Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Obesity Patterns, Metabolic Abnormality, and Diabetic Kidney Disease in Patients with Type 2 Diabetes
Authors Zhang K, Zhang W, Xia F , Wang N, Lu Y , Sui C, Wang B
Received 11 September 2023
Accepted for publication 29 November 2023
Published 7 December 2023 Volume 2023:16 Pages 3999—4011
DOI https://doi.org/10.2147/DMSO.S436046
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Kun Zhang, Wen Zhang, Fangzhen Xia, Ningjian Wang, Yingli Lu, Chunhua Sui, Bin Wang
Institute and Department of Endocrinology and Metabolism, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200011, People’s Republic of China
Correspondence: Bin Wang; Chunhua Sui, Institute and Department of Endocrinology and Metabolism, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, 639 Zhizaoju Road, Shanghai, 200011, People’s Republic of China, Tel +86-21-23271699 ; +86-21-53315139, Email [email protected]; [email protected]
Objective: Obesity has been identified as a risk factor for chronic kidney disease. However, the impact of obesity, with or without a metabolically healthy condition, on diabetic kidney disease (DKD) remains unclear. We aimed to examine the associations of obesity patterns and metabolic abnormalities with the prevalence of DKD.
Methods: This cross-sectional study included 4079 patients with type 2 diabetes from eleven communities in Shanghai, China. General obesity was assessed by body mass index (BMI) and abdominal obesity assessed by waist-to-hip ratio. Metabolic abnormalities were determined according to the Adult Treatment Panel III criteria. DKD was defined as estimated glomerular filtration rate < 60 mL/min/1.73 m2 or urinary albumin-creatinine ratio ≥ 30 mg/g. Poisson regression model with inverse probability of treatment weighting was used to estimate prevalence ratios (PRs) and 95% CIs.
Results: Higher BMI and WHR were each associated with a greater prevalence of DKD after mutual adjustment. When considered jointly, patients with both general obesity and abdominal obesity had the highest odds of DKD (PR 1.51, 95% CI 1.29– 1.76). The associations of BMI and WHR with prevalent DKD were mainly observed in patients with use of antidiabetic drugs but not in those without drug use. Compared with normal-weight patients with 0– 1 metabolic abnormality, patients who were overweight or obese with 0– 1 metabolic abnormality showed increased odds of DKD. The PRs (95% CI) of DKD for patients with both overweight/obesity and abdominal obesity who had 0– 1, 2, and 3 metabolic abnormalities were 1.59 (1.20– 2.10), 1.68 (1.29– 2.18), and 2.16 (1.67– 2.78), respectively, relative to those with normal BMI and no abdominal obesity who had 0– 1 metabolic abnormality.
Conclusion: BMI and WHR were positively associated with DKD prevalence. Obesity composite and metabolic abnormalities had an additive effect on the odds of DKD. Further longitudinal studies are warranted to elucidate the role of obesity and metabolic abnormalities in the development of DKD.
Keywords: abdominal obesity, general obesity, diabetic kidney disease, metabolic abnormality
Introduction
Type 2 diabetes poses a major threat to public health. It is estimated that 537 million people had diabetes worldwide in 2021, and China has the world’s largest diabetes epidemic.1 Nearly 20%-40% of patients with diabetes are complicated with diabetic kidney disease (DKD), which is now the leading cause of chronic kidney disease and end-stage renal disease.2 In 2017, DKD accounted for a third of 35.8 million disability-adjusted life-years resulted from chronic kidney disease globally.3 Besides, patients with DKD are at higher risks of developing cardiovascular events and excess mortality than patients without DKD.4 For this reason, identifying the underlying modifiable risk factors is essential to reduce the burden of DKD and related complications.
Obesity is rapidly reaching epidemic proportions in parallel with the increase in the prevalence of diabetes. Obesity is a known risk factor for diabetes, cardiovascular disease, and chronic kidney disease.5–7 General and abdominal obesity are the major subtypes of obesity. Investigations on the relationships between obesity types and DKD have been performed, but the results were inconsistent.8–12 For instance, one study reported that patients with overweight or obesity rather than abdominal obesity were more likely to have DKD.9 However, in two other studies, abdominal obesity was more closely associated with DKD than general obesity.10 In addition, diabetic patients with higher body mass index (BMI) have been shown to exhibit a lower risk of DKD and kidney function decline,11,12 suggesting a paradoxical protective effect of a high BMI.
Metabolic disorders like diabetes, dyslipidemia, and hypertension are thought to mediate the increased risk of morbidity associated with obesity.13 Even so, the prognostic value of obesity on its own is still debatable.14 There is some evidence that obese individuals without metabolic abnormalities, described as having metabolically healthy obesity (MHO), may not be at a higher risk of chronic kidney disease compared to individuals with metabolically healthy nonobesity.15 In contrast, more recent studies have consistently indicated a significant association between MHO and chronic kidney disease.16–19 Obesity and metabolic abnormalities are more prevalent in patients with diabetes, but whether obesity with no or fewer metabolic abnormalities confers a higher risk of DKD remains unclear. Moreover, the combined effect of general and abdominal obesity and metabolic status on DKD is yet to be elucidated.
To this end, using data from a well-characterized sample of Chinese adults with type 2 diabetes, we examined the associations of general obesity (assessed by BMI) and abdominal obesity (assessed by waist-to-hip ratio [WHR]) with DKD and further evaluated the odds of DKD in relation to the combination of composite obesity patterns and metabolic abnormalities.
Methods
Design and Population
We undertook a population-based, cross-sectional study with the aim to investigate risk factors for vascular complications in Chinese adults with type 2 diabetes. The detailed design and methods of the study have been described elsewhere.20,21 Briefly, between May and August 2018, local residents with type 2 diabetes were enrolled from eleven communities in Huangpu and Pudong District, Shanghai, based on the registration system of each community healthcare center. The diagnosis criteria for type 2 diabetes included fasting plasma glucose ≥7.0 mmol/L, or glycated hemoglobin (HbA1c) ≥6.5%, or a previous diagnosis by physicians. Among the eligible individuals, one-half were randomly selected for participation. In total, 4813 patients with type 2 diabetes were recruited and completed a comprehensive survey including a standard questionnaire, physical examination, and sample collection. We excluded participants with missing data on BMI, waist circumference (WC), or hip circumference (n = 128), blood pressure (BP) (n = 112), or urinary measurements (n = 237). We further excluded participants with kidney disease (eg, acute or chronic nephritis, hydronephrosis) or urological neoplasms (n = 103) and those who were receiving glucocorticoid medication (n = 154). Finally, 4079 patients were included in the primary analysis (Supplementary Figure 1). The study protocol was approved by the Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. All participants provided written informed consent and received an ethical agreement from official authorities.
Data Collection
A detailed questionnaire comprising information on demographic characteristics, education attainment, lifestyle behaviors, and medical history was administered through personal interviews. Education level was categorized into high school or above and less than high school. The type and frequency of alcohol consumption and smoking habits were recorded. Participants were classified as never, former, or current drinkers or smokers. Diabetes duration was determined as the time from the date of diabetes onset to the date of enrollment. Anthropometric measurements and blood parameters were performed by trained staff according to a standard protocol. Height and weight were measured with participants wearing lightweight clothes and no shoes. Waist and hip circumferences were measured horizontally at the midpoint between the rib cage and iliac crest and at the greatest protrusion of the buttocks, respectively. BP was tested three times using an automated device (Omron Model HEM-752 FUZZY) after a 5-min rest, and readings were averaged for analysis.
Venous blood samples were drawn after an overnight fast of at least 8 h. Blood specimens were centrifuged on site within 2 h of collection and shipped in dry ice to a central laboratory, which is certified by the College of American Pathologists Laboratory Accreditation Program. Plasma glucose level was measured using the hexokinase method. HbA1c was assessed by high-performance liquid chromatography (MQ-2000PT, Medconn, Shanghai, China). Serum fasting C-peptide and insulin were assessed by chemiluminescence assay (Abbott i2000 SR, USA). Serum creatinine, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides were measured using a Beckman Coulter AU 680 (Brea, USA). Morning spot urine samples were collected under normative retention guidelines. Urinary albumin and creatinine were measured using a turbidimetric immunoassay and an enzymatic method separately on an automated analyzer (Beckman Coulter AU680, Brea, USA).
Assessment of Obesity Patterns and Metabolic Health Status
BMI was calculated as weight in kilograms divided by height in meters squared. General obesity was determined by the BMI according to the Chinese specific cut-off values,22 categorized as underweight (<18.5 kg/m2), normal (18.5–23.9 kg/m2), overweight (24.0–27.9 kg/m2) and obesity (≥28.0 kg/m2). Given the limited number of underweight patients (n = 86), these were grouped into the normal BMI group in analysis. WHR was calculated as WC divided by hip circumference. WHR was categorized as normal (<0.90 for men and <0.85 for women), moderate (0.90–0.94 for men and <0.85–0.89 for women) and high (≥0.95 for men and ≥0.90 for women), and abdominal obesity was defined as WHR ≥0.90 for men and ≥0.85 for women.23
Metabolic health status was assessed by using National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP-III) components except for fasting glucose and WC.24 Specifically, metabolic abnormalities were considered as the presence of the following conditions: (1) elevated BP (systolic BP ≥130 mm Hg and/or diastolic BP ≥85 mm Hg) or on antihypertensive medications; (2) high triglyceride level (≥1.7 mmol/L), or on lipid-lowering medications; (3) low HDL-C level (<1.04 mmol/L in men or <1.29 mmol/L in women). The number of metabolic abnormalities was summed and classified into three categories as 0–1, 2, and 3 metabolic abnormalities.
Definition of DKD
The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation:25 eGFR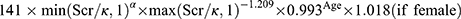 , where Scr is serum creatinine expressed in mg/dL, κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min and max indicate the minimum and maximum of Scr/κ or 1, respectively. Urinary albumin-to-creatinine ratio (UACR) was recorded to represent the urinary albumin excretion rate. DKD was defined as an eGFR of <60 mL/min/1.73 m2 or albuminuria (UACR ≥30 mg/g).2
, where Scr is serum creatinine expressed in mg/dL, κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min and max indicate the minimum and maximum of Scr/κ or 1, respectively. Urinary albumin-to-creatinine ratio (UACR) was recorded to represent the urinary albumin excretion rate. DKD was defined as an eGFR of <60 mL/min/1.73 m2 or albuminuria (UACR ≥30 mg/g).2
Statistical Analysis
The characteristics of study participants were summarized as mean ± SD or median (interquartile range) for continuous variables and number (percentage) for categorical variables. Between-group differences in characteristics by the presence of DKD were tested using the Student’s t test or Mann–Whitney U-test for continuous variables and the chi-square test for categorical variables.
Poisson regression model with a robust error variance was used to calculate prevalence ratios (PRs) and 95% confidence intervals (CIs) for DKD associated with BMI and WHR, given that there is no longer good justification for fitting logistic regression model when outcomes are not rare.26 In the multivariable analysis, an incremental adjustment was performed. Model 1 was adjusted for age and sex. Model 2 was additionally adjusted for education, smoking status, alcohol drinking, diabetes duration, HbA1c, use of antidiabetic agents, systolic BP, use of antihypertensive agents, HDL-C, and triglycerides. In model 3, BMI and WHR were further mutually adjusted to examine their independent effect on DKD. We also evaluated the association of a combination of general obesity and abdominal obesity with DKD prevalence. The final Poisson regression model showed an acceptable goodness of fit (P>0.60). We applied inverse probability of treatment weighting (IPTW) to match different BMI and WHR groups or their combination groups in terms of population characteristics including age, sex, education, smoking status, alcohol drinking, diabetes duration, and medication use. Given the impact of antidiabetic medication on DKD progression, stratified analyses according to the use or non-use of antidiabetic drugs were also conducted.
To investigate the joint association of obesity indices and metabolic status with DKD, participants were divided into 9 groups based on their BMI or WHR categories and metabolic abnormalities (0–1, 2, and 3), with normal BMI or WHR category with 0–1 metabolic abnormality as reference. The odds of DKD were estimated using the Poisson regression analyses adjusting for age, sex, education, smoking status, alcohol drinking, diabetes duration, HbA1c, use of antidiabetic agents, and WHR (or BMI). In addition, we estimated the odds of DKD according to combined obesity patterns and metabolic abnormalities by creating a 12-category variable. Because patients who were obese without abdominal obesity (n = 59) were insufficient to configure a cross- categorization, overweight and obesity were grouped, and patients who had normal BMI and no abdominal obesity with 0–1 metabolic abnormality were set as the reference.
Subgroup analyses were conducted to assess potential effect modification for sex, age (≤65 years and >65 years), and glycemic control (HbA1c <7% and ≥7%). The interaction was tested by using a likelihood ratio test comparing models with and without the cross-product term. For sensitivity analysis, we used WC as an alternative indicator of abdominal obesity. Furthermore, we repeated the analyses after excluding participants with cardiovascular disease or those with retinopathy. All statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC). A two-sided P value <0.05 was considered statistically significant.
Results
Population Characteristics
Table 1 shows the characteristics of the 4079 patients with type 2 diabetes. Overall, the mean age was 67.3 (SD 8.7) years and 2196 (53.8%) were women. The mean BMI and WHR values were 24.9 (SD 3.6) kg/m2 and 0.91 (SD 0.06), respectively. Among the patients, 1122 (27.5%) had DKD. Patients with DKD were more likely to be older, less educated, and to have a longer duration of diabetes and a higher proportion of antidiabetic medications. The levels of BP, triglycerides, fasting glucose, HbA1c, C-peptide, fasting insulin, BMI, WC, and WHR were higher in patients with DKD than in those without DKD. Population characteristics by BMI and WHR categories are presented in Supplementary Table 1. Patients with higher BMI and WHR tended to be less educated and had poor metabolic profiles. There was an increase in the mean age of patients with higher WHR but lower BMI levels.
 |
Table 1 Characteristics of the Study Population with and without DKD |
Obesity Patterns and DKD
BMI and WHR were positively associated with the prevalence of DKD (Table 2). These associations persisted after additional adjustment for potential confounders including socioeconomic status, lifestyles, medication use, and metabolic parameters. In the full adjusted model that included BMI and WHR simultaneously, each 1 kg/m2 increment in BMI and 0.1 increment in WHR were associated with 4% and 12% higher odds of DKD, respectively. Compared with patients who had a normal weight, the PR (95% CI) of DKD was 1.09 (0.97–1.23) for those with overweight and 1.44 (1.26–1.65) for those with obesity. The risk gradient was attenuated across WHR categories (moderate: PR 0.98, 95% CI 0.85–1.13; high: PR 1.10, 95% CI 0.96–1.27). When stratified by antidiabetic medication, the associations of BMI and WHR with prevalent DKD were mainly observed in patients with use of antidiabetic drugs but not in those without use of antidiabetic drugs (P for interaction >0.05). Such results were confirmed by using spline regression splines (Supplementary Figure 2).
 |
Table 2 Prevalence Ratios (95% CIs) of DKD According to BMI and WHR Categories |
We then performed a joint analysis of two obesity measures (Table 3). Patients with both obesity types (PR 1.51, 95% CI 1.29–1.76) rather than those with either abdominal obesity (PR 1.06, 95% CI 0.93–1.22) or general obesity (PR 1.23, 95% CI 0.81–1.87) had increased odds of DKD compared with patients without any type of obesity. Similar results were found in patients with use of antidiabetic agents, and the joint association of obesity measures with DKD was largely attenuated in those without antidiabetic medication use.
 |
Table 3 Prevalence Ratios (95% CIs) of DKD According to Obesity Patterns |
Obesity Patterns, Metabolic Abnormalities and DKD
The associations of BMI or WHR categories and metabolic abnormalities with DKD are shown in Table 4. Compared with normal-weight patients with 0–1 metabolic abnormality, patients who were overweight or obese with 0–1 metabolic abnormality had increased odds of DKD (overweight: PR 1.33, 95% CI 1.06–1.67; obese: PR 1.82, 95% CI 1.37–2.44). Within each BMI or WHR category, the prevalence of DKD increased with an increasing number of metabolic abnormalities (Supplementary Table 2). There was no significant interaction between BMI or WHR and metabolic abnormalities. The highest odds of DKD was found for patients with all 3 metabolic abnormalities and general obesity (PR 2.39, 95% CI 1.94–2.95) or abdominal obesity (PR 1.71, 95% CI 1.36–2.16). Generally, the association of WHR categories and metabolic abnormalities with DKD was more evident among patients with use of antidiabetic agents than among those without use.
 |
Table 4 Associations of BMI and WHR Categories and Metabolic Status with Prevalent DKD |
We further evaluated the association of combined obesity patterns and metabolic abnormalities with DKD (Figure 1). The prevalence of DKD was minimal in patients who had normal BMI without abdominal obesity and 0–1 metabolic abnormality (15.6%, the reference group), and maximal in patients with overweight/obesity and abdominal obesity plus 3 metabolic abnormalities (37.8%). Compared with the reference group, the PRs (95% CI) of DKD were 1.59 (1.20–2.10), 1.68 (1.29–2.18), and 2.16 (1.67–2.78) for patients who had overweight/obesity and abdominal obesity with 0–1, 2, and 3 metabolic abnormalities, respectively. Similar pattern of results was observed for albuminuria (Figure 1).
Subgroup Analyses and Sensitivity Analyses
The association of BMI or WHR categories and metabolic status with DKD was consistent across stratification by sex, age, and HbA1c levels (all P for interaction >0.05) (Supplementary Tables 3 and 4). Analyses of the associations between WC as well as its combination with metabolic abnormalities and DKD showed similar results to those for WHR in the main analyses (Supplementary Tables 5 and 6). Moreover, the results did not change appreciably after excluding patients with cardiovascular disease or retinopathy (Supplementary Table 7).
Discussion
In this Chinese population with type 2 diabetes, we found that higher BMI and WHR were individually associated with a greater prevalence of DKD. Patients with both general and abdominal obesity were more likely to have DKD than those with either type of obesity. Within each BMI or WHR category, there was a graded association between more metabolic abnormalities and higher odds of DKD. The combination of overweight/obesity and abdominal obesity and all 3 metabolic abnormalities conferred the highest odds of DKD. These findings suggest that general and abdominal obesity may contribute to kidney function decline in type 2 diabetes, particularly when metabolic abnormalities are concurrently present.
Obesity is judged as a risk factor for chronic kidney disease in the general population.27 However, results regarding the association between obesity and DKD in patients with diabetes have been mixed.8–12 In a prospective analysis of Chinese adults with diabetes, high WC and low BMI were shown to predict the development of DKD over 5 years of follow-up.11 Hu et al reported a relationship between abdominal obesity and DKD independent of BMI, whereas BMI was not significantly associated with DKD after adjustment for abdominal obesity parameters.10 On the contrary, a multiethnic study in Asian population found that high BMI (ie, overweight and obesity), but not abdominal obesity parameters, was associated with greater odds of having DKD.9 These conflicting results may be partially attributed to heterogeneity in patients’ characteristics, study design, definition of kidney outcomes, and potential confounders. Consistent with our findings, a longitudinal study in Taiwan reported that diabetic patients with obesity and high WC were at a higher risk of developing DKD.28 Similarly, a recent study among patients with diabetes showed significant associations of BMI and various abdominal obesity indices including WHR with albuminuria and advanced kidney disease.29 Although abdominal obesity measures such as WC and WHR have been supposed to be more strongly associated with metabolic disorders and cardiovascular risk than BMI,30,31 the relative contribution of abdominal obesity and general obesity to the development of DKD is still uncertain and warrants further clarification.
The joint analysis demonstrated an additive effect of general and abdominal obesity on DKD. Of note, in the current study, only 59 patients were categorized as being obese and without abdominal obesity, which precluded sufficient statistical power to obtain precise effect estimates. Coexistence of both obesity types is presumably linked with a higher risk of metabolic dysfunctions that promote chronic diabetes complications. In this light, determining both general and abdominal obesity among patients with diabetes might be more helpful for DKD risk stratification than using each obesity type alone.
Abundant evidence showing increased risks of chronic kidney disease in MHO suggests the MHO phenotype not to be a benign or harmless condition.16–19 To date, the association of combined obesity patterns and metabolic status with DKD has not been documented. In this study, we observed that diabetic patients with overweight or obesity and 0–1 metabolic abnormality still had significantly higher odds of prevalent DKD compared with their normal-weight counterparts. The results were similar but less evident among patients with higher WHR. Additionally, patients with 2 or more metabolic abnormalities were more likely to have DKD even though they had normal weight, in accordance with previous studies demonstrating a positive association between metabolic abnormalities and chronic kidney disease in normal-weight individuals.19,32 These findings suggest the negative impact of obesity on DKD even under a healthy metabolic condition and also the DKD risk associated with metabolic abnormalities regardless of body weight status. When we finally divided patients into 12 subphenotypes according to obesity patterns and metabolic status, coexistence of both overweight/obesity and abdominal obesity and all 3 metabolic abnormalities conferred the highest odds of prevalent DKD and albuminuria. Prior studies have reported a graded relationship between the number of metabolically unhealthy components and incident chronic kidney disease.11,19,33 Intriguingly, as the number of metabolic abnormalities increased, there was an increase in the odds of DKD with higher WHR and a slight decrease in the odds of DKD with higher BMI. Although the underlying cause is unclear, such findings may suggest the distinct role of central fat distribution in DKD development among diabetic patients with comorbid metabolic disorders.
We noticed that the associations of BMI and WHR with prevalent DKD were largely present among patients with use of antidiabetic drugs rather than among those without drug use, even though no significant interaction between BMI or WHR categories and antidiabetic medication was observed. In this study, 71.8% of patients with diabetes took antidiabetic drugs. Patients with use of antidiabetic drugs had worse glycemic control and longer duration of diabetes than those without drug use (mean HbA1c, 7.6 vs 7.1%; mean fasting glucose, 8.0 vs 7.1 mmol/L; median diabetes duration, 10 vs 6 years). Generally, patients who receive antidiabetic drug medication are supposed to have more serious disease; therefore, these individuals might be at higher risk of suffering kidney impairment associated with obesity and other metabolic disorders. Further prospective studies are needed to verify our findings.
The exact mechanisms by which obesity initiates and exacerbates DKD remain elusive. A putative explanation is obesity-induced abnormality in kidney hemodynamics and function.34 Obesity-related glomerulopathy (ORG) is an increasing condition characterized by glomerulomegaly in the presence or absence of focal and segmental glomerulosclerosis lesions.35,36 The well-recognized mechanisms involved in ORG include glomerular hyperfiltration, overactivation of the renin-angiotensin-aldosterone system (RAAS), insulin resistance, inflammation, apoptosis, and ectopic lipid accumulation and lipotoxicity.37–39 For example, obesity is closely associated with insulin resistance and compensatory hyperinsulinemia that have been shown to reduce bioavailable nitric oxide, increase oxidative stress, and cause activation of insulin-like growth factor 1 and adipokines, all of which promote glomerular hyperfiltration and fibrotic processes in the kidney, contributing to the pathogenesis and progression of DKD.40–42 Excess visceral adipose tissue and circulating insulin can inappropriately activate the renin-angiotensin-aldosterone system that lead to endothelial dysfunction and tubulointerstitial fibrosis.34,43 Furthermore, visceral adipose tissue has been regarded as an active source of inflammatory adipocytokines including leptin and interleukin-6, which have a direct effect on endothelial function and the kidney.44 Chronic low-grade inflammation correlated with obesity is also postulated to play a part in kidney damage.45 It is noteworthy that weight loss, RAAS blockade, and certain antidiabetic agents such as sodium-glucose cotransporter 2 inhibitors (SGLT2i) or glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are potentially effective treatment methods for ORG.36,38
Our study may have important clinical and public health implications. For diabetic population, simple measurable indicators such as BMI and WHR are useful for predicting high-risk groups for DKD progression. These indicators can be easily measured at home and primary clinics and widely used for both individual-level and population-level screening. Our results could not only help to raise the knowledge of the combined effect of general obesity, abdominal obesity and metabolic status on DKD but also provide evidence on intervention strategies targeting obesity and concomitant metabolic abnormalities for prevention and delayed onset of DKD.
To our knowledge, this study is the first analysis of the association between combined obesity types, metabolic status, and DKD in patients with type 2 diabetes. The major strength of our study included the relatively large sample size, detailed subphenotyping according to anthropometric measurements and metabolic risk factors, and comprehensive adjustment for potential confounders. Our study also has several limitations. First, because of the cross-sectional study design, causal inferences between obesity phenotypes and DKD could not be established. However, consistent results among patients without cardiovascular disease and those without retinopathy indicate the potential reverse causality of vascular complications preceding obesity might be less likely. Second, UACR and eGFR were assessed based on a single collection and might not fully reflect the background kidney function, but this measurement error tends to introduce non-differential misclassification of DKD status. Third, we were unable to verify if the DKD cases in our study were a consequence of diabetes, as several other non-diabetic causes such as ischemic nephrosclerosis and glomerulonephritis could also lead to kidney injury.11 Lastly, although we carefully adjusted for multiple potential confounders, the possibility of unmeasured or residual confounding cannot be ruled out.
Conclusions
In this study, higher BMI and WHR were associated with higher odds of DKD in a Chinese population with type 2 diabetes. Obesity composite and metabolic abnormalities had an additive effect on prevalent DKD. Our results lend support for early interventions targeting general obesity and abdominal obesity in concert to prevent or delay the onset of DKD, particularly among those with abnormal metabolic features. Longitudinal studies are warranted to further elucidate the role of obesity and concomitant metabolic abnormalities in the development of DKD.
Data Sharing Statement
Requests for the dataset analyzed for the current study can be made by contacting the corresponding author.
Statement of Ethics
All individuals provided written informed consent after being fully briefed and advised about the study procedures. This study was approved by the Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. It was conducted in compliance with the Declaration of Helsinki.
Acknowledgments
Thanks to all the patients who participated in the study and the staff of Endocrinology and Metabolism, Shanghai Ninth People’s Hospital for their involvement in this project.
Funding
This study was supported by the National Natural Science Foundation of China (82000750, 82120108008), Postdoctoral Scientific Research Foundation of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (Kun Zhang-2021-startup funding), Fundamental research program funding of Shanghai Ninth People’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine (JYZZ171), Science and Technology Commission of Shanghai Municipality (19140902400, 18410722300), and Major Science and Technology Innovation Program of Shanghai Municipal Education Commission (2019-01-07-00-01-E00059).
Disclosure
All authors have declared no conflict of interest and agreed to publish the data presented in this manuscript.
References
1. International Diabetes Federation. IDF diabetes atlas, 10th edition. Brussels; 2021. Available from: http://www.diabetesatlas.org/.
2. American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl1):S135–S151 . doi:10.2337/dc20-S011
3. Bikbov B, Purcell CA, Levey AS, GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733. doi:10.1016/S0140-6736(20)30045-3
4. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37(10):2864–2883. doi:10.2337/dc14-1296
5. Vazquez G, Duval S, Jacobs DR, et al. Comparison of body mass index, waist circumference, and waist/Hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev. 2007;29(1):115–128. doi:10.1093/epirev/mxm008
6. Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9(1):88. doi:10.1186/1471-2458-9-88
7. Chang AR, Grams ME, Ballew SH, et al. Adiposity and risk of decline in glomerular filtration rate: meta-analysis of individual participant data in a global consortium. BMJ. 2019;364(k 5301). doi:10.1136/bmj.k5301
8. Xia Y, Jiang C, Lu J, et al. Associations between obesity and kidney disease in Chinese men and women with type 2 diabetes: a retrospective cohort study. Can J Diabetes. 2022;46(1):47–52.e3. doi:10.1016/j.jcjd.2021.05.005
9. Man REK, Gan ATL, Fenwick EK, et al. The relationship between generalized and abdominal obesity with diabetic kidney disease in type 2 diabetes: a multiethnic Asian study and meta-analysis. Nutrients. 2018;10(11):1685. doi:10.3390/nu10111685
10. Hu J, Yang S, Zhang A, et al. Abdominal obesity is more closely associated with diabetic kidney disease than general obesity. Diabetes Care. 2016;39(10):e179–e180. doi:10.2337/dc16-1025
11. Luk AO, So WY, Ma RC, et al. Metabolic syndrome predicts new onset of chronic kidney disease in 5829 patients with type 2 diabetes: a 5-year prospective analysis of the Hong Kong Diabetes Registry. Diabetes Care. 2008;31(12):2357–2361. doi:10.2337/dc08-0971
12. Huang WH, Chen CY, Lin JL, et al. High body mass index reduces glomerular filtration rate decline in type II diabetes mellitus patients with stage 3 or 4 chronic kidney disease. Medicine. 2014;93(7):e41. doi:10.1097/MD.0000000000000041
13. Lu Y, Hajifathalian K, Ezzati M, et al. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:9921):970–983.
14. Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions? A systematic review and meta-analysis. Ann Intern Med. 2013;159(11):758–769. doi:10.7326/0003-4819-159-11-201312030-00008
15. Hashimoto Y, Tanaka M, Okada H, et al. Metabolically healthy obesity and risk of incident CKD. Clin J Am Soc Nephrol. 2015;10(4):578–583. doi:10.2215/CJN.08980914
16. Lin L, Peng K, Du R, et al. Metabolically healthy obesity and incident chronic kidney disease: the role of systemic inflammation in a prospective study. Obesity. 2017;25(3):634–641. doi:10.1002/oby.21768
17. Chang Y, Ryu S, Choi Y, et al. Metabolically healthy obesity and development of chronic kidney disease: a cohort study. Ann Intern Med. 2016;164(5):305–312. doi:10.7326/M15-1323
18. Nam KH, Yun HR, Joo YS, et al. Changes in obese metabolic phenotypes over time and risk of incident chronic kidney disease. Diabetes Obes Metab. 2018;20(12):2778–2791. doi:10.1111/dom.13458
19. Wang J, Niratharakumar K, Gokhale K, et al. Obesity without metabolic abnormality and incident CKD: a population-based British cohort study. Am J Kidney Dis. 2022;79(1):24–35.e1. doi:10.1053/j.ajkd.2021.05.008
20. Wang C, Zhang W, Wang Y, et al. Novel associations between sex hormones and diabetic vascular complications in men and postmenopausal women: a cross-sectional study. Cardiovasc Diabetol. 2019;18(1):97. doi:10.1186/s12933-019-0901-6
21. Wang B, Wan H, Cheng J, et al. Blood lead, vitamin D status, and albuminuria in patients with type 2 diabetes. Environ Pollut. 2021;276:116653. doi:10.1016/j.envpol.2021.116653
22. Chen C, Lu FC. Department of Disease Control Ministry of Health, PR China. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17(Suppl):1–36.
23. World Health Organization. Waist Circumference and Waist-Hip Ratio. Report of a WHO Expert Consultation. Geneva: World Health Organization; 2011.
24. National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421.
25. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006
26. Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3(1):21. doi:10.1186/1471-2288-3-21
27. Garofalo C, Borrelli S, Minutolo R, et al. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 2017;91(5):1224–1235. doi:10.1016/j.kint.2016.12.013
28. Chung HF, Al Mamun A, Huang MC, et al. Obesity, weight change, and chronic kidney disease in patients with type 2 diabetes mellitus: a longitudinal study in Taiwan. J Diabet. 2017;9(11):983–993. doi:10.1111/1753-0407.12514
29. Ou YL, Lee MY, Lin IT, et al. Obesity-related indices are associated with albuminuria and advanced kidney disease in type 2 diabetes mellitus. Ren Fail. 2021;43(1):1250–1258. doi:10.1080/0886022X.2021.1969247
30. Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79(3):379–384. doi:10.1093/ajcn/79.3.379
31. Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366(9497):1640–1649. doi:10.1016/S0140-6736(05)67663-5
32. Zhang J, Jiang H, Chen J. Combined effect of body mass index and metabolic status on the risk of prevalent and incident chronic kidney disease: a systematic review and meta-analysis. Oncotarget. 2017;8(22):35619–35629. doi:10.18632/oncotarget.10915
33. Thomas G, Sehgal AR, Kashyap SR, et al. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2011;6(10):2364–2373. doi:10.2215/CJN.02180311
34. Whaley-Connell A, Sowers JR. Obesity and kidney disease: from population to basic science and the search for new therapeutic targets. Kidney Int. 2017;92(2):313–323. doi:10.1016/j.kint.2016.12.034
35. D’Agati VD, Chagnac A, de Vries AP, et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12(8):453–471. doi:10.1038/nrneph.2016.75
36. Xu T, Sheng Z, Yao L. Obesity-related glomerulopathy: pathogenesis, pathologic, clinical characteristics and treatment. Front Med. 2017;11(3):340–348. doi:10.1007/s11684-017-0570-3
37. Felizardo RJ, da Silva MB, Aguiar CF, Câmara NO. Obesity in kidney disease: a heavyweight opponent. World J Nephrol. 2014;3(3):50–63. doi:10.5527/wjn.v3.i3.50
38. Martínez‐Montoro JI, Morales E, Cornejo‐Pareja I, Tinahones FJ, Fernández‐García JC. Obesity-related glomerulopathy: current approaches and future perspectives. Obes Rev. 2022;23(7):e13450. doi:10.1111/obr.13450
39. Wei L, Li Y, Yu Y, et al. Obesity-related glomerulopathy: from mechanism to therapeutic target. Diabetes Metab Syndr Obes. 2021;14:4371–4380. doi:10.2147/DMSO.S334199
40. Sarafidis PA, Ruilope LM. Insulin resistance, hyperinsulinemia, and renal injury: mechanisms and implications. Am J Nephrol. 2006;26(3):232–244. doi:10.1159/000093632
41. Suzuki D, Miyata T, Saotome N, et al. Immunohistochemical evidence for an increased oxidative stress and carbonyl modification of proteins in diabetic glomerular lesions. J Am Soc Nephrol. 1999;10(4):822–832. doi:10.1681/ASN.V104822
42. Prabhakar SS. Role of nitric oxide in diabetic nephropathy. Semin Nephrol. 2004;24(4):333–344. doi:10.1016/j.semnephrol.2004.04.005
43. Hall JE, Do Carmo JM, da Silva AA, et al. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116(6):991–1006. doi:10.1161/CIRCRESAHA.116.305697
44. Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15(11):2792–2800. doi:10.1097/01.ASN.0000141966.69934.21
45. Ellulu MS, Patimah I, Khaza’ai H, et al. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13(4):851–863. doi:10.5114/aoms.2016.58928
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

