Available from: Https://clinicaltrials.gov/ct2/show/NCT03451851. NLM identifier: NCT03451851.
91. UCB Biopharma S.P.R.L. A study to evaluate the efficacy, safety, and drug concentration of Certolizumab Pegol (CZP) in children and adolescent study participants with moderate to severe chronic plaque psoriasis (PSO) (CIMcare). Available from: Https://clinicaltrials.gov/ct2/show/NCT04123795. NLM identifier: NCT04123795.
92. Sun Pharma Global FZE. A study of tildrakizumab in pediatric subjects with chronic plaque psoriasis. Available from: https://clinicaltrials.gov/ct2/show/NCT03997786. NLM identifier: NCT03997786.
93. Bausch Health Americas, Inc. An open-label, single-dose study to evaluate safety, tolerability, and pharmacokinetics of brodalumab in pediatric subjects. Available from: Https://clinicaltrials.gov/ct2/show/NCT03240809. NLM identifier: NCT03240809.
94. Siegfried EC, Jaworski JC, Kaiser JD, Hebert AA. Systematic review of published trials: long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatr. 2016;16(1):75. doi:10.1186/s12887-016-0607-9
95. Oquendo M, Abramovits W, Morrell P. Topical vitamin D analogs available to treat psoriasis. Skinmed. 2012;10(6):356–360.
96. Pink AE, Jalili A, Berg P, et al. Rapid onset of action of calcipotriol/betamethasone dipropionate cutaneous foam in psoriasis, even in patients with more severe disease. J Eur Acad Dermatol Venereol. 2019;33(6):1116–1123. doi:10.1111/jdv.15398
97. Bronckers IMGJ, Seyger MMB, West DP, et al. Safety of systemic agents for the treatment of pediatric psoriasis. JAMA Dermatol. 2017;153(11):1147–1157. doi:10.1001/jamadermatol.2017.3029
98. Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J Eur Acad Dermatol Venereol. 2015;29(6):1128–1134. doi:10.1111/jdv.12768
99. Prodanovich S, Ma F, Taylor JR, Pezon C, Fasihi T, Kirsner RS. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52(2):262–267. doi:10.1016/j.jaad.2004.06.017
100. Iversen L, Eidsmo L, Austad J, et al. Secukinumab treatment in new-onset psoriasis: aiming to understand the potential for disease modification - rationale and design of the randomized, multicenter STEPIn study. J Eur Acad Dermatol Venereol. 2018;32(11):1930–1939. doi:10.1111/jdv.14979
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

