Back to Journals » Nutrition and Dietary Supplements » Volume 16
Observational Study to Evaluate Efficacy and Safety of Vidaslim®, a Medical Nutrition Therapy, for Weight Loss in Subjects with Obesity
Authors Kalra S, Kapoor N , Joseph J, Arun A, Bhattacharyya S, Dalai SP
Received 6 October 2023
Accepted for publication 23 January 2024
Published 5 March 2024 Volume 2024:16 Pages 27—35
DOI https://doi.org/10.2147/NDS.S432746
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Sanjay Kalra,1 Nitin Kapoor,2 Jeevan Joseph,3 Abhishek Arun,4 Supratik Bhattacharyya,5 Siba Prasad Dalai6
1Endocrinology Department, Bharti Hospital, Karnal, Haryana, India; 2Endocrinology Department, Christian Medical College, Vellore, Tamil Nadu, India; 3Endocrinology Department, Vimala Hospital, Ettumanoor, Kerala, India; 4Endocrinology Department, Vishudh Diabetes Clinic, Lucknow, Uttar Pradesh, India; 5Endocrinology Department, Apollo Sugar Clinic, Kolkata, West Bengal, India; 6Endocrinology Department, IMS & SUM Hospital, Bhubaneshwar, Odisha, India
Correspondence: Sanjay Kalra, Bharti Hospital, BRIDE Kunjpura Road, Model Town, Near State Bank of India, Sector 12, Karnal, Haryana, 132001, India, Tel +91 98960 48555, Fax +91 124-407 8652, Email [email protected]
Purpose: The study aimed to evaluate the effect of Vidaslim®, meal replacement on weight, body mass index (BMI), waist circumference (WC), hip circumference (HC), and other cardiometabolic parameters and to see the impact of timing of Vidaslim meal replacement on the level of weight loss achieved.
Methods: In a prospective, observational study, male and female subjects with obesity based on inclusion and exclusion criteria were administered Vidaslim (60 g in 200 mL water) once daily as a meal replacement (lunch or dinner) for 16 weeks.
Results: A total of 107 subjects (mean age 34.09± 8.26 years) were included in the study. There was a significant reduction in weight by 13.4 kg (p=0.0001), BMI by 5.5 kg/m2 (p=0.00001), WC by 3.7 inches (p=0.0001), and HC by 4.5 inches (p=0.0001) at the end of 16 weeks. Statistically significant reductions were noted in heart rate, fasting blood glucose, postprandial blood glucose, lipid profile (p=0.0001 for all), and systolic blood pressure (p=0.002) at 16 weeks. A significant change in hunger (p=0.0001), appetite (p=0.014), mood (p=0.021), sleep quality (p=0.0001), and general well-being (p=0.013) was observed at the end of 16 weeks. Replacing lunch or dinner with Vidaslim resulted in a significant reduction in weight (− 9.8 kg versus − 14.3 kg, respectively; p=0.0001 for both) compared to replacing breakfast (− 3 kg, p=0.205) with Vidaslim over 16 weeks. No subject reported any side effects post–Vidaslim intervention.
Conclusion: The medical nutrition therapy, Vidaslim, was found to be an effective and safe meal (lunch or dinner)-replacement strategy and brought a significant reduction in weight, WC, and HC over 16 weeks in overweight and obese individuals.
Keywords: food supplement, overweight, anthropometric, cardiometabolic, meal replacement, blood glucose
Introduction
Obesity is a global health problem and has attained similar status in India.1 National Family Health Survey 4 data shows that 21% of women and 19% of men in India are overweight or obese.2 Obesity is linked to an increased risk of many chronic non-communicable diseases, such as diabetes and hypertension, and myocardial infarction.1,3 Compared to ideal-weight individuals, those with obesity are at 2.9 times increased risk of developing diabetes.1,3 Also, obesity can lead to sleep disturbances, anxiety, and depression.3 Hence, it is important to treat obesity and ensure that the weight lost is not regained.
Weight loss in overweight and obesity requires energy expenditure in excess of energy intake. This can be achieved through lifestyle interventions such as exercise and well-designed diet, medical nutrition therapy (MNT), and pharmacotherapy.1 Lifestyle interventions have their own challenges, as they are dependent on patient attitude. Pharmacotherapy is seldom effective alone, needs to be combined with lifestyle interventions, is expensive, and may cause adverse outcomes.4 Hence, pharmacotherapy is recommended and individualized only after weighing the pros and cons of therapy, and prescribed for maintaining weight loss achieved through lifestyle interventions.4,5 Physical activity and proper diet are the backbone of any guideline recommended for an obesity management program.1,5,6 The optimal weight-loss diet should be efficacious, safe, healthy, nutritionally adequate, affordable, and culturally acceptable, and should ensure long-term compliance and result in healthy weight loss.1,7 MNT is the cornerstone of obesity management.1,5 MNT supplements (MNSs) used as meal replacement are an accepted and effective weight-loss strategy.8 Since obesity is linked to diabetes and cardiovascular diseases, MNSs that lower the risk of these conditions would be ideal for weight loss.
Many MNSs are available in the market to aid in weight loss. However, a majority have no proven efficacy or safety.9,10 MNSs rich in fiber, proteins,
Vidaslim® is a scientifically designed low-calorie, high-fiber, and protein-rich MNS (Box 1) for weight loss in overweight and obese adults. The quantity of the 38 key nutrients in the Vidaslim supplement match the recommended dietary allowance by the Indian Council of Medical Research. The nutrients help in increasing lean muscle mass, providing satiety, and sustained weight loss and body fat without causing any nutritional deficiencies. However, there are no human clinical studies evaluating the efficacy and safety of Vidaslim for weight loss or its effect on appetite and mood. Therefore, this study aimed to evaluate the efficacy and safety of Vidaslim in reducing weight in overweight and obese individuals, assess its impact on satiety and mood, and see if the timing of meal replacement with Vidaslim impacts the degree of weight loss over a 16-week period.
 |
Box 1 Signature nutrients in Vidaslim® |
Methods
This study was performed in compliance with the principles of the Declaration of Helsinki, the International Conference of Harmonization Guidelines for Good Clinical Practice, and applicable regulatory requirements. All participants provided written informed consent. The study was done with ethics permission from the Good Society for Ethical Research (GSER-BMR-2020-005). This study was also registered on the Clinical Trial Registry — India (2021/09/046987).
This was an observational, single-center, prospective interventional study that observed the effects of Vidaslim on weight, body mass index (BMI), waist circumference (WC), hip circumference (HC), satiety, mood, sleep, and general well-being, and whether the timing of meal replacement with Vidaslim affected the outcomes of interest. The study was conducted from September 15, 2021 to January 15, 2022, and both males and females were included.
The study enrolled 110 overweight and obese subjects aged 18–50 years with abdominal obesity defined as WC ≥102 cm and ≥88 cm in men and women, respectively, who were classified as overweight or obese according to their BMI: 25–29.9 kg/m2 (overweight) and ≥30 kg/m2 (obese). Subjects were excluded if they had any chronic metabolic disease (eg, type 2 diabetes [HbA1c >6.5]), polycystic ovary disease, or major systemic illness, had used lipid-lowering drugs in the previous 3 months, if obesity were related to any underlying medical condition (eg, type 2 diabetes, hypothyroidism, Cushing’s syndrome), if they were using any medications that were likely to influence their weight, and pregnant or lactating women.
The subjects were administered Vidaslim (60 g in 200 mL water) once daily as a meal replacement (lunch or dinner) for 16 weeks. Weight, BMI (height measured at baseline), anthropometric measures (WC, HC), heart rate, blood pressure, laboratory parameters, appetite, hunger, mood change, sleep, and general well-being was assessed at baseline and at week 8 after the start of Vidaslim supplementation. Laboratory investigation included monitoring of blood lipid profile (fasting) and plasma glucose level (fasting and postprandial). The fasting blood lipid profile included low-density lipoprotein, very low–density lipoprotein, triglycerides, and total cholesterol.
The primary outcomes were changes in weight, BMI, WC, HC, heart rate, blood pressure, and laboratory parameters over 16 weeks. The secondary outcomes assessed were mood, appetite, hunger, sleep, and general well-being over 16 weeks. Data were entered in Microsoft Excel and analyzed using descriptive statistical methods. Categorical data are represented as frequencies and percentages. One-group pretest–posttest measures were used. The X2 test was used to assess differences in categorical values. Quantitative data are given as means ± SD. Student’s t-test was used to assess differences in qualitative values. SPSS 26.0 was used for all statistical analysis, and p<0.05 was considered significant. Subjects’ responses to changes in appetite, hunger, sleep, mood, and general well-being were recorded on a five-point Likert scale (Box 2). Similar five-point Likert scales have been effectively used to assess appetite, hunger, sleep, mood, and general well-being in other publications.17,20–22
 |
Box 2 Likert values used for each parameter |
Results
A total of 107 subjects (mean age 34.09±8.26 years), 23 men and 84 women completed the study, with a dropout of 2.7%. The study flowchart is given in Figure 1. The baseline characteristics are given in Table 1. There was a significant reduction in weight by 13.38 kg (p=0.0001), BMI by 5.5 kg/m2 (p=0.00001), WC by 3.7 inches (p=0.0001), and HC by 4.5 inches (p=0.0001) at the end of 16 weeks. Statistically significant reductions were noted in heart rate, fasting blood glucose, postprandial blood glucose, lipid profile (p=0.0001 for all), and systolic blood pressure (p=0.002) at the end of 16 weeks. The decrease in diastolic blood pressure (p=0.195) was insignificant at the end of 16 weeks. Details of these findings are given in Table 2.
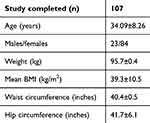 |
Table 1 Participant demography |
 |
Table 2 Baseline and 16-week parameters |
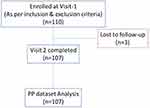 |
Figure 1 Study flowchart. |
Significant changes in hunger, appetite, mood, sleep quality, and general well-being were observed at 16 weeks. At baseline, 47.22% were hungry most of the time, but at the end of 16 weeks only 6.48% were hungry most of the time. No subject was hungry all the time at baseline or at 16 weeks (p=0.0001). “Very good” appetite was seen in 17.59% of subjects at baseline, while only 8.33% reported “very good” appetite at the end of 16 weeks (p=0.014). The overall feeling of well-being had also improved significantly at the end of 16 weeks, as no one was feeling “in very low spirits” (p=0.013). Mood improved significantly over the 16-week period (p=0.0001): 68.52% of the subjects were happy and 14.81% very happy at the end of 16 weeks in comparison to 27.78% and 0%, respectively, at baseline (p=0.021). The sleep quality of the study participants improved significantly: 21.30% of the subjects were experiencing “very good” sleep quality at the end of 16 weeks in comparison to 2.78% subjects at baseline (p=0.0001). The details of these findings are given in Figure 2. Replacing lunch or dinner with Vidaslim resulted in a significant reduction in weight (9.8 kg versus 14.3 kg, p=0.0001 for both) compared to replacing breakfast (3 kg, p=0.205) with Vidaslim over 16 weeks. However, replacing dinner with Vidaslim resulted in an additional weight loss of 4.5 kg compared to replacing lunch with Vidaslim. No subject reported any side effect due to Vidaslim.
 |
Figure 2 Change in appetite (A), hunger (B), mood (C), sleep (D) and general well-being (E) over 16 weeks. |
Discussion
Vidaslim is a nutrient-rich blend of proteins, dietary fibers, green tea extract, L-carnitine, and white kidney bean extract. This is the first human study to show that Vidaslim can be effectively and safely used for weight loss. Replacing one major meal (lunch or dinner) with 60 g of Vidaslim in 200 mL water had significantly reduced weight, improved anthropometric and cardiometabolic profile, mood, sleep, and general well-being, and reduced hunger and appetite at 16 weeks. The study also showed that though both lunch and dinner replacement with Vidaslim resulted in significant weight loss, replacing dinner with Vidaslim resulted in an additional weight loss of 4.5 kg compared to replacing lunch with Vidaslim. No adverse effects were reported during the study.
Even though this is the first human study demonstrating the efficacy and safety of Vidaslim for weight loss, the key ingredients of Vidaslim have been amply tested, even in Asian populations, and found to be an effective weight-loss strategy in human clinical trials. Individually, these ingredients also affect satiety, mood, sleep, or general well-being and help in reducing one or more cardiovascular risk factors. The protein blend in Vidaslim consists of whey, soy, and casein. Together, these proteins provide a balanced protein intake of highest quality (protein digestibility–corrected amino acid score of 1).23 High-quality evidence through randomized controlled trials (RCTs) and systemic reviews and meta-analyses show the benefits of using whey, soy, and casein as a weight-loss supplement.3,15,24
A meta-analysis of nine RCTs showed that whey protein supplementation in overweight and obese individuals resulted in significant reductions in body weight, lean mass, and fat mass compared to placebo, with pooled mean differences of 0.56, 0.77, and 1.12, respectively.15 Whey protein supplementation significantly improved cardiometabolic profile compared to placebo with respect to systolic blood pressure, diastolic blood pressure, blood glucose, high-density lipoprotein, and total cholesterol (p<0.05 for all).15
There are no meta-analyses studying the effect of casein alone on body weight. However, a meta-analysis of 29 RCTs (24 RCTs enrolled overweight and obese) evaluating the effect of supplementing dairy products as a weight-loss strategy found that dairy products aid in weight loss over short periods if combined with an energy-restricted diet.24 The results cannot be extrapolated for casein completely, as dairy products include protein (casein and whey), calcium, and other bioactive compounds. These components together are likely to favorably increase energy expenditure.24 Clinical data also indicate that whey results in short-term satiety and casein induces long-term satiety.25 Therefore, casein exerts a favorable effect in managing weight by providing long-term satiety, which subsequently reduces the overall daily calorie intake.
Another meta-analysis of 22 trials (870 overweight or obese Asians) showed that soy use resulted in significant reduction in body weight by 0.37 kg (p=0.010), BMI by 0.27 kg/m2 (p=0.042), body fat by 0.36% (p=0.032), and WC by 0.35 cm (p=0.049).3 The weight-loss benefit was reported to be due to the soy protein, isoflavone, and soy fiber in the soy products used in these trials. Soy protein, fiber, and isoflavone contribute towards weight loss by achieving early satiety, reducing fat accumulation by inhibiting fat production, and increasing fat oxidation by delaying carbohydrate absorption.3 The effect was more pronounced in overweight or obese non-menopausal women with significant reduction in body weight by 0.59 kg (p=0.041), BMI by 0.59 kg/m2 (p=0.041), and WC by 0.59 cm (p=0.041).3 This finding of more pronounced benefit in women is important, because 84 of 107 participants of this study were female.
Polydextrose is the soluble dietary fiber in Vidaslim. It belongs to the digestion-resistant dextrin group and helps achieve satiety, subsequently reducing overall daily calorie intake, which may help reduce body weight.17,26,27 Fermentation of dietary fibers by the gut microbiota releases short-chain fatty acids, which have been shown to increase energy expenditure and fat oxidation.28,29 Evidence shows that polydextrose inhibits energy intake, achieves early satiety, reduces gastrointestinal transit time and macronutrient absorption, thereby reducing glycemic and insulinemic responses, and increases energy and fat metabolism via short-chain fatty acids.29 A randomized crossover study comparing polydextrose supplementation versus maltodextrin showed that polydextrose achieved satiety and decreased hunger, prospective food consumption, and desire to eat (p<0.001 for all) compared to maltodextrin.30 Energy intake during a meal and blood sugar levels were significantly lower for the polydextrose group than for the maltodextrin group.30
A systematic review and meta-analysis of three RCTs (275 Asians with overweight and obesity; 8–12 weeks’ duration) showed that resistant dextrin supplementation significantly decreased BMI by 0.39 kg/m2 (p<0.01) and body weight by 0.81 kg (p<0.01)). An RCT comparing dextrin supplementation versus placebo in 100 otherwise healthy overweight adults in China showed dose-dependent increases in satiety (assessed on a visual analogue scale) and time to hunger (assessed on a Likert scale). Catechins in green tea extracts (epigallocatechin gallate; EGCG) have been reported to have antiobesity effects.18,31 An RCT showed that 12 weeks’ treatment with high-dose EGCG resulted in significant decreases in weight (p=0.025), BMI (p=0.018), and WC (p=0.023).18 There was a trend toward decreased low-density lipoprotein and total cholesterol.18 Long-term EGCG use is also associated with a reduction in cardiovascular risk factors.32
L-carnitine plays an important role in the transportation of fatty acids for its oxidation, and thus helps reduce obesity.14 A meta-analysis of nine studies showed that L-carnitine supplementation resulted in significantly higher weight loss and BMI decrease compared to controls, with a mean difference of 1.33 kg and 0.47 kg/m2, respectively.33 Another systematic review and meta-analysis of 37 RCTs (n=2292 with obesity) showed that L-carnitine supplementation significantly reduced body weight, BMI, and fat mass compared to controls, with weighted mean differences of 1.21 kg (p<0.001), 0.24 kg/m2 (p=0.001), and 2.08 kg (p=0.003), respectively. No significant association was seen for WC or body fat percentage.12 Another large systematic review and meta-analysis of 43 RCTs also showed that L-carnitine supplementation significantly reduced body weight, BMI, and fat mass compared to controls, with weighted mean differences of 1.129 kg, 0.359 kg/m2, and 1.158 kg, respectively.14 Similarly to the previously cited meta-analysis, this meta-analysis too failed to show any significant association between L-carnitine supplementation, WC, and body fat percentage.14 A subgroup analysis revealed that the antiobesity effects of L-carnitine supplementation were seen only in overweight and obese subjects.14 A systematic review and meta-analysis of 10 RCTs showed that L-carnitine supplementation significantly decreased diastolic blood pressure in overweight and obese participants by 1.232 mmHg (p=0.023), with a decrease of 1.639 mmHg achieved at doses <2 g/day (p=0.022).16
White kidney bean (Phaseolus vulgaris) extract has been shown to produce significant decreases in body fat while maintaining lean body mass.34 In an RCT of 60 overweight volunteers, a 30-day supplementation of Phaseolus vulgaris extract given along with a carbohydrate-rich 2000- to 2200-calorie diet resulted in significantly greater reductions in body weight, BMI, waist, hip, and thigh circumference, fat mass, and adipose tissue thickness compared to volunteers receiving a placebo (p<0.001).34 Additionally, the supplementations helped maintain lean body mass.34 A systematic review and meta-analysis of 11 studies on weight loss (n=573) and three studies on body fat reduction (n=110) showed that Phaseolus vulgaris supplementation for 8 weeks resulted in an average weight loss of 1.08 kg (p<0.00001) and average reduction in body fat by 3.26 kg (p=0.02).13 Apart from a significant decrease in weight and body fat, an RCT showed that Phaseolus vulgaris supplementation reduced WC by an average of 2.7 cm and hip circumference by 2.3 cm (p<0.01 for both).35
None of the studies or meta-analyses discussed reported any adverse effects attributed to the supplement investigated. Similarly, in this study as well, none of the subjects reported any adverse effect of Vidaslim. The 2019 European guidelines for management of adult obesity in primary care36 and the South Asian consensus on MNT in diabesity (CoMeND)1 give importance to a decrease in WC over weight loss alone, as a decrease in WC is associated with a decrease in visceral fat and its associated cardiometabolic risks.36 In this study as well, Vidaslim meal replacement was associated with a significant reduction in WC and hip circumference at the end of 16 weeks, apart from a significant reduction in weight and BMI. Along with this, there was significant improvement in mood, sleep, satiety, and general well-being. The increase in satiety provided by the protein blend and fiber in Vidaslim is likely to be responsible for the reduction in hunger and appetite seen over the 16 weeks. All these factors are important to bring about a behavior change for sustained and maintained weight loss.36
Strengths and Limitations
This is the first clinical study to demonstrate the efficacy and safety of Vidaslim as a meal-replacement strategy in overweight and obese adults. This is also the first study to report that the timing of meal replacement is important. Significant weight loss is achieved only by replacing lunch or dinner and not breakfast. The study showed that replacing dinner with Vidaslim was a better strategy than replacing lunch with Vidaslim. However, we did not assess energy expenditure or control for energy intake, except for replacing one meal with Vidaslim. This was a 16-week study. Though there were significant reductions in weight, WC, HC, hunger, and appetite and significant improvement in mood, sleep, and general well-being, longer-duration studies are required to see if the weight-loss effect observed is sustained and maintained.
Conclusion
The MNS Vidaslim was found to be an effective and safe meal (lunch or dinner)-replacement therapy and brought about significant reductions in weight, WC, and HC over a period of 16 weeks in overweight and obese individuals. Long-term Vidaslim use is likely to help maintain the weight loss, as the MNS was also found to significantly reduce hunger and appetite and significantly improve mood, sleep, and general well-being, factors necessary to bring about behavior change for sustained and maintained weight loss.
Acknowledgments
The authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this manuscript, take responsibility for the integrity of the work, and have given final approval to the version to be published. The authors thank Dr. Punit Srivastava and Dr. Kokil Mathur of Mediception Science (www.mediception.com) for providing medical writing support in the preparation of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kapoor N, Sahay R, Kalra S, et al. Consensus on Medical Nutrition Therapy for Diabesity (CoMeND) in Adults: a South Asian Perspective. Diabetes Metab Syndr Obes Targets Ther. 2021;14:1703–1728.
2. Ministry of Health and Family Welfare. National Family Health Survey (NFHS-4). International Institute for Population Sciences Deonar, Mumbai; 2015. Available from: http://rchiips.org/nfhs/nfhs-4Reports/India.pdf.
3. Mu Y, Kou T, Wei B, et al. Soy products ameliorate obesity-related anthropometric indicators in overweight or obese asian and non-menopausal women: a meta-analysis of randomized controlled trials. Nutrients. 2019;11:11.
4. Tak YJ, Lee SY. Anti-obesity drugs: long-term efficacy and safety: an updated review. World J Men's Health. 2021;39(2):208–221. doi:10.5534/wjmh.200010
5. Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ Can Med Assoc J. 2020;192:31.
6. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–138. doi:10.1161/01.cir.0000437739.71477.ee
7. Koliaki C, Spinos T, Spinou Μ, Brinia ΜE, Mitsopoulou D, Katsilambros N. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare. 2018;6(3):73. doi:10.3390/healthcare6030073
8. Astbury NM, Piernas C, Hartmann-Boyce J, Lapworth S, Aveyard P, Jebb SA. A systematic review and meta-analysis of the effectiveness of meal replacements for weight loss. Obes Rev. 2019;20(4):569–587. doi:10.1111/obr.12816
9. Dwyer JT, Allison DB, Coates PM. Dietary Supplements in Weight Reduction. J Am Diet Assoc. 2005;105(5 Supplement):80.
10. Ronis MJJ, Pedersen KB, Watt J. Adverse effects of nutraceuticals and dietary supplements. Annu Rev Pharmacol Toxicol. 2018;58:583–601. doi:10.1146/annurev-pharmtox-010617-052844
11. Ugartemendia L, Bravo R, Castaño MY, et al. Influence of diet on mood and social cognition: a pilot study. Food Funct. 2020;11(9):8320–8330. doi:10.1039/d0fo00620c
12. Talenezhad N, Mohammadi M, Ramezani-Jolfaie N, Mozaffari-Khosravi H, Salehi-Abargouei A. Effects of l-carnitine supplementation on weight loss and body composition: a systematic review and meta-analysis of 37 randomized controlled clinical trials with dose-response analysis. Clin Nutr ESPEN. 2020;37:9–23. doi:10.1016/j.clnesp.2020.03.008
13. Udani J, Tan O, Molina J. Systematic review and meta-analysis of a proprietary alpha-amylase inhibitor from white bean (Phaseolus vulgaris L) on weight and fat loss in humans. Foods Basel Switz. 2018;7:4.
14. Askarpour M, Hadi A, Miraghajani M, Symonds ME, Sheikhi A, Ghaedi E. Beneficial effects of l-carnitine supplementation for weight management in overweight and obese adults: an updated systematic review and dose-response meta-analysis of randomized controlled trials. Pharmacol Res. 2020;151:104554. doi:10.1016/j.phrs.2019.104554
15. Wirunsawanya K, Upala S, Jaruvongvanich V, Sanguankeo A. Whey protein supplementation improves body composition and cardiovascular risk factors in overweight and obese patients: a systematic review and meta-analysis. J Am Coll Nutr. 2018;37:1.
16. Askarpour M, Hadi A, Dehghani Kari Bozorg A, et al. Effects of L-carnitine supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Hum Hypertens. 2019;33(10):725–734. doi:10.1038/s41371-019-0248-1
17. Guérin-Deremaux L, Pochat M, Reifer C, Wils D, Cho S, Miller LE. The soluble fiber NUTRIOSE induces a dose-dependent beneficial impact on satiety over time in humans. Nutr Res. 2011;31(9):665–672.
18. Chen IJ, Liu CY, Chiu JP, Hsu CH. Therapeutic effect of high-dose green tea extract on weight reduction: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr Edinb Scotl. 2016;35(3):592–599.
19. Kim JY. Optimal diet strategies for weight loss and weight loss maintenance. J Obes Metab Syndr. 2021;30(1):20–31. doi:10.7570/jomes20065
20. Outlaw J, Wilborn C, Smith A, et al. Effects of ingestion of a commercially available thermogenic dietary supplement on resting energy expenditure, mood state and cardiovascular measures. J Int Soc Sports Nutr. 2013;10(1):25. doi:10.1186/1550-2783-10-25
21. Owens M, Watkins E, Bot M, et al. Acceptability and feasibility of two interventions in the MooDFOOD Trial: a food-related depression prevention randomised controlled trial in overweight adults with subsyndromal symptoms of depression. BMJ Open. 2020;10:9.
22. Wallace CJK, Milev RV. The efficacy, safety, and tolerability of probiotics on depression: clinical results from an open-label pilot study. Front Psychiatry. 2021;3:12.
23. Hoffman JR, Falvo MJ. Protein – which is best? J Sports Sci Med. 2004;3(3):118–130.
24. Chen M, Pan A, Malik VS, Hu FB. Effects of dairy intake on body weight and fat: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96(4):735–747. doi:10.3945/ajcn.112.037119
25. Kondrashina A, Brodkorb A, Giblin L. Dairy-derived peptides for satiety. J Funct Foods. 2020;66:103801.
26. Namazi N, Larijani B, Azadbakht L. Are isolated and complex fiber supplements good choices for weight management? A systematic review. Arch Iran Med. 2017;20(11):704–713.
27. Kondo T, Handa K, Genda T, Hino S, Hamaguchi N, Morita T. Digestion-resistant dextrin derivatives are moderately digested in the small intestine and contribute more to energy production than predicted from large-bowel fermentation in rats. J Nutr. 2017;147(3):330–336. doi:10.3945/jn.116.239855
28. van Baak MA, Mariman ECM. Dietary strategies for weight loss maintenance. Nutrients. 2019;11(8):1916. doi:10.3390/nu11081916
29. Canfora EE, Blaak EE. The role of polydextrose in body weight control and glucose regulation. Curr Opin Clin Nutr Metab Care. 2015;18(4):395–400. doi:10.1097/MCO.0000000000000184
30. Alptekin İM, Erdoğan E, Işler A, Yanalak EC, Çakiroğlu FP, Aras S. Short-term effects of milkshake containing polydextrose and maltodextrin on subjective feelings of appetite, energy intake and blood glucose in healthy females. Nutr Food Sci. 2021;52(1):151–162.
31. Chantre P, Lairon D. Recent findings of green tea extract AR25 (Exolise) and its activity for the treatment of obesity. Phytomedicine. 2002;9(1):3–8.
32. Hartley L, Flowers N, Holmes J, et al. Green and black tea for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;18(6):CD009934.
33. Pooyandjoo M, Nouhi M, Shab-Bidar S, Djafarian K, Olyaeemanesh A. The effect of (L-)carnitine on weight loss in adults: a systematic review and meta-analysis of randomized controlled trials. Obes Rev off J Int Assoc Study Obes. 2016;17(10):970–976.
34. Celleno L, Tolaini MV, D’Amore A, Perricone NV, Preuss HG. A Dietary supplement containing standardized Phaseolus vulgaris extract influences body composition of overweight men and women. Int J Med Sci. 2007;4(1):45–52.
35. Wang S, Chen L, Yang H, Gu J, Wang J, Ren F. Regular intake of white kidney beans extract (Phaseolus vulgaris L) induces weight loss compared to placebo in obese human subjects. Food Sci Nutr. 2020;8(3):1315–1324. doi:10.1002/fsn3.1299
36. Durrer Schutz D, Busetto L, Dicker D, et al. European practical and patient-centred guidelines for adult obesity management in primary care. Obes Facts. 2019;12(1):40–66. doi:10.1159/000496183
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
