Back to Journals » ImmunoTargets and Therapy » Volume 9
Novel Resolution Mediators of Severe Systemic Inflammation
Authors Gudernatsch V, Stefańczyk SA , Mirakaj V
Received 21 December 2019
Accepted for publication 19 February 2020
Published 6 March 2020 Volume 2020:9 Pages 31—41
DOI https://doi.org/10.2147/ITT.S243238
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Michael Shurin
Verena Gudernatsch, Sylwia Anna Stefańczyk, Valbona Mirakaj
Molecular Intensive Care Medicine, Department of Anesthesiology and Intensive Care Medicine, University Hospital Tübingen, Eberhard Karls University Tübingen, Tübingen, Germany
Correspondence: Valbona Mirakaj
Molecular Intensive Care Medicine, Department of Anesthesiology and Intensive Care Medicine, University Hospital Tübingen, Eberhard Karls University Tübingen, Hoppe-Seyler-Straße 3, Tübingen 72076, Germany
Tel +49 7071 29-86622
Fax +49 7071 29-5533
Email [email protected]
Abstract: Nonresolving inflammation, a hallmark of underlying severe inflammatory processes such as sepsis, acute respiratory distress syndrome and multiple organ failure is a major cause of admission to the intensive care unit and high mortality rates. Many survivors develop new functional limitations and health problems, and in cases of sepsis, approximately 40% of patients are rehospitalized within three months. Over the last few decades, better treatment approaches have been adopted. Nevertheless, the lack of knowledge underlying the complex pathophysiology of the inflammatory response organized by numerous mediators and the induction of complex networks impede curative therapy. Thus, increasing evidence indicates that resolution of an acute inflammatory response, considered an active process, is the ideal outcome that leads to tissue restoration and organ function. Many mediators have been identified as immunoresolvents, but only a few have been shown to contribute to both the initial and resolution phases of severe systemic inflammation, and these agents might finally substantially impact the therapeutic approach to severe inflammatory processes. In this review, we depict different resolution mediators/immunoresolvents contributing to resolution programmes specifically related to life-threatening severe inflammatory processes.
Keywords: inflammation, resolution, specialized lipid mediators, neuronal guidance protein, sepsis, immunoresolvents
Inflammation and the Immune Response
Systemic responses to severe injury or major trauma may lead to activation of the immune, endocrine and nervous systems.1 Additional factors triggering these systems involve blood products and the imbalance between perfusion and coagulation.2 The dysfunction of vascular permeability induced by the loss of endothelial integrity facilitates the entrance of proinflammatory mediators and immune cells.3 However, the unsuccessful restoration of homeostasis may ultimately lead to the development of multiorgan failure accompanied by sepsis.4–6
Initial Phase of Sterile Inflammation
Among the first responders, neutrophils (polymorphonuclear leukocytes (PMNs)) are one of the first immune cells to respond to infection or tissue damage.7,8 The first signals responsible for the inflammatory processes underlying sterile inflammation are initially activated by damage-associated molecular patterns (DAMPs) at the tissue injury site. DAMP molecules such as DNA, high mobility group protein B1 (HMGB1), N-formyl peptide, extracellular components, ATP and uric acid9 are released from damaged and necrotic cells to induce the recruitment of neutrophils via their cell-surface DAMP receptors— the so-called pattern recognition receptors (PRRs). Known PRRs are categorized into the main classes: Toll-like receptors, C-type lectin receptors, and retinoic acid-inducible gene 1-like receptors.10 Moreover, DAMPs induce the exposure of hydrogen peroxide (H2O2) to activate the early recruitment of neutrophils through the SRC family kinase LYN.7 For further neutrophil recruitment, DAMPs activate the production of proinflammatory mediators such as CXC-chemokine ligand 8 (CXCL8) family chemokines and lipid mediators, e.g. leukotriene B4 (LTB4), within the surrounding immune cells.11,12 In turn, the activated neutrophils induce the release of CXCL8 family chemokines and LTB4 to mediate neutrophil migration to the site of injury. Further components of the inflammatory response involve the rapid activation of the complement system, particularly with the increased generation of C3a and C5a.13,14 These factors lead to the extended participation of immune competent cells, including macrophages (MΦ), dendritic cells, mast cells and T-cells.15–18
Onset of Nonsterile Inflammation
In addition to the concept of DAMPs, pathogen-associated molecular patterns (PAMPs) expressed by pathogens and PRRs characterize a major variable in the control of the initial immune response to nonsterile inflammation.19 From a molecular viewpoint, the initial inflammatory response to infection is largely similar to that of sterile inflammation caused by trauma, ischaemic reperfusion injury or burns.20 This point may explain the difficulties in making precise diagnoses and finally elucidating a better targeted therapy based on only clinical signs.
Resolution and the Main Players
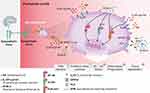
The ideal outcomes of an acute inflammatory response are the termination of inflammation, prevention of excessive tissue injury and, ultimately, the restoration of tissue homeostasis (Figure 1). Various molecules belonging to a continually growing superfamily of proresolving molecules actively mediate key factors of resolution, including the limitation of PMN tissue infiltration, promotion of macrophage (MΦ) reprogramming and MΦ phagocytosis of apoptotic PMNs and cell debris, relief of pain, and counterregulation of inflammatory mediators. Central members of these immunoresolvents are specialized proresolving mediators (SPMs), which are derived from polyunsaturated fatty acids, such as arachidonic acid (AA), docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), and comprise the lipoxin, resolvin, protectin and maresin families. During the onset of an acute inflammatory response, the basis for SPM generation in the resolution phase is established by a lipid mediator class switch. Here, AA-derived mediator synthesis switches from proinflammatory leukotrienes (LTs) and prostaglandins (PGs) to proresolving lipoxins, resolvins and protectins. Interestingly, PGs can exhibit diverse functions: they are essential inducers of inflammation during the onset phase, can indirectly function as proresolvers by inducing the expression of enzymes necessary for the biosynthesis of SPMs, and can evoke anti–inflammatory and immunosuppressive responses. PGE2 not only stimulates LTB4-mediated PMN recruitment to sites of inflammation21 but also promotes the translation of the enzyme 15-lipoxygenase (LOX) type I to enhance the generation of SPMs.22,30 Importantly, immunoresolvents such as lipoxins promote the resolution of inflammation without suppressing the immune system of the host and thus represent an attractive new therapeutic approach for inflammatory conditions.
Immune Cell Function During Resolution
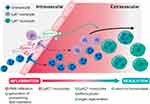
At the cellular level, resolution depends on clearance of the inflammatory infiltrate, which is achieved by several central processes. First, SPMs such as lipoxin A4 (LXA4)23 and Resolvin E1 (RvE1)24 inhibit further PMN recruitment. Second, to remove the already infiltrated neutrophils from the tissue, PMNs become apoptotic and are ingested by MΦ (so-called efferocytosis). Efferocytosis is essential since apoptotic cells can become secondarily necrotic and trigger non-resolving, pathological inflammatory responses.25 Briefly, efferocytosis is defined as a carefully orchestrated process by which professional or non-professional phagocytes are designed to act in the characterized four phases: (1) apoptotic cell finding, (2) apoptotic cell binding, (3) apoptotic cell internalization and (4) apoptotic cell degeneration.25,26 During this process apoptotic cells have an active role in their own clearance. First, they release distinct molecules such as chemokines, nucleotides ATP and UTP, sphingosine-1-phosphate (S1P), lysophophatidylcholine (LPC) or fractalkine to activate the mobilization of phagocytic cells. Phagocytotic cells sense these signals through their correspondent receptors (P2Y purinoceptor 2 (P2Y2), S1PRs, G-protein-coupled receptor G2A, and CXCR3). Second, apoptotic cells express further signals that engage phagocytic receptors and bridging molecules to facilitate engulfment. During this process phosphatidylserine (PtdSer or PS), interacts with the PtdSer-specific receptors, T cell immunoglobulin mucin receptors (TIM1, TIM3, TIM4), brain-specific angiogenesis inhibitor 1 (BAI1), stabilin-2, and RAGE, as well as the PS-specific bridging molecules milk fat globule-EGF factor 8 (MFG-E8), growth arrest-specific protein 6 (Gas6), and protein S. These bridging molecules activate other surface engulfment receptors (integrin αvβ3 or Tryo3-Axl-Mer (TAM)) to accelerate uptake and then finally to induce the process of internalization and degeneration of apoptotic cells.25,26 This apoptosis of neutrophils is crucial for an efficient resolution27 because it prevents exuberant PMN activation and, together with the engulfment by MΦ, promotes an MΦ phenotypic switch from the classical inflammatory phenotype towards a proresolving, alternatively activated MΦ phenotype. This phenotypic switch is possible due to the plasticity of MΦ and represents another fundamental process during the resolution of inflammation. Classical MΦs shut down their generation of proinflammatory mediators and activate a transcriptional programme resulting in the release of anti–inflammatory cytokines (eg IL-10 and transforming growth factor β (TGFβ)) and various growth factors (such as amphiregulin and vascular endothelial growth factor α (VEGFα)). Thus, these alternatively activated MΦs dampen the acute inflammatory response and are crucial regulators of tissue repair by stimulating cellular proliferation and angiogenesis. Third, nonapoptotic leucocytes exit the site of inflammation via lymphatics or reverse migration.28–32
Post-Resolution Phase
To ultimately restore tissue function, repair and regeneration mechanisms must be activated. Various cell types are involved in their regulation; however, MΦs play a central role. In particular, alternatively activated MΦs exhibit a crucial regulatory activity to promote the proliferation of parenchymal and stromal cells, angiogenesis to restore the oxygen supply, fibroblast differentiation into myofibroblasts to stimulate wound closure and the generation of extracellular matrix components.31,32 Interestingly, the MΦ-derived immunoresolvent Maresin 1 (MaR1) not only promotes the resolution of acute inflammation but also enhances tissue regeneration.30,33 Other exogenous SPMs, such as Resolvin D1, accelerate the healing of diabetic wounds in a mouse model.34
Role of the Vagus Nerve in Resolution
Increasing evidence suggests that the nervous system and immune system interact and communicate to regulate the host immune response. These inflammatory reflex circuits comprise afferent nerves that sense peripheral inflammation, injury and infection and transmit the signal to the brain stem, which in turn activates efferent nerves to relay signals to modulate immune responses.
Cholinergic Anti-Inflammatory Pathway
A prominent inflammatory reflex involves the vagus nerve, which is termed the cholinergic anti–inflammatory pathway.35 Here, sensory vagus nerves can be activated by inflammatory mediators such as cytokines via their PRR and transmit the signal to the brain stem, resulting in stimulation of efferent vagus nerve fibres. These signals from the vagus nerves are delivered to splenic nerve fibres, which induce the release of acetylcholine (ACh) from T-cells. Subsequent activation of the α7 nicotinic ACh receptor (α7nAChR) on MΦs inhibits the synthesis of proinflammatory mediators and thus suppresses the inflammatory response.35–37 Hence, vagotomy intensifies inflammatory responses and increases tissue damage in conditions such as colitis38 and pancreatitis.39
Vagus Nerve During Resolution of Sterile and Infectious Peritonitis
Recently, the vagal regulation of the resolution phase and SPM generation was revealed.40,41 In one study,40 unilateral vagotomy resulted in intensified sterile murine peritonitis (increased leucocyte numbers, PMNs and enhanced levels of myeloperoxidase (MPO), cytokines and chemokines) and delayed resolution with a longer resolution interval Ri30 of 37 h compared to 24 h in sham-operated mice. Furthermore, vagotomy shifted the lipid mediator profile in exudates during sterile peritonitis by stimulating the synthesis of proinflammatory lipid mediators such as leukotriene B4 (LTB4) while inhibiting the generation of immunoresolvents such as Resolvin D1 (RvD1).40 Interestingly, the administration of RvD1 rescues this hyperinflammatory response in vagotomized mice by limiting excessive PMN infiltration and regulating monocyte recruitment and cytokine production. In addition, the vagus nerve controls the expression of the neuronal guidance protein (NGP) Netrin-1,40 which regulates not only neuronal development but also inflammatory responses.42–45 After vagotomy and peritonitis, the expression of Netrin-1 was substantially decreased, indicating an involvement of Netrin-1 in this neuroimmune interaction.40 During peritonitis, Netrin-1 shortens the time required for resolution and promotes the synthesis of endogenous proresolving mediators. Notably, deficient Netrin-1 expression in Netrin-1 heterozygous mice delayed the resolution time compared to that in wild-type animals, and acceleration with an additional injection of RvD1 was not observed in Netrin+/- mice like it was in wild-type mice, suggesting a bidirectional interaction between Netrin-1 and resolvins. In human MΦs, Netrin-1 upregulated the biosynthesis of SPMs, the efferocytosis of apoptotic PMNs and, synergistically with RvD1, the phagocytosis rate of zymosan A particles. Overall, this study illustrated a novel collaboration of Netrin-1 and RvD1, which are both regulated by the vagus nerve, during the resolution of acute sterile inflammation.
In another report, vagotomy decreased peritoneal immunoresolvent levels and delayed the resolution of inflammation during infectious E. coli peritonitis.41 In this work, Dalli and colleagues demonstrated that vagotomy delayed resolution processes through the dysregulation of tissue retinoic acid-related orphan receptor γ t (RORγt) CD335+ group 3 innate lymphoid cells (ILC3) and changes in the lipid mediator profile of resident peritoneal MΦs. Vagotomy decreases PCTR1 levels before exposure to E. coli infection. Treatment of vagotomized mice with PCTR1 recovered the macrophage phenotypes and partly protected them from infectious phenomena. The authors also showed that acetylcholine, a crucial transmitter in the vagal response, controls the PCTR biosynthetic pathway in ILC3s, which in turn impacts the resident phenotype of peritoneal MΦs.
Role of the Sympathetic Nerve in Resolution
Efforts to better understand inflammatory reflex circuits and the neural regulation of immunity revealed that the classic neuronal terminology of sympathetic and parasympathetic neurons is inaccurate and limited. Cooperation among somatosensory, sensory autonomic and efferent neurons via neurotransmitters such as acetylcholine and catecholamines was demonstrated in some circuits.46
Sympathetic Nerve in Resolution and Tissue Regeneration
Thus, a recent report highlighted a novel facet of inflammatory reflexes in that adrenergic nerve fibres of the sympathetic nervous system together with the NGP Repulsive Guidance Molecule A (RGM-A) regulate the resolution of acute inflammation and promote tissue regeneration (Figure 2).47 The protein RGM-A was shown to substantially influence the initial phase of acute inflammation and the pathobiology of autoimmune encephalomyelitis.43,48,49 In a study by Körner and colleagues,47 RGM-A was shown to induce a phenotypic switch from monocyte-derived human MΦs towards alternatively activated (M2) MΦs, affect PMN and MΦ chemotaxis and stimulate the efferocytosis of apoptotic PMNs. Moreover, RGM-A alone promotes the resolution of acute murine peritonitis by inhibiting PMN recruitment, thus accelerating the resolution interval from 30 h in vehicle controls to 9 h while stimulating the phagocytosis rate and the biosynthesis of exudate SPMs such as LXA4, Mar1 and Protectin DX (PDX). Interestingly, RGM-A increases the expression of the sympathetic β2 adrenergic receptor (β2AR) on human MΦs, while α-adrenergic receptors are not affected or suppressed. The β2AR agonist formoterol, in turn, stimulates the expression of RGM-A on MΦs and in murine peritoneal tissue, thus indicating an interaction between β2 adrenergic receptors and RGM-A signalling. Furthermore, the β2AR agonist alone stimulates key aspects of resolution, but not as substantially as that achieved with RGM-A alone. Notably, the synergistic effect of RGM-A and the β2AR agonist induces an even stronger promotion of the resolution as that achieved with one substance alone, as demonstrated by the biosynthesis of immunoresolvents. Chemical sympathectomy, however, inhibits the resolution during acute peritonitis, as reflected by the enhanced infiltration of PMNs and classical monocytes, decreased clearance of PMNs and generation of SPMs. Additional administration of RGM-A, however, rescues this hyperinflammatory response to chemical sympathectomy by inhibiting the elevated PMN influx and controlling monocyte extravasation as well as the biosynthesis of immunoresolvents. Additionally, RGM-A and/or the β2AR agonist inhibit NF-κB signalling and activate RICTOR as well as PI3K/AKT signalling pathways in peritoneal monocytes.
Role of Neuronal Guidance Proteins in Resolution
The superfamily of neuronal guidance proteins (NGPs) was originally discovered in the developing nervous system and shown to guide growing axons by chemoattraction and chemorepulsion. Over the last few years, NGPs have been identified in peripheral tissues and shown to modulate immune reactions, especially leucocyte migration, through their chemoattractive and chemorepulsive properties. However, recent studies have highlighted various additional immune functions of NGPs in acute and chronic inflammation conditions as well as during the resolution (Figure 3).43
Function of Netrin-1 During Resolution and Regeneration of Sterile Liver Inflammation
The NGP Netrin-1 promotes the resolution of acute inflammation not only during peritonitis40 but also during acute liver inflammation in ischaemia/reperfusion injury (IR).42 The disruption of blood flow (ischaemia) followed by its restoration (reperfusion) results in an intense inflammatory response and represents a major adverse complication in the liver during surgery, transplantation and haemorrhagic shock.50 Interestingly, in Netrin-1-deficient mice, the infiltration of PMNs is enhanced, while nonclassical monocytes and thus efferocytosis are inhibited in the late phase of liver IR compared to that in wild-type (WT) animals, indicating a delayed resolution.42 Treatment of WT mice with Netrin-1 during the ischaemic phase, however, promotes the resolution of liver inflammation by limiting the PMN and classical monocyte numbers, increasing nonclassical monocytes and consequently increasing the MΦ uptake of apoptotic PMNs. Notably, Netrin-1 induces resident MΦs of the liver, Kupffer cells, and their efferocytosis rate. When Netrin-1 is administered in the resolution phase, its stimulating effects on resolution and regeneration during hepatic injury are even stronger than those exhibited at other phases of administration. Furthermore, Netrin-1 is expressed on human MΦs and robustly stimulates the phagocytosis rate of apoptotic PMNs. Netrin-1 not only promotes the biosynthesis of immunoresolvents such as LXA4 during liver inflammation but also stimulates the expression of key growth factors of liver regeneration, such as hepatocyte growth factor (HGF), heparin-binding epidermal growth factor (HB-EGF) and its receptor EGFR. Additionally, the expression of angiogenic vascular endothelial growth factor A (VEGF-A) and its receptors VEGF-R1 and VEGF-R2 as well as the proliferation rate of hepatocytes are robustly increased by Netrin-1 treatment compared to those in controls.42 Together, the results of this study reveal a novel role of the NGP Netrin-1 during the onset, resolution and regeneration phases of acute liver inflammation.42
Impact of Neogenin on Inflammation Resolution and Tissue Regeneration
The receptor of Netrin-1 and RGM-A, Neogenin 1 (Neo1), plays an important role during the onset of acute inflammation.51–53 A recent report identified a previously unknown role of Neo1 during the resolution of inflammation and during regeneration.54 Functional inhibition of Neo1 stimulated the apoptosis of human PMNs and activated find-me signals on apoptotic PMNs as well as find-me and eat-me receptors on human MΦs. Together with the increased phagocytosis rate of MΦs and the stimulated expression of G-protein coupled receptors (GPCRs) for SPMs on human MΦs, the inhibition of Neo1 promotes resolution. Consistently, the lack of Neo1 inhibits the recruitment of PMNs while stimulating their apoptosis as well as the efferocytosis rate of MΦs and the biosynthesis of immunoresolvents such as LXA4 and Mar1 in a murine peritonitis model compared to those in WT controls. Notably, the expression of Neo1 is restricted to classical Ly6Chi monocytes in the peritoneal cavity during acute inflammation, while Neo1 deficiency elicits a phenotype switch towards nonclassical Ly6Clo monocytes by stimulating PI3K/AKT and inhibiting the TGFβ signalling pathway. Endogenous Neo1 repression stimulates tissue repair and regeneration by enhancing IL-10 and TGFβ levels and promoting the proliferative response in the peritoneum during acute inflammation. Functional exogenous blockade of Neo1 with an antibody mitigates the onset phase of acute peritonitis and accelerates the resolution. Additionally, in a cohort of critically ill paediatric ICU (PICU) patients, the plasma levels of Neo1 were shown to correlate with abdominal compartment syndrome (ACS), the intraabdominal hypertension (IAH) grade, the Pediatric Risk of Mortality III (PRISM-III) score, the length of stay in the ICU and survival. This report identified Neo1 as a regulator of the resolution of inflammation and tissue regeneration as well as a predictor of the severity and survival of critically ill paediatric patients.54
Role of Nutrition in Resolution
Ω-3-Enriched Lipid Emulsions for Critically Ill Patients
Ideally, the onset phase of acute inflammation is followed by the resolution phase, which is actively mediated by immunoresolvents derived from AA as well as the two Omega-3 (Ω-3) fatty acids DHA and EPA. Hence, the administration of lipid emulsions (LEs) with Ω-3 supplementation was evaluated in critically ill patients to identify a possible reduction of inflammation and improvement of clinical outcomes.55–57 The results, however, were controversial, and due to the lack of data, no recommendation for the nutritional treatment of critically ill patients was made.58 Additional debates remain about the composition and amount as well as the time point of administration and indication for parenteral LEs.58 The shortage of data about the effects of nutritional support extends to the possible impact of LEs during the initial and resolution phases as well as during tissue regeneration.
Ω-3-Enriched Lipid Emulsions in Sterile Peritonitis and Sepsis
However, a recent report highlights the role of Ω-3-enriched LEs (Ω-3+ LEs) containing long- and medium-chain fatty acids and fish oil (50:40:10) during the resolution of acute murine peritonitis and murine sepsis.59 In a self-limited model of peritonitis, WT mice were treated with Ω-3+ LEs 24 h prior to zymosan A injection, resulting in robust decreases in infiltrating leucocytes such as PMNs, classical monocytes and MΦs, enhanced nonclassical monocyte numbers and phagocytosis rates, and reduced cytokine levels of IL-6 and keratinocyte chemoattractant (murine IL-8 homologue) compared to those in vehicle controls. Furthermore, Ω-3+ LEs decrease leucocyte adherence to the endothelium as well as their migration, whereas they stimulate the rolling velocity of leucocytes during murine peritonitis compared to that in controls as evaluated by intravital microscopy. Consequently, Ω-3+ LE treatment accelerates resolution, as a strong reduction in the resolution interval Ri was observed in Ω-3+ LE-treated mice. Moreover, Ω-3+ LEs stimulate the generation of immunoresolvents such as LXA4, PDX and Mar1 and their precursors and induce a mediator class switch from prostaglandins to SPMs during murine peritonitis in comparison to these features in control animals. Ω-3+ LEs attenuate not only the initial phase of acute inflammation but also stimulate the resolution phase as well as peritoneal regeneration, as demonstrated by a robust enhancement of exudate IL-10 and TGFβ amounts as well as an increased proliferation rate of peritoneal cells in a mouse model of zymosan A peritonitis.59
In human MΦs, Ω-3+ LEs significantly enhance the phagocytosis rates of apoptotic PMNs, zymosan A particles and Escherichia coli compared to those in cells treated with nonenriched Ω-3 (Ω-3−) LEs composed of long- and medium-chain fatty acids (50:50). Additionally, the expression of receptors for SPMs, such as ALX/FRP2, DRV1/GPR32 and ERV/ChemR23, on human MΦs is enhanced by stimulation with Ω-3+ LEs and tumour necrosis factor (TNF) α, whereas treatment with Ω-3− LEs and TNF α fails to induce these proresolving effects.59
Severe conditions such as sepsis due to infection or sterile injury, ischaemia/reperfusion injury and cancer are associated with metabolic and immune alterations, which can cause dysregulated inflammatory responses leading to organ dysfunction and a high morbidity and mortality.58,60 The treatment options for sepsis, however, are unspecific and concentrate on symptomatic therapy.60 Körner and colleagues demonstrated that only dietary Ω-3+ LEs improved survival and protected against weight loss and hypothermia in a murine polymicrobial sepsis model compared to that in vehicle and Ω-3− LEs controls.59 In addition, Ω-3+ LEs decrease leucocyte infiltration, especially PMNs, and enhance the generation of immunoresolvents such as LXA4, Mar1 and PDX compared to those in vehicle- and Ω-3− LE-treated animals. Together, the results of this study highlight the beneficial effects of dietary Ω-3+ LE treatment on murine sepsis as well as the regulation of Ω-3+ LEs during the initial and resolution phases and during tissue regeneration and repair.
Concluding Remarks
In translational terms, new evidence points to that excessive, uncontrolled inflammatory response can lead to tissue dysfunction and in extreme cases to tissue damage with loss of organ function. It is proven that the inflammatory response can be subdivided in three main phases: (1) the onset, (2) resolution and (3) post-resolution phase.30,61 Up to the present, we consider the acute inflammation as a temporal crescendo of the resolution mediators and decrescendo of the initiating inflammatory chemical mediators. As described above, upon initiation of an inflammatory event the innate immune system induces the tissue-resident cells to perceive the inflammatory stimulus and to express inflammatory mediators such as chemokines, cytokines, classic eicosanoids, free radicals and vasoactive amins.3,61 This response is associated with the recruitment and accumulation of leukocytes in the affected tissues. In the course of this, the key characteristic programs of inflammation resolution become activated to achieve a state of tissue homeostasis. Firstly, the release of “stop signals” such as transcription factors, anti–inflammatory cytokines, miRNA genes and NGP3,43,61 etc. is crucial to dampen the leukocyte accumulation. Inflammation-initiating molecules such as cyclooxygenase derived prostaglandin E2 (PGE2) or PGD2 within the neutrophils convert through lipid-mediator class switching to anti–inflammatory and pro-resolving molecules, such as lipoxins, resolvins and protectins.30 This PMN phenotype switch implies the strong connection between the initiation and resolution phase, because SPMs also induces MΦ phenotype reprograming toward a pro-resolving phenotype.30,61 The clearance or elimination of inflammatory cells can be further initiated by: reverse neutrophil migration, leukocyte apoptosis in the inflamed tissue, efferocytosis and phagocytosis. Thus, these processes indicate that the acute inflammatory response is a highly coordinated process in which the initiation and resolution phases are closely linked.
The biological actions of immunoresolvents are important in the treatment of severe systemic disease, as the approach is altered from “combating inflammation” to “focusing and promoting inflammation resolution”.30 A deeper understanding of the complex pathophysiology of severe systemic diseases is elementary for generating curative approaches. Of substantial importance are mediators that have proven to exert potent proresolving effects on severe systemic models (Table 1). Recently, specialized proresolving mediators, such as RvE1, RvD1-D5 and PD1, were identified as potent proresolving targets in lung injury, sterile and infectious peritonitis, colitis and sepsis.30 The fact that some of these targets are currently being investigated in the clinic holds promise for new therapeutic approaches even in cases of severe systemic inflammation. However, the mechanisms, particularly the downstream pathways captured by these lipid mediators, have not been clearly elucidated, and additional investigations are needed to learn more about their specific effects and interactions with each other.
 |
Table 1 Resolution Mediators in Severe Systemic Inflammation |
An additional mediator class, NGPs, is becoming increasingly important in the therapeutic concepts underlying severe systemic inflammation. The peculiarity of NPGs lies in their influence of different and specific pathophysiological cornerstones. These events include the following: (1) alterations during the initial, resolution and regeneration phases of inflammation; (2) changes in the coagulation system, which can be associated with vessel barrier disorder and local cellular/tissue hypoxia; (3) dysregulation of the neuroendocrine system; and (4) changes in the function of immune cells that can manifest in immune paralysis.5,43,47,54,62 In particular, the intersection of the neuronal system (vagus nerve and sympathetic nerve) with the NGP Netrin-1 or RGM-A in association with SPMs substantiates the broader impact on the inflammatory networks of severe inflammation.40,47
Data from basic science, preclinical and clinical/translational studies corroborate the variety of mediators influencing the network of resolution programmes. Therefore, it is of great importance to more closely investigate the mechanisms and effects of these and newly detected mediators on the pathophysiology of severe systemic inflammation to generate potential new treatment concepts.
Funding
This study was supported by 2 grants from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation): DFG-MI 1506/5-1 and Projektnummer 374031971 – TRR 240 (to V.M.), a grant from the European Commission “Horizon 2020 Framework Programme” (MSCA-ITN-ETN, 812890).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lord JM, Midwinter MJ, Chen YF, et al. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. 2014;384(9952):1455–1465. doi:10.1016/S0140-6736(14)60687-5
2. Gruen RL, Brohi K, Schreiber M, et al. Haemorrhage control in severely injured patients. Lancet. 2012;380(9847):1099–1108. doi:10.1016/S0140-6736(12)61224-0
3. Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140(6):771–776. doi:10.1016/j.cell.2010.03.006
4. Wafaisade A, Lefering R, Bouillon B, et al. Epidemiology and risk factors of sepsis after multiple trauma: an analysis of 29,829 patients from the Trauma Registry of the German Society for Trauma Surgery. Crit Care Med. 2011;39(4):621–628. doi:10.1097/CCM.0b013e318206d3df
5. Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(21):2063. doi:10.1056/NEJMra1208623
6. Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. 2015;160(5):816–827. doi:10.1016/j.cell.2015.02.010
7. de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol. 2016;16(6):378–391. doi:10.1038/nri.2016.49
8. Hind LE, Huttenlocher A. Neutrophil reverse migration and a chemokinetic resolution. Dev Cell. 2018;47(4):404–405. doi:10.1016/j.devcel.2018.11.004
9. Venereau E, Ceriotti C, Bianchi ME. DAMPs from cell death to new life. Front Immunol. 2015;6:422. doi:10.3389/fimmu.2015.00422
10. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi:10.1016/j.cell.2010.01.022
11. Middleton J, Neil S, Wintle J, et al. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91(3):385–395. doi:10.1016/S0092-8674(00)80422-5
12. Powell WS, Gravel S, MacLeod RJ, Mills E, Hashefi M. Stimulation of human neutrophils by 5-oxo-6,8,11,14-eicosatetraenoic acid by a mechanism independent of the leukotriene B4 receptor. J Biol Chem. 1993;268(13):9280–9286.
13. Burk AM, Martin M, Flierl MA, et al. Early complementopathy after multiple injuries in humans. Shock. 2012;37(4):348–354. doi:10.1097/SHK.0b013e3182471795
14. Huber-Lang M, Kovtun A, Ignatius A. The role of complement in trauma and fracture healing. Semin Immunol. 2013;25(1):73–78. doi:10.1016/j.smim.2013.05.006
15. Abtin A, Jain R, Mitchell AJ, et al. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat Immunol. 2014;15(1):45–53. doi:10.1038/ni.2769
16. Schiwon M, Weisheit C, Franken L, et al. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell. 2014;156(3):456–468. doi:10.1016/j.cell.2014.01.006
17. Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10(6):440–452. doi:10.1038/nri2782
18. Krishna S, Miller LS. Innate and adaptive immune responses against Staphylococcus aureus skin infections. Semin Immunopathol. 2012;34(2):261–280. doi:10.1007/s00281-011-0292-6
19. Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227(1):221–233. doi:10.1111/imr.2008.227.issue-1
20. Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi:10.1146/annurev-immunol-030409-101323
21. Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1998;101(4):819–826. doi:10.1172/JCI1578
22. Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2(7):612–619. doi:10.1038/89759
23. Papayianni A, Serhan CN, Brady HR. Lipoxin A4 and B4 inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells. J Immunol. 1996;156(6):2264–2272.
24. Arita M, Bianchini F, Aliberti J, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201(5):713–722. doi:10.1084/jem.20042031
25. Doran AC, Yurdagul A
26. Elliott MR, Ravichandran KS. The dynamics of apoptotic cell clearance. Dev Cell. 2016;38(2):147–160. doi:10.1016/j.devcel.2016.06.029
27. Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109(1):41–50. doi:10.1172/JCI0211638
28. Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol. 2016;16(1):51–67. doi:10.1038/nri.2015.4
29. Spite M, Claria J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014;19(1):21–36. doi:10.1016/j.cmet.2013.10.006
30. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128(7):2657–2669. doi:10.1172/JCI97943
31. Sugimoto MA, Vago JP, Perretti M, Teixeira MM. Mediators of the resolution of the inflammatory response. Trends Immunol. 2019. doi:10.1016/j.it.2019.01.007
32. Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. doi:10.1016/j.immuni.2016.02.015
33. Serhan CN, Dalli J, Karamnov S, et al. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26(4):1755–1765. doi:10.1096/fj.11-201442
34. Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes. 2013;62(2):618–627. doi:10.2337/db12-0684
35. Chavan SS, Pavlov VA, Tracey KJ. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity. 2017;46(6):927–942. doi:10.1016/j.immuni.2017.06.008
36. Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9(6):418–428. doi:10.1038/nri2566
37. Dalli J, Serhan CN. Immunoresolvents signaling molecules at intersection between the brain and immune system. Curr Opin Immunol. 2018;50:48–54. doi:10.1016/j.coi.2017.10.007
38. Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131(4):1122–1130. doi:10.1053/j.gastro.2006.08.016
39. van Westerloo DJ, Giebelen IA, Florquin S, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130(6):1822–1830. doi:10.1053/j.gastro.2006.02.022
40. Mirakaj V, Dalli J, Granja T, Rosenberger P, Serhan CN. Vagus nerve controls resolution and pro-resolving mediators of inflammation. J Exp Med. 2014;211(6):1037–1048. doi:10.1084/jem.20132103
41. Dalli J, Colas RA, Arnardottir H, Serhan CN. Vagal regulation of group 3 innate lymphoid cells and the immunoresolvent PCTR1 controls infection resolution. Immunity. 2017;46(1):92–105. doi:10.1016/j.immuni.2016.12.009
42. Schlegel M, Kohler D, Korner A, et al. The neuroimmune guidance cue netrin-1 controls resolution programs and promotes liver regeneration. Hepatology. 2016;63(5):1689–1705. doi:10.1002/hep.v63.5
43. Mirakaj V, Rosenberger P. Immunomodulatory functions of neuronal guidance proteins. Trends Immunol. 2017;38(6):444–456. doi:10.1016/j.it.2017.03.007
44. Ly NP, Komatsuzaki K, Fraser IP, et al. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc Natl Acad Sci U S A. 2005;102(41):14729–14734. doi:10.1073/pnas.0506233102
45. Ramkhelawon B, Hennessy EJ, Menager M, et al. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat Med. 2014;20(4):377–384. doi:10.1038/nm.3467
46. Pavlov VA, Tracey KJ. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat Neurosci. 2017;20(2):156–166. doi:10.1038/nn.4477
47. Korner A, Schlegel M, Kaussen T, et al. Sympathetic nervous system controls resolution of inflammation via regulation of repulsive guidance molecule A. Nat Commun. 2019;10(1):633. doi:10.1038/s41467-019-08328-5
48. Mirakaj V, Brown S, Laucher S, et al. Repulsive guidance molecule-A (RGM-A) inhibits leukocyte migration and mitigates inflammation. Proc Natl Acad Sci U S A. 2011;108(16):6555–6560. doi:10.1073/pnas.1015605108
49. Muramatsu R, Kubo T, Mori M, et al. RGMa modulates T cell responses and is involved in autoimmune encephalomyelitis. Nat Med. 2011;17(4):488–494. doi:10.1038/nm.2321
50. Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med. 2011;17(11):1391–1401. doi:10.1038/nm.2507
51. Schlegel M, Granja T, Kaiser S, et al. Inhibition of neogenin dampens hepatic ischemia-reperfusion injury. Crit Care Med. 2014;42(9):e610–e619. doi:10.1097/CCM.0000000000000485
52. Konig K, Gatidou D, Granja T, Meier J, Rosenberger P, Mirakaj V. The axonal guidance receptor neogenin promotes acute inflammation. PLoS One. 2012;7(3):e32145. doi:10.1371/journal.pone.0032145
53. Mirakaj V, Jennewein C, Konig K, Granja T, Rosenberger P. The guidance receptor neogenin promotes pulmonary inflammation during lung injury. FASEB J. 2012;26(4):1549–1558. doi:10.1096/fsb2.v26.4
54. Schlegel M, Korner A, Kaussen T, et al. Inhibition of neogenin fosters resolution of inflammation and tissue regeneration. J Clin Invest. 2018;128(10):4711–4726. doi:10.1172/JCI96259
55. Rice TW, Wheeler AP, Thompson BT, deBoisblanc BP, Steingrub J, Rock P. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306(14):1574–1581. doi:10.1001/jama.2011.1435
56. Barbosa VM, Miles EA, Calhau C, Lafuente E, Calder PC. Effects of a fish oil containing lipid emulsion on plasma phospholipid fatty acids, inflammatory markers, and clinical outcomes in septic patients: a randomized, controlled clinical trial. Crit Care. 2010;14(1):R5. doi:10.1186/cc8844
57. Pontes-Arruda A, Martins LF, de Lima SM, et al. Enteral nutrition with eicosapentaenoic acid, gamma-linolenic acid and antioxidants in the early treatment of sepsis: results from a multicenter, prospective, randomized, double-blinded, controlled study: the INTERSEPT study. Crit Care. 2011;15(3):R144. doi:10.1186/cc10267
58. Patel JJ, Codner P. Controversies in critical care nutrition support. Crit Care Clin. 2016;32(2):173–189. doi:10.1016/j.ccc.2015.11.002
59. Korner A, Schlegel M, Theurer J, et al. Resolution of inflammation and sepsis survival are improved by dietary omega-3 fatty acids. Cell Death Differ. 2018;25(2):421–431. doi:10.1038/cdd.2017.177
60. Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity. 2014;40(4):463–475. doi:10.1016/j.immuni.2014.04.001
61. Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15(8):551–567. doi:10.1038/nrd.2016.39
62. Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562–572. doi:10.1056/NEJMra1608077
63. El Kebir D, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc Natl Acad Sci U S A. 2012;109(37):14983–14988. doi:10.1073/pnas.1206641109
64. Arita M, Yoshida M, Hong S, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102(21):7671–7676. doi:10.1073/pnas.0409271102
65. Spite M, Norling LV, Summers L, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461(7268):1287–U1125. doi:10.1038/nature08541
66. Wu B, Capilato J, Pham MP, et al. Lipoxin A4 augments host defense in sepsis and reduces Pseudomonas aeruginosa virulence through quorum sensing inhibition. FASEB J. 2016;30(6):2400–2410. doi:10.1096/fsb2.v30.6
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.