Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Obesity and COVID-19 Pandemics: Epidemiology, Mechanisms, and Management
Received 6 October 2023
Accepted for publication 8 December 2023
Published 20 December 2023 Volume 2023:16 Pages 4147—4156
DOI https://doi.org/10.2147/DMSO.S441762
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Yanping Yang,1 Yuanlin Song,1– 3 Dongni Hou2
1Shanghai Institute of Infectious Disease and Biosecurity, Fudan University, Shanghai, People’s Republic of China; 2Shanghai Key Laboratory of Lung Inflammation and Injury, Department of Pulmonary Medicine, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 3Shanghai Respiratory Research Institute, Shanghai, People’s Republic of China
Correspondence: Dongni Hou, Shanghai Key Laboratory of Lung Inflammation and Injury, Department of Pulmonary Medicine, Zhongshan Hospital, Fudan University, 180 Fenglin Road, Shanghai, People’s Republic of China, Email [email protected]
Abstract: Obesity is a principle causative factor of various metabolic dysfunctions, chronic inflammation, and multi-organ impairment. The global epidemic of obesity has constituted the greatest threat to global health. Emerging evidence has associated obesity with an increased risk of severe infection and poor outcomes from coronavirus disease 2019 (COVID-19). During current COVID-19 pandemic, the interaction between COVID-19 and obesity has exaggerated the disease burden of obesity more than ever before. Thus, there is an urgent need for consideration of universal measures to reduce the risk of complications and severe illness from severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in obesity population. In this review, we first summarized the clinical evidence on the effect of obesity on susceptibility, severity, and prognosis of COVID-19. Then we discussed and the underlying mechanisms, including respiratory pathophysiology of obesity, dysregulated inflammation, upregulated angiotensin-converting enzyme 2 (ACE2) expression, hyperglycemia, and adipokines. Finally, we proposed recommendations on how to reduce the spread and pandemic of SARS-CoV-2 infection by prevention and treatment of obesity.
Keywords: obesity, COVID-19, metabolic syndrome, severe acute respiratory syndrome coronavirus 2, angiotensin-converting enzyme 2, inflammation
Background
Since the emergence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in December 2019, it has caused a global health crisis and affected more than 200 countries. Approximately 772 million cases of coronavirus disease- 2019 (COVID-19) and six million deaths have been reported globally as of November 2023.1 Acute SARS-CoV-2 infection can range from asymptomatic to fatal disease, and the symptoms vary (fever, myalgia, headache, respiratory symptoms, loss of taste and smell, cardiovascular complications and gastrointestinal symptoms) and may persist for more than 4 weeks after initial diagnosis.2
Overwhelming studies have investigated the risk factors of for severe outcomes after SARS-CoV-2 infection, and obesity has been found as one of the most common underlying medical conditions associated with disease severity and mortality after adjusting for age and other potential confounders.3–5 In addition to the direct risk, obesity causes metabolic syndrome and increases the risk of developing diabetes, hypertension, and cardiovascular disease. These underlying diseases are also related to an increased risk of severe COVID-19.6–8 Furthermore, higher susceptibility to SARS-CoV-2 infection and serious long-term consequences, increased complications, and possible reduction in vaccine efficacy adds to the risk of acquiring SARS-CoV-2 infection in individuals with obesity.
Most of the existing studies discuss the impact of obesity on COVID-19 from a single perspective. The exact mechanism between obesity and the risk of COVID-19 infection is still unclear. Further studies are needed to understand the relationship and develop and customize intervention and prevention strategies suitable for person with obesity. In this review, we aim to summarize the evidence implicating the higher risk of severity COVID-19 in person with obesity, and discuss the mechanisms of how obesity might predispose patients to develop severe COVID-19. Additionally, we provided practical measures for governments, health care systems, and the public to reduce the transmission and pandemic of SARS-CoV-2 infection by prevention and treatment of obesity. This is important for understanding the etiology of the COVID-19 pandemic, developing intervention strategies, and raising public awareness and health management. To some extent, it can protect the health of patients and reduce the risk of the spread of the epidemic and the deterioration of the disease.
Epidemiology
Although vaccines against SARS-CoV-2 have proved highly effective at preventing COVID-19-associated hospitalization and death, some vaccinated individuals still develop severe outcomes. The rate of severe outcome including acute respiratory failure need hospitalization, need for noninvasive ventilation (NIV) or invasive ventilation, admission to ICU, or death were 18.0 per 10,000 in persons who completed primary vaccination.3 With the rise of new variants of SARS-CoV-2 like Omicron,9 the COVID-19 pandemic continues to threaten public health and the stability of medical systems worldwide. Identifying at risk population and understanding the underlying mechanisms will be important to reduce poor outcomes related to COVID-19. In addition to older age, risk factors for severe COVID-19 outcomes were male agender and underlying conditions such as obesity, diabetes, immunosuppression, chronic renal disease, organ transplantation.3,4 Notably, up to 50% of death related to COVID-19 were found in people who had metabolic and vascular disorders.5 There has been overwhelming evidence suggested obesity is associates with increased incidence, complications, severity and mortality of COVID-19.10
Before the pandemic of SARS-CoV-2, the prevalence of obesity increased dramatically. Obesity is defined as a BMI ≥ 30 kg/m2, and overweight as a BMI ≥25 kg/m2 for adults.11 The incidence of obesity has been nearly tripled worldwide since 1975.11 According to WHO, more than 650 million adults were person with obesity, making up of 13% of the whole population aged 18 years and older, along with 1.9 billion adults over-weight in 2016. Obesity may exert physical, metabolic and molecular effects on multiple organ systems, and is a major risk factor for multiple comorbidities in critically ill patients.12
Susceptibility
Epidemiological studies have found that people with obesity are more susceptible to SARS-CoV-2 infection. In a cross-sectional study, BMI was associated with a positive test of SARS-CoV-2 independent of ethnicity, deprivation, population density, and smoking.13 A meta-analysis of 20 studies showed the risk of testing positive for COVID-19 in individuals with obesity were 46% higher than non-obese individuals (OR: 1.46; 95% CI: 1.30–1.65).14 The increase in odds of SARS-CoV-2 positivity related to person with obesity reported in two other meta-analyses was even higher, up to 78%.15 Even in persons have been fully vaccinated, higher rate of breakthrough infection were also found in person with obesity.16
Severity
For persons infected with SARS-CoV-2, obesity is also one of the most important risk factors predicting severe COVID-19. A large nationwide study from England, the OpenSAFELY study, included more than 17 million adults had COVID-19 showed obesity and diabetes mellitus were independently associated with increased COVID-19 related mortality.17 A body mass index (BMI) of over 40 kg/m2 was associated with nearly twice as much mortality related to COVID-19 when compared with those without obesity. In Qresearch database from England, BMI ≥23kg/m2 was related to a linear increase in the risk of hospitalization and death from COVID-19.10 Moreover, there have been several meta-analyses reported the association between obesity and adverse outcomes of COVID-19. With various outcomes and prevalence of obesity in these studies, the results constantly supported BMI as a risk factor for poor outcomes in COVID-19. Higher BMI was associated with 2~4 times higher risk for hospitalization, 21–88% higher risk for ICU admission or 66–113% higher risk for invasive mechanical ventilation support.18–23 The risk related to obesity differs in old and young population. The higher risk due to obesity was particularly notable in younger adults.10 In consistent, a nationwide study in two representative British birth cohorts found that an earlier age of first obesity was associated with 2–3 times higher risk of long COVID-19 and also hospitalization.24
In addition, certain socioeconomic variables may increase the risk of obesity, thereby increasing the severity and transmission risk of COVID-19. Studies had found that people living in areas with low socioeconomic indices had high rates of overweight or obesity, and that existing negative sociodemographic factors (unemployment, income insecurity, and education) seemed to have a cumulative effect.25 They may face more economic pressures and restrictions and choose cheap and unhealthy foods,26,27 leading to unhealthy dietary choices and lifestyles. The impact was particularly severe for both disproportionately affecting marginalized/disadvantaged populations. Faced with greater barriers to health care services and relatively insufficient medical resources, problems such as obesity cannot be diagnosed and treated in time, which may increase the risk of COVID-19 and poor prognosis.28
Complications
The effect of obesity on the outcome of COVID-19 may be partially attributed to increased complications. Adipose tissue led to increased baseline pleural pressure from the abdominal and chest wall, susceptibility to atelectasis, and collapse of the upper airway, as seen in obstructive sleep apnea. Severe obesity also increases the work load of breathing and hypoventilation may cause hypercapnia.12 Acute respiratory distress syndrome (ARDS), a significant cause of death from COVID-19, develops as a result of excess inflammation after SARS-CoV-2 infection, while inflammatory process has been recognized as a common characteristic of obesity.29,30 Person with obesity with COVID-19 also have predisposition to cardiovascular complications such as hypertension, cardiomyopathy, dysrhythmias, and stroke, due to an increase in total blood volume and cardiac output,31 hypoxia and inflammatory stress,32 and hypercoagulability.33 A study by Onder et al indicated that obesity was associated with the increased probability of acute renal failure in COVID-19 patients,34 while the later contributed significantly to the higher mortality.35 It is also reported that short-term risk of post-COVID-19 venous thromboembolism in person with obesity was about twice of the risk in the counterpart.36
Vaccine Efficacy
As a major risk for poor outcomes in COVID-19, the efficacy of vaccines against SARS-CoV-2 in person with obesity has been of concern. By now, solid evidence suggesting the effect of obesity on SARS-CoV-2 breakthrough the infection or waning of immune memory from vaccines is lacking. A preliminary retrospective study of subject with obesity found that grade III obesity was more common in patients with COVID-19 vaccine breakthrough than those without.37 These findings were in line with the study by Juthani et al which showed the overweight was one of the pre-existing comorbidities in patients with severe break-through infection.16 A cohort study showed that higher waist circumference but not BMI was associated with lower SARS-CoV-2 antibody titers after two COVID-19 mRNA vaccine (Pfizer/BioNTech),38 while another study found higher anti-Spike protein IgG concentrations in obesity people.39 However, the RCT studies which evaluated efficacy of Pfizer-BioNTech and Moderna COVID-19 vaccines showed comparable efficacy between obese and non-obese subgroup within 2 months after vaccination.40,41 Additional studies evaluating long-term maintenance of vaccine induced immunity in person with obesity are warranted.
Viral Load
Studies have shown that COVID-19 patients with obesity also had higher SARS-CoV-2 viral loads in the upper respiratory tract (log10 1.89 genome equivalents for the N1 gene and log10 2.62 for the N2 gene),39,42 implying uncontrolled virus replication in the upper respiratory tract and/or an inefficient immune response in person with obesity. Notably, patients with high viral load tended to have a higher risk of death and incubation.42
Genetic
Albeit overwhelming epidemiological evidence on associations between obesity and susceptibility, there is confounding that additional evidence of causal effect of BMI on susceptibility or severe outcome of COVID-19 is still limited. Freuer et al conducted a multivariable two-sample Mendelian randomization (MR) study which used summary statistics of genome-wide association studies to analyze the causal impact of overall obesity on the susceptibility and severity of COVID-19 disease. The result showed BMI was strongly associated susceptibility after adjustment for genetically predicted visceral obesity traits.43
Mechanisms Linking Obesity to COVID-19
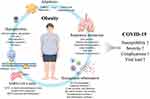
Obesity is a heterogeneous disease, and the effect of obesity on patients with COVID-19 may vary substantially (Figure 1.). Some of the physical, metabolic, and molecular effects may only occur in patients with severe obesity (BMI ≥40–50 kg/m2), others may depend on the adipose distribution or lean muscle mass to BMI.12
Respiratory Pathophysiology of Obesity
Excessive subcutaneous adipose tissue around the chest and abdomen restricts lung expansion and result in decreased vital capacity. Shallow breathing in obesity facilitates respiratory tract infections. In addition, increased baseline pleural pressure leads to reduced expiratory reserve volume and functional residual capacity, which increases the risk of collapse of peripheral dependent airways and atelectasis. In this condition, tidal breathing initiates from low end-expiratory, where the lung are less compliant.44 Basilar atelectasis may cause ventilation/perfusion mismatch and resultant hypoxemia. In addition to subcutaneous adipose, visceral fat depots cause high peritoneal cavity pressure and restrict diaphragm movement and limit lung activity. Visceral fat depots have been reported as the risk factor for severe COVID-19 and a predictor for ICU admission.45,46
In addition, excess parapharyngeal adipose is associated with higher airway resistance and causes the upper airway obstruction or collapse, as reported in obstructive sleep apnea.47 Severe obesity increases respiratory load, resulting in higher oxygen consumption dedicated to respiratory work at baseline,48 and also substantially increases neural respiratory drive to 2–3 times that of non-obese subjects, especially at spine position.49 In patients with Obesity hypoventilation syndrome, when the increased neural drive cannot be maintained, hypercapnia occurs due to hypoventilation. These patients prone to have acute- on -chronic respiratory failure and require non-invasive ventilation in COVID-19.50
Dysregulated Inflammation
There has been substantial evidence suggesting a low grade chronic inflammatory state in obesity. It is characterized by the higher concentration of pro-inflammatory cytokines such as TNF-α, IL-1β, MCP-1 and IL-6, and reduced anti-inflammatory cytokines, such as IL-10, IL-4 and IL-13.51 Previous studies have attributed obesity-related inflammation to activated macrophages in adipose tissue.52 The number of macrophages in adipose tissue in obesity people is increased. Meanwhile, macrophages in adipose tissue are activated and polarized into M1-like macrophages, which secrete proinflammatory cytokines, rather than M2-like macrophages producing anti-inflammatory cytokines. In addition, the inflammation in the adipose tissue induces adipocyte apoptosis and the production of chemotactic mediators, which promote leukocyte infiltration and subsequent accumulation of CD8+ T cells and CD4+ Th1 cells. The leukocytes and T cells, in turn, induce M1 and adipocytes to produce pro-inflammatory, which causing a vicious cycle of inflammation.53 Similar to obesity, SARS-CoV-2 infection also induces an excess production of pro-inflammatory cytokines, which is a quick and robust immune response that is closely associated with severe infection and leads to multiorgan failure.54 Obesity-related chronic low-grade inflammation potentiates dysregulated cytokine response in SARS-CoV-2 infection, and contributes to more severe COVID-19 and mortality.
Obesity has been also associated with increased number but reduced activity in NK cells,55 dendritic cells,56 increased T cell exhaustion and reduced T cell proliferation,57 and defects in antibody production and class-switch recombination in B cells.58 Overall, chronic inflammation and impaired innate and adaptive immunity in obesity increases the risk of severe COVID-19.
SARS-CoV-2 Entry
SARS-CoV-2 uses the ACE2 receptor to enter the host cells and transmembrane protease serine 2 (TMPRSS2) for cleaving the spike protein of the virus to facilitate the fusion of virus and host cell membranes. Spike (S) protein priming. Mice with diet-induced obesity showed increased expression of the ACE2 gene in the lungs.59 In addition, ACE2 expressed in adipose tissue can also serve as a target of SARS-CoV-2 virus, and gene expression profile have shown that ACE gene expression is higher in the visceral and subcutaneous tissues than lung tissue,60 suggesting excess adipose tissue in obesity subjects may serve as a reservoir for the virus. Consistently, in COVID-19 patients, high serum ACE2 level was associated with obesity.61 Studies have found that ACE2 expression can be upregulated by a wide range of pro-inflammatory cytokines,62,63 which is increased in individuals with obesity as previously mentioned. Overall, these findings imply the elevated expression of ACE2 in person with obesity may play a role in facilitating SARS-CoV-2 entry, and thus result in severe COVID-19. In addition to ACE2, another receptor that provides viral entry for SARS-CoV-2 is glucose-regulated protein 78 (GRP78). GRP78 is overexpressed and translocated to the cell membrane or cell surface, when ER stress is induced in obesity and related insulin resistance, nutritional imbalance and excessive fat storage.64,65 Thus increased cell surface GRP78 in patients with obesity may facilitate entry of SARS-CoV-2 into host cells.66
Hyperglycemia
Hyperglycemia and type 2 diabetes are common metabolic dysregulation in obesity. Studies have shown that despite strict glycemic control, diabetic patients have a higher risk of developing ICU-acquired blood stream infection than non-diabetic patients.67 Similarly, diabetes is independently associated with a higher risk of mild and moderate patients developing to severe type of COVID-19 and death.68,69 Another study found that uncontrolled hyperglycemia had a particularly high mortality compared to diabetic patients, underscoring the importance of in-hospital glycemic management.70 Possible molecular mechanisms of correlation between hyperglycemia and SARS-CoV-2 have been proposed. Given both the ACE2 receptor and the spike protein are heavily glycosylated, hyperglycemia might contribute to glycosylation of ACE2 and the viral spike protein, which increase the binding activity of ACE2 and SARS-CoV-2 in obesity and facilitate viral entry. The glycosylation of human ACE2 at the N322 site interacts with the receptor binding domain of the virus spike protein and strengthens the complex, while glycosylation at the N90 site have opposite effects.71 The factors that determine the glycosylation site remain unclear. After viral entry into human cells, excess glucose may promote fast viral replication from the hexosamine biosynthetic pathway (HBP) hijacking substrates from the metabolic environment, which induces overexpression of interferon 5, leading to cytokine overexpression and endoplasmic reticulum (ER) stress.72
Hypertriglyceridemia
Person with obesity tend to have high blood lipid content, especially triglyceride, which is one of the common biochemical indexes of these people. Most person with obesity have triglyceride levels well above normal values.73 Obesity and hypertriglyceridemia are important risk factors for severe COVID-19 and mortality, which are negatively correlated with the prognosis.74,75 Caricchio et al76 found that triglycerides could well explain the relationship between obesity and the severity of COVID-19. Cytokine storm was the main cause of poor prognosis of person with obesity and the triglyceride contents were found to be twice as high in patients who experienced cytokine storms as in those who did not. There is a hypothesis that explains this phenomenon from the perspective of ACE2 and fat metabolism. After COVID-19, the virus enters and infects adipocytes by binding to the ACE2 of adipocytes through surface spike proteins. Adipocytes with high triglyceride levels rapidly promote virus reproduction and release, which further aggravates infections.77–79 In addition, adipocytes can produce angiotensinogen, which promotes adipocytes to convert excess glucose into triglycerides. Then ACE2 converts angiotensin I / II into angiotensin 1–7, which reduces the production of triglycerides and inhibits the inflammatory response of macrophages.80–82 After COVID-19, massive virus replication consumes ACE2, resulting in reduced inhibition of triglycerides and a large number of inflammatory reactions.
Adipokines
Adipokines are cytokines produced by adipocytes to regulate and maintain energy metabolic homeostasis. Leptin and adiponectin are crucial adipokines reported to play a role in severe COVID-19.83,84 Leptin is a pro-inflammatory adipokine highly produced in obesity, which contributes to obesity-related chronic low-grade inflammation.85 Higher levels of serum leptin were associated with the risk for mechanical ventilation in COVID-19 patients.83 In contrast, as an anti-inflammatory adipokine, adiponectin is decreased in obesity subjects.86 Adiponectin reduces T cell response to pathogens, modulates macrophage phenotype to M2 type, and stimulates the production of anti-inflammatory IL-10 by macrophages.87 Clinical study also found reduced antiprotection level in the circulation of COVID-19 patients.88 Alterations in leptin and adiponectin levels in obesity may lead to a dysregulated immune response to SARS-CoV-2 infection.
Clinical Implications
Growing evidence has identified obesity or ectopic fact deposition as independent risk factor for COVID-19 severity and death as indicated. Overlapping with the COVID-19 pandemic, the global pandemic of obesity and its comorbidities are great challenges for preventing morbidity and mortality from COVID-19 and other future pandemic viral infections. Moreover, extended self-quarantine, widespread shutdown duration, and adverse psychological reactions result from measures for reducing transmission of SARS-CoV-2 have led to unintended consequence of weight gain,89 which in turn could increase the risk for poor prognosis of COVID-19.
Management
Obesity is one of the most common modifiable risk factors for COVID-19, more efforts in improvements in lifestyle as well as additional measure to control obesity may benefit in reducing transmission and pandemic of SARS-CoV-2 infection.90 Weight loss is a fundamental way to reduce the negative effects of obesity. Physical exercise, dietary changes and both surgical and non-surgical weight loss strategies are suggested as measures to reverse obesity and treat its associated co-morbidities.53 Appropriate physical activities can be added according to their own physical conditions, such as light aerobic exercise, family exercises or stretching exercises, to promote physical recovery and improve cardiopulmonary function.91 Evidence has showed benefit of intermittent fasting regimens in improve glucose homeostasis and insulin sensitivity.92 Future clinical studies testing the role of dietary strategies, including IF, in improving antiviral immunity and reducing the severity of COVID-19 is needed. Obesity is associated with an increased risk of chronic diseases such as diabetes and hypertension. Continuous monitoring of blood pressure, blood glucose and blood lipid levels is needed.93,94 Meanwhile, more investment in health care systems to work with physicians, professional dietitians, and other healthcare professionals to provide timely mental health support and facilitate access to interventions to determine the best management strategy for the individual.95,96 Currently, the efficacy of anti-inflammatory drugs (eg Tocilizumab, non-steroidal anti-inflammatory drugs, etc.) or combination therapy of anti-viral with anti-inflammatories is yet to be evaluated in person with obesity. No strong evidence suggested reduced efficacy of vaccination against SARS-CoV-2, thus vaccination is still the best choice for prevention and control of COVID-19 for person with obesity.
Conclusions
The overlapping two pandemics of SARS-CoV-2 and obesity have resulted in a major global health crisis. In this review, we summarized the clinical evidence for the impact of obesity on susceptibility, severity, and prognosis COVID-19, and discussed and the underlying mechanisms. Success in reducing the obesity-related disease burden of COVID-19 will require not only individual lifestyles changes but also coordinated efforts and investments from governments, health care systems, and the public in obesity prevention and treatments. Further research should focus on a deeper understanding of how obesity and obesity-related metabolic dysregulation modulate the specific inflammatory pathways involved in SARS-CoV-2 infection and new drug targets that preferentially suppress detrimental inflammatory responses in person with obesity.
Abbreviations
COVID-19, Coronavirus disease 2019; SARS-CoV-2, Severe acute respiratory syndrome coronavirus-2; ACE2, Angiotensin-converting enzyme 2; NIV, Noninvasive ventilation; BMI: Body mass index; ARDS, Acute respiratory distress syndrome; TNF-α, Tumor necrosis factor –α; IL, Interleukin; MCP-1, Monocyte chemoattractant protein-1; NK cell, natural killer cell; TMPRSS2, transmembrane protease serine 2; GRP78, Glucose-regulated protein 78; HBP, Hexosamine biosynthetic pathway.
Funding
This work is partly supported by grants from the Shanghai Municipal Science and Technology Major Project (ZD2021CY001), Shanghai Municipal Key Clinical Specialty (shslczdzk02201), National Natural Science Foundation of China (82130001, 82070045), Science and Technology Commission of Shanghai Municipality (20Z11901000, 20DZ2261200, 20XD1401200), and Clinical Research Plan of SHDC (SHDC2020CR5010-002).
Disclosure
The authors declare that they have no competing interests.
References
1. Coronavirus disease (covid-19) situation reports (no date) World Health Organization. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
2. Cevik M, Kuppalli K, Kindrachuk J, Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020;371:m3862. doi:10.1136/bmj.m3862
3. Kim L, Garg S, O’Halloran A, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis. 2021;72(9):e206–e14. doi:10.1093/cid/ciaa1012
4. Hippisley-Cox J, Coupland CA, Mehta N, et al. Risk prediction of covid-19 related death and hospital admission in adults after covid-19 vaccination: national prospective cohort study. BMJ. 2021;374:n2244. doi:10.1136/bmj.n2244
5. Thakur B, Dubey P, Benitez J, et al. A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Sci Rep. 2021;11(1):8562. doi:10.1038/s41598-021-88130-w
6. Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782–792. doi:10.1016/S2213-8587(20)30238-2
7. Batabyal R, Freishtat N, Hill E, Rehman M, Freishtat R, Koutroulis I. Metabolic dysfunction and immunometabolism in COVID-19 pathophysiology and therapeutics. Int J Obes. 2021;45(6):1163–1169. doi:10.1038/s41366-021-00804-7
8. Costa FF, Rosario WR, Ribeiro Farias AC, de Souza RG, Duarte Gondim RS, Barroso WA. Metabolic syndrome and COVID-19: an update on the associated comorbidities and proposed therapies. Diabetes Metab Syndr. 2020;14(5):809–814. doi:10.1016/j.dsx.2020.06.016
9. Dhama K, Nainu F, Frediansyah A, et al. Global emerging Omicron variant of SARS-CoV-2: impacts, challenges and strategies. J Infect Public Health. 2023;16(1):4–14. doi:10.1016/j.jiph.2022.11.024
10. Gao M, Piernas C, Astbury NM, et al. Associations between body-mass index and COVID-19 severity in 6.9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2021;9(6):350–359. doi:10.1016/S2213-8587(21)00089-9
11. Obesity and overweight (no date) World Health Organization. Available from: https://www.who.int/zh/news-room/fact-sheets/detail/obesity-and-overweight.
12. Anderson MR, Shashaty MGS. Impact of Obesity in Critical Illness. Chest. 2021;160(6):2135–2145. doi:10.1016/j.chest.2021.08.001
13. de Lusignan S, Dorward J, Correa A, et al. Risk factors for SARS-CoV-2 among patients in the oxford royal college of general practitioners research and surveillance centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20(9):1034–1042. doi:10.1016/S1473-3099(20)30371-6
14. Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21(11):e13128. doi:10.1111/obr.13128
15. Yang J, Tian C, Chen Y, Zhu C, Chi H, Li J. Obesity aggravates COVID-19: an updated systematic review and meta-analysis. J Med Virol. 2021;93(5):2662–2674. doi:10.1002/jmv.26677
16. Juthani PV, Gupta A, Borges KA, et al. Hospitalisation among vaccine breakthrough COVID-19 infections. Lancet Infect Dis. 2021;21(11):1485–1486. doi:10.1016/S1473-3099(21)00558-2
17. Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected - obesity, impaired metabolic health and COVID-19. Nat Rev Endocrinol. 2021;17(3):135–149. doi:10.1038/s41574-020-00462-1
18. Huang Y, Lu Y, Huang YM, et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020;113:154378. doi:10.1016/j.metabol.2020.154378
19. Foldi M, Farkas N, Kiss S, et al. Obesity is a risk factor for developing critical condition in COVID-19 patients: a systematic review and meta-analysis. Obes Rev. 2020;21(10):e13095. doi:10.1111/obr.13095
20. Poly TN, Islam MM, Yang HC, et al. Obesity and mortality among patients diagnosed with COVID-19: a systematic review and meta-analysis. Front Med. 2021;8:620044. doi:10.3389/fmed.2021.620044
21. Soeroto AY, Soetedjo NN, Purwiga A, et al. Effect of increased BMI and obesity on the outcome of COVID-19 adult patients: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14(6):1897–1904. doi:10.1016/j.dsx.2020.09.029
22. Malik P, Patel U, Patel K, et al. Obesity a predictor of outcomes of COVID-19 hospitalized patients-A systematic review and meta-analysis. J Med Virol. 2021;93(2):1188–1193. doi:10.1002/jmv.26555
23. Yang Y, Wang L, Liu J, Fu S, Zhou L, Wang Y. Obesity or increased body mass index and the risk of severe outcomes in patients with COVID-19: a protocol for systematic review and meta-analysis. Medicine. 2022;101(1):e28499. doi:10.1097/MD.0000000000028499
24. Bridger Staatz C, Bann D, Ploubidis GB, Goodman A, Silverwood RJ. Age of first overweight and obesity, COVID-19 and long COVID in two British birth cohorts. J Epidemiol Glob Health. 2023;13(1):140–153. doi:10.1007/s44197-023-00093-5
25. Diamantis DV, Karatzi K, Kantaras P, et al. Prevalence and socioeconomic correlates of adult obesity in Europe: the Feel4Diabetes study. Int J Environ Res Public Health. 2022;19(19):12572. doi:10.3390/ijerph191912572
26. van Diepen RJ, van Erpecum CL, Tabak D, van Zon SKR, Bültmann U, Smidt N. Neighborhood socioeconomic differences in BMI: the role of fast-food outlets and physical activity facilities. Obesity. 2023;31(2):506–514. doi:10.1002/oby.23617
27. Allen J. The indirect effects of food insecurity on obesogenic environments. Front Public Health. 2023;10:1052957. doi:10.3389/fpubh.2022.1052957
28. De Lorenzo A, Cenname G, Marchetti M, et al. Social inequalities and nutritional disparities: the link between obesity and COVID-19. Eur Rev Med Pharmacol Sci. 2022;26(1):320–339. doi:10.26355/eurrev_202201_27784
29. Marques MB, Langouche L. Endocrine, metabolic, and morphologic alterations of adipose tissue during critical illness. Crit Care Med. 2013;41(1):317–325. doi:10.1097/CCM.0b013e318265f21c
30. Heymsfield SB, Wadden TA, Longo DL. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376(3):254–266. doi:10.1056/NEJMra1514009
31. Park JB, Kim DH, Lee H, et al. Obesity and metabolic health status are determinants for the clinical expression of hypertrophic cardiomyopathy. Eur J Prev Cardiol. 2020;27(17):1849–1857. doi:10.1177/2047487319889714
32. Kayser B, Verges S. Hypoxia, energy balance, and obesity: an update. Obes Rev. 2021;22(Suppl 2):e13192.
33. Zhang S, Zhang J, Wang C, et al. COVID19 and ischemic stroke: mechanisms of hypercoagulability (Review). Int J Mol Med. 2021;47(3). doi:10.3892/ijmm.2021.4854
34. Onder G, Palmieri L, Vanacore N, Giuliano M, Brusaferro S; Italian National Institute of Health C-MG. Nonrespiratory complications and obesity in patients dying with COVID-19 in Italy. Obesity. 2021;29(1):20–23. doi:10.1002/oby.23007
35. Nimkar A, Naaraayan A, Hasan A, et al. Incidence and risk factors for acute kidney injury and its effect on mortality in patients hospitalized from COVID-19. Mayo Clin Proc Innov Qual Outcomes. 2020;4(6):687–695. doi:10.1016/j.mayocpiqo.2020.07.003
36. Xie J, Prats-Uribe A, Feng Q, et al. Clinical and genetic risk factors for acute incident venous thromboembolism in ambulatory patients with COVID-19. JAMA Intern Med. 2022;182(10):1063. doi:10.1001/jamainternmed.2022.3858
37. Muscogiuri G, Barrea L, Verde L, Vetrani C, Savastano S, Colao A. The ”identikit” of subject with obesity and COVID-19 vaccine breakthrough. EXCLI J. 2022;21:687–694. doi:10.17179/excli2022-4864
38. Watanabe M, Balena A, Tuccinardi D, et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab Res Rev. 2022;38(1):e3465. doi:10.1002/dmrr.3465
39. Epsi NJ, Richard SA, Laing ED, et al. Clinical, immunological, and virological SARS-CoV-2 phenotypes in obese and nonobese military health system beneficiaries. J Infect Dis. 2021;224(9):1462–1472. doi:10.1093/infdis/jiab396
40. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi:10.1056/NEJMoa2034577
41. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi:10.1056/NEJMoa2035389
42. Maltezou HC, Raftopoulos V, Vorou R, et al. Association between upper respiratory tract viral load, comorbidities, disease severity, and outcome of patients with SARS-CoV-2 infection. J Infect Dis. 2021;223(7):1132–1138. doi:10.1093/infdis/jiaa804
43. Freuer D, Linseisen J, Meisinger C. Impact of body composition on COVID-19 susceptibility and severity: a two-sample multivariable Mendelian randomization study. Metabolism. 2021;118:154732. doi:10.1016/j.metabol.2021.154732
44. Behazin N, Jones SB, Cohen RI, Loring SH. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol. 2010;108(1):212–218. doi:10.1152/japplphysiol.91356.2008
45. Chandarana H, Dane B, Mikheev A, Taffel MT, Feng Y, Rusinek H. Visceral adipose tissue in patients with COVID-19: risk stratification for severity. Abdom Radiol. 2021;46(2):818–825. doi:10.1007/s00261-020-02693-2
46. Pepin JL, Timsit JF, Tamisier R, Borel JC, Levy P, Jaber S. Prevention and care of respiratory failure in obese patients. Lancet Respir Med. 2016;4(5):407–418. doi:10.1016/S2213-2600(16)00054-0
47. Busetto L, Enzi G, Inelmen EM, et al. Obstructive sleep apnea syndrome in morbid obesity: effects of intragastric balloon. Chest. 2005;128(2):618–623. doi:10.1378/chest.128.2.618
48. Kress JP, Pohlman AS, Alverdy J, Hall JB. The impact of morbid obesity on oxygen cost of breathing (VO(2RESP)) at rest. Am J Respir Crit Care Med. 1999;160(3):883–886. doi:10.1164/ajrccm.160.3.9902058
49. Steier J, Jolley CJ, Seymour J, Roughton M, Polkey MI, Moxham J. Neural respiratory drive in obesity. Thorax. 2009;64(8):719–725. doi:10.1136/thx.2008.109728
50. Masa JF, Pepin JL, Borel JC, Mokhlesi B, Murphy PB, Sanchez-Quiroga MA. Obesity hypoventilation syndrome. Eur Respir Rev. 2019;28(151):180097. doi:10.1183/16000617.0097-2018
51. Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015;3(3):207–215. doi:10.1016/S2213-8587(14)70134-2
52. Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13(8):707–712. doi:10.1038/ni.2343
53. Morais AHA, Passos TS, de Lima Vale SH, da Silva Maia JK, Maciel BLL. Obesity and the increased risk for COVID-19: mechanisms and nutritional management. Nutr Res Rev. 2021;34(2):209–221. doi:10.1017/S095442242000027X
54. Moreno-Fernandez J, Ochoa J, Ojeda ML, Nogales F, Carreras O, Díaz-Castro J. Inflammation and oxidative stress, the links between obesity and COVID-19: a narrative review. J Physiol Biochem. 2022;78(3):581–591. doi:10.1007/s13105-022-00887-4
55. Bahr I, Spielmann J, Quandt D, Kielstein H. Obesity-associated alterations of natural killer cells and immunosurveillance of cancer. Front Immunol. 2020;11:245. doi:10.3389/fimmu.2020.00245
56. O’Shea D, Corrigan M, Dunne MR, et al. Changes in human dendritic cell number and function in severe obesity may contribute to increased susceptibility to viral infection. Int J Obes. 2013;37(11):1510–1513. doi:10.1038/ijo.2013.16
57. Wang Z, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25(1):141–151. doi:10.1038/s41591-018-0221-5
58. Farnsworth CW, Schott EM, Benvie A, et al. Exacerbated staphylococcus aureus foot infections in obese/diabetic mice are associated with impaired germinal center reactions, Ig class switching, and humoral immunity. J Immunol. 2018;201(2):560–572. doi:10.4049/jimmunol.1800253
59. Al Heialy S, Hachim MY, Senok A, et al. Regulation of angiotensin- converting Enzyme 2 in obesity: implications for COVID-19. Front Physiol. 2020;11:555039. doi:10.3389/fphys.2020.555039
60. Al-Benna S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes Med. 2020;19:100283. doi:10.1016/j.obmed.2020.100283
61. Makhoul E, Aklinski JL, Miller J, et al. A review of COVID-19 in relation to metabolic syndrome: obesity, hypertension, diabetes, and dyslipidemia. Cureus. 2022;14(7):e27438. doi:10.7759/cureus.27438
62. Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–35 e19. doi:10.1016/j.cell.2020.04.035
63. Hennighausen L, Lee HK. Activation of the SARS-CoV-2 receptor Ace2 by cytokines through pan JAK-STAT enhancers. SSRN. 2020;3601827. doi:10.2139/ssrn.3601827
64. Kawasaki N, Asada R, Saito A, Kanemoto S, Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep. 2012;2(1):799. doi:10.1038/srep00799
65. Amen OM, Sarker SD, Ghildyal R, Arya A. Endoplasmic reticulum stress activates unfolded protein response signaling and mediates inflammation, obesity, and cardiac dysfunction: therapeutic and molecular approach. Front Pharmacol. 2019;10:977. doi:10.3389/fphar.2019.00977
66. Ha DP, Van Krieken R, Carlos AJ, Lee AS. The stress-inducible molecular chaperone GRP78 as potential therapeutic target for coronavirus infection. J Infect. 2020;81(3):452–482. doi:10.1016/j.jinf.2020.06.017
67. Michalia M, Kompoti M, Koutsikou A, et al. Diabetes mellitus is an independent risk factor for ICU-acquired bloodstream infections. Intensive Care Med. 2009;35(3):448–454. doi:10.1007/s00134-008-1288-0
68. Wu J, Huang J, Zhu G, et al. Elevation of blood glucose level predicts worse outcomes in hospitalized patients with COVID-19: a retrospective cohort study. BMJ Open Diabetes Res Care. 2020;8(1):e001476. doi:10.1136/bmjdrc-2020-001476
69. Yang P, Wang N, Wang J, Luo A, Gao F, Tu Y. Admission fasting plasma glucose is an independent risk factor for 28-day mortality in patients with COVID-19. J Med Virol. 2021;93(4):2168–2176. doi:10.1002/jmv.26608
70. Bode B, Garrett V, Messler J, et al. Glycemic Characteristics and Clinical Outcomes of COVID-19 Patients Hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813–821. doi:10.1177/1932296820924469
71. Mehdipour AR, Hummer G. Dual nature of human ACE2 glycosylation in binding to SARS-CoV-2 spike. Proc Natl Acad Sci U S A. 2021;118(19):e2100425118.
72. Laviada-Molina HA, Leal-Berumen I, Rodriguez-Ayala E, Bastarrachea RA. Working hypothesis for glucose metabolism and sars-cov-2 replication: interplay Between the Hexosamine Pathway and Interferon RF5 triggering hyperinflammation. Role of BCG vaccine? Front Endocrinol. 2020;11:514. doi:10.3389/fendo.2020.00514
73. Ni SJ, Gao JL, Shen SS. 倪苏婕,高健林,沈树泉.肥胖和高甘油三酯血症与重症新型冠状病毒肺炎及其死亡相关联的临床证据及可能机制 [Obesity and hypertriglyceridemia: high risks for disease severity and mortality in COVID-19 patients]. Sheng Li Xue Bao. 2022;74(5):783–791. Chinese.
74. Qin C, Minghan H, Ziwen Z, Yukun L. Alteration of lipid profile and value of lipids in the prediction of the length of hospital stay in COVID-19 pneumonia patients. Food Sci Nutr. 2020;8(11):6144–6152. doi:10.1002/fsn3.1907
75. Zhong P, Wang Z, Du Z. Serum triglyceride levels and related factors as prognostic indicators in COVID-19 patients: a retrospective study. Immun Inflamm Dis. 2021;9(3):1055–1060. doi:10.1002/iid3.469
76. Caricchio R, Gallucci M, Dass C, et al. Preliminary predictive criteria for COVID-19 cytokine storm. Ann Rheum Dis. 2021;80(1):88–95. doi:10.1136/annrheumdis-2020-218323
77. Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1–7 axis of the renin-angiotensin system in heart failure. Circ Res. 2016;118(8):1313–1326. doi:10.1161/CIRCRESAHA.116.307708
78. Samavati L, Uhal BD. ACE2, much more than just a receptor for SARS-COV-2. Front Cell Infect Microbiol. 2020;10:317. doi:10.3389/fcimb.2020.00317
79. Sanchis-Gomar F, Lavie CJ, Mehra MR, Henry BM, Lippi G. Obesity and outcomes in COVID-19: when an epidemic and pandemic collide. Mayo Clin Proc. 2020;95(7):1445–1453. doi:10.1016/j.mayocp.2020.05.006
80. Mori J, Patel VB, Ramprasath T, et al. Angiotensin 1–7 mediates renoprotection against diabetic nephropathy by reducing oxidative stress, inflammation, and lipotoxicity. Am J Physiol Renal Physiol. 2014;306(8):F812–F821. doi:10.1152/ajprenal.00655.2013
81. Patel VB, Mori J, McLean BA, et al. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. Diabetes. 2016;65(1):85–95. doi:10.2337/db15-0399
82. Yasue S, Masuzaki H, Okada S, et al. Adipose tissue-specific regulation of angiotensinogen in obese humans and mice: impact of nutritional status and adipocyte hypertrophy. Am J Hypertens. 2010;23(4):425–431. doi:10.1038/ajh.2009.263
83. Larsson A, Lipcsey M, Hultstrom M, Frithiof R, Eriksson M. Plasma leptin is increased in intensive care patients with COVID-19-an investigation performed in the PronMed-Cohort. Biomedicines. 2021;10(1):4. doi:10.3390/biomedicines10010004
84. Di Filippo L, De Lorenzo R, Sciorati C, et al. Adiponectin to leptin ratio reflects inflammatory burden and survival in COVID-19. Diabetes Metab. 2021;47(6):101268. doi:10.1016/j.diabet.2021.101268
85. Jung CH, Kim MS. Molecular mechanisms of central leptin resistance in obesity. Arch Pharm Res. 2013;36(2):201–207. doi:10.1007/s12272-013-0020-y
86. Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18(6):1321. doi:10.3390/ijms18061321
87. Choi HM, Doss HM, Kim KS. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int J Mol Sci. 2020;21(4):1219.
88. Kearns SM, Ahern KW, Patrie JT, Horton WB, Harris TE, Kadl A. Reduced adiponectin levels in patients with COVID-19 acute respiratory failure: a case-control study. Physiol Rep. 2021;9(7):e14843. doi:10.14814/phy2.14843
89. Zeigler Z. COVID-19 self-quarantine and weight gain risk factors in adults. Curr Obes Rep. 2021;10(3):423–433. doi:10.1007/s13679-021-00449-7
90. Ho FK, Celis-Morales CA, Gray SR, et al. Modifiable and non-modifiable risk factors for COVID-19, and comparison to risk factors for influenza and pneumonia: results from a UK Biobank prospective cohort study. BMJ Open. 2020;10(11):e040402. doi:10.1136/bmjopen-2020-040402
91. Lino RS, Silva MSP, Jesus DS, et al. Molecular aspects of COVID-19 and its relationship with obesity and physical activity: a narrative review. Sao Paulo Med J. 2023;141(1):78–86. doi:10.1590/1516-3180.2021.1038.r1.06072022
92. Sattar N, Valabhji J. Obesity as a risk factor for severe COVID-19: summary of the best evidence and implications for health care. Curr Obes Rep. 2021;10(3):282–289. doi:10.1007/s13679-021-00448-8
93. Dissanayake H. COVID-19 and metabolic syndrome. Best Pract Res Clin Endocrinol Metab. 2023;37(4):101753. doi:10.1016/j.beem.2023.101753
94. Dohet F, Loap S, Menzel A, et al. Obesity considerations during the COVID-19 outbreak. Int J Vitam Nutr Res. 2022;92(1):67–79. doi:10.1024/0300-9831/a000695
95. Hauner H. The COVID-19 pandemic: challenges for obesity management - a call for providing reliable data and solutions. Obes Facts. 2022;15(3):303–304. doi:10.1159/000524424
96. Melamed OC, Selby P, Taylor VH. Mental health and obesity during the COVID-19 pandemic. Curr Obes Rep. 2022;11(1):23–31. doi:10.1007/s13679-021-00466-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.