Back to Journals » ImmunoTargets and Therapy » Volume 12
NOTCH1 Mutations Predict Superior Outcomes of Immune Checkpoint Blockade in Non-Small Cell Lung Cancer
Authors Huang Q , Cao H, Yao Q, Zhou X, Li H, Bai Q , Hu H
Received 6 August 2023
Accepted for publication 7 November 2023
Published 5 December 2023 Volume 2023:12 Pages 165—173
DOI https://doi.org/10.2147/ITT.S433555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Michael Shurin
Qingyuan Huang,1– 3,* Hang Cao,1– 3,* Qianlan Yao,3– 5,* Xiaoyan Zhou,3– 5 Hang Li,1– 3 Qianming Bai,3– 5 Hong Hu1– 3
1Departments of Thoracic Surgery, Fudan University Shanghai Cancer Center, Shanghai, 200032, People’s Republic of China; 2Institute of Thoracic Oncology, Fudan University, Shanghai, 200032, People’s Republic of China; 3Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, 200032, People’s Republic of China; 4Department of Pathology, Fudan University Shanghai Cancer Center, Shanghai, 200032, People’s Republic of China; 5Institute of Pathology, Fudan University, Shanghai, 200032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hong Hu; Qianming Bai, Email [email protected]; [email protected]
Background: NOTCH1 is frequently mutated in non-small cell lung cancer (NSCLC), and also is a poor therapeutic target. It is of clinical importance to investigate the effects of NOTCH1 mutations on anti-tumor immunity and response to immune checkpoint blockade (ICB).
Methods: An observational study with targeted sequencing in 963 NSCLC patients at our center were performed (FUSCC cohort). Data of the Cancer Genome Atlas Pan-Lung Cancer study (TCGA cohort) were analyzed, and gene set enrichment analysis (GSEA) was performed. The Samstein et al cohort included 350 patients with advanced NSCLC undergoing genomic profiling with the MSK-IMPACT assay, and receiving at least one dose of ICB therapy.
Results: NOTCH1 mutations were more common in smokers and patients with squamous-cell carcinoma (SCC) (all P value < 0.05). For patients who did not receive ICB therapy (TCGA cohort), the overall survival (OS) of NOTCH1-mutant and -WT patients were comparable (log-rank P = 0.72), while for patients who received ICB therapy in the Samstein et al cohort, NOTCH1-mutant patients had significantly superior OS than WT patients (log-rank P = 0.041). On multivariate Cox analysis, the predictive value of NOTCH1 mutations reached marginal statistical significance (HR, 0.42; 95% CI, 0.17– 1.04; P = 0.059). The median of TMB for NOTCH1-mutant tumors was significantly higher than that for NOTCH1-WT tumors, and GSEA revealed that NOTCH1 mutations manifested various defects in the repair of DNA damage. NOTCH1-mutant tumors displayed an inflamed tumor microenvironment (TME), manifesting as increased PD-L1 expression and tumor-infiltrating CD8+ T cells.
Conclusion: NOTCH1 mutations define a molecular subtype of NSCLC, which are more common in smokers and patients with SCC, are characterized with higher TMB, inflamed TME, and display improved survival of ICB therapy for NSCLC patients.
Keywords: NOTCH1, non-small cell lung cancer, immunotherapy, survival, tumor mutational burden, tumor microenvironment
Introduction
Lung cancer remains the leading cause of cancer-related mortality worldwide, and the majority are non-small-cell lung cancer (NSCLC).1,2 Genetic variation is a typical feature of NSCLC that drives cancer initiation and progression.3 Understanding the role of mutated genes in NSCLC is the basis of the development of novel treatment modalities, and precision treatment.4 Mammals have four Notch paralogue genes (NOTCH1, NOTCH 2, NOTCH 3, and NOTCH 4) and five membrane-bound ligand genes (Delta-like 1, Delta-like 3, Delta-like 4, Jagged-1, and Jagged-2).5 The Notch signaling is a highly conserved cell-cell interaction mechanism a diverse, cell context-specific, signaling output,6 and plays vital roles in cancer-related functions such as proliferation, tumor angiogenesis, stemness maintenance and epithelial-to-mesenchymal transition.7,8 In 2004, Dr. Weng et al identified frequent NOTCH1 mutations in heterodimerization domain and/or the C-terminal PEST domain among about half of patients with primary T-cell acute lymphoblastic leukemias, which were considered to be oncogenic drivers.9 On the other hand, NOTCH1 can also act as a tumor suppressor gene in a variety of malignancies, including NSCLC, myeloid leukaemia, and head and neck squamous cell carcinoma.10–12 In this setting, the mutations generally result in the functional inactivation of NOTCH1 gene, making it a poor therapeutic target so far.
Immunotherapy has dramatically impacted the current landscape of cancer treatment. Immune checkpoint blockade (ICB) targeting the programmed cell death (ligand) 1 [PD-(L)1] has been the standard therapy as first-line and second-line treatment in patients with advanced NSCLC, whether as monotherapy or in combination with chemotherapy.13,14 Despite the improved survival of ICB for NSCLC patients, only about 20%-30% of patients could benefit from the treatment, which necessitates further investigation into the biomarkers to identify the patients who are more likely to respond to ICB.13 To date, PD-L1 expression and tumor mutational burden (TMB) are two critical biomarkers which have been validated. PD-L1 expression has been approved by Food and Drug Administration (FDA) as a biomarkers for NSCLC,13,15,16 however, it has intrinsic limitations and remains imperfect.17,18 This raises the question of whether there are some other features simultaneously affecting factors to serve as a powerful predictor for therapeutic outcomes.19 Previous studies have revealed the interplay of tumor genetic variations and immune response, and demonstrated that mutations of oncogenic genes and tumor suppressor genes, such as EGFR, KRAS, and P53, could reprogram the tumor microenvironment (TME) and thus affect the response to immunotherapy.20–22
In order to summarize the characteristics of NSCLC patients harboring NOTCH1 mutations, and systematically address the influence of NOTCH1 mutations on the anti-tumor immunity to NSCLC, we performed integrative analyses of clinical and genomic data from our center and public databases. We found that NOTCH1 mutations were associated with higher TMB, inflamed microenvironment, and improved survival of ICB therapy for NSCLC patients, implying the potential of NOTCH1 as a therapeutic target or predictive marker for NSCLC.
Patients and Methods
Ethics Statement
This study was approved by the institutional review board of FUSCC (2008223-9). Written informed consent was exempted because of retrospective analysis. This study was conducted in accordance with the Declaration of Helsinki and patient confidentiality is guaranteed.
Patients
Patients diagnosed with NSCLC treated at Fudan University Shanghai Cancer Center (FUSCC) from June 2018 to December 2019 were screened. Patients were included in the FUSCC cohort whose tumors underwent targeting next-generation sequencing (68-gene panel, Burning Rock Company, China), covering the whole exon regions of NOTCH1 gene. The processes of genomic DNA sequencing and variants calling were described previously.23 The demographic, clinical and pathological information, including the PD-L1 tumor proportion score (TPS), were collected from the electronic medical records.
Data of TCGA Pan-Lung Cancer study was retrieved from https://www.cbioportal.org/ (TCGA cohort). Data of the Samstein et al cohort were retrieved from the published article.16 The cohort included 350 patients with advanced NSCLC receiving at least one dose of ICB therapy. In total, 329 patients received anti-PD-1/PD-L1 monotherapy, and 21 received combination therapy with anti-CTLA-4. Patients underwent genomic profiling with the Memorial Sloan Kettering Cancer Center Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) assay as part of clinical care, identifying somatic exon variations in a predefined set of 468 cancer-related genes.
Gene Set Enrichment Analysis
For gene set enrichment analysis (GSEA),24 the javaGSEA Desktop Application (GSEA 4.0.1) was downloaded from http://software.broadinstitute.org/gsea/index.jsp. GSEA was used to associate the gene signature with the NOTCH1 mutation status (NOTCH1-mut vs NOTCH1-wt). The normalized enrichment score (NES) is the primary statistic for examining gene set enrichment results. Nominal p-values were calculated empirically using 1000 random phenotype label permutations, and multiple testing correction was applied to generate False Discovery Rate (FDR) -adjusted P values. A gene set with an FDR cutoff <0.25 and nominal p-value <0.05 was considered to be significantly enriched in genes.
Statistical Analysis
The demographic and clinicopathological data were compared by Student’s t test or Mann–Whitney U-test as appropriate. The Kaplan–Meier method was applied to delineate the curve of overall survival (OS), and the Log-rank method was applied to evaluate the significance. The Cox proportional-hazards regression analysis was implemented to calculate the HR of OS in both univariate and multivariate analyses, and Backward Stepwise (Wald) method was used in multivariate analysis. Patients with specific missing clinicopathological data were not included in relevant specific clinicopathological characteristic analyses. The statistical analyses mentioned above were performed using GraphPad Prism 7 or IBM SPSS Statistics 22. Level of significance was set as P < 0.05 (two-tailed) (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001).
Results
NOTCH1 Mutations Were More Common in Smokers and Patients with Squamous Cell Carcinoma
To investigate the clinical and pathologic characteristics of NOTCH1-mutant NSCLC, medical record information of 963 patients were collected, whose tumors underwent targeted next-generation sequencing at FUSCC from June 2018 to December 2019. Among them, NOTCH1 mutations were detected in tumors of 55 patients, accounting for 5.7%. As shown in Table 1, patients harboring NOTCH1 mutation displayed distinct clinical and pathologic characteristics. In the FUSCC cohort, NOTCH1 mutations were more common in male patients at least 65 years old, smokers and patients with squamous-cell carcinoma (SCC) (all P value <0.05).
 |
Table 1 Baseline Clinicopathologic Characteristics of NSCLC Patients in the FUSCC Cohort, TCGA Cohort, and Samstein et al Cohort |
We further analyzed the data of two publicly available cohorts of NSCLC patients, the TCGA cohort and Samstein et al16 cohort. In the TCGA cohort, NOTCH1 mutations were found in 6.8% (78/1141) of patients. Former/current smokers and patients with SCC were more likely to harbor NOTCH1 mutations (both P value <0.05). The Samstein et al cohort collected 350 patients with advanced NSCLC treated with ICB, and NOTCH1 mutations were found in 14 (4.0%) patients. NOTCH1-mutant tumors had significantly higher proportion of SCC (Fisher’s exact P value = 0.005), while the information of smoking status was unavailable in this cohort. The difference of the distribution in gender and age were equivalent between NOTCH1-mutant and -WT patients in both cohorts. The majority of patients in the FUSCC cohort and TCGA cohort were diagnosed with early-stage disease, and the stage of NOTCH1-mutant and -WT patients were comparable in both cohorts. The data of three NSCLC cohorts with different context collectively shown that smokers and patients with SCC were more likely to be NOTCH1 mutant.
NOTCH1 Mutations Predicted Improved Survival of ICB Therapy for NSCLC Patients
The prognostic and predictive value of NOTCH1 mutations for patients with NSCLC were subsequently investigated. Patients of the TCGA cohort did not receive ICB therapy. The 5-year OS of NOTCH1-mutant and -WT patients were 39.1% (95% confidence interval [CI], 15.0%-50.4%) and 40.5% (95% CI, 34.6%-46.3%), respectively (HR, 1.08; 95% CI, 0.69–1.68; log-rank P = 0.72; Figure 1A). This data suggested that NOTCH1 mutant status was not a prognostic indicator for patients with NSCLC who did not receive ICB.
 |
Figure 1 NSCLC patients harboring NOTCH1 mutations could benefit from ICB therapy. (A) Overall survival of NOTCH1-WT and -mutant NSCLC patients, who did not receive ICB therapy, was comparable in the TCGA dataset. (B) Overall survival of NOTCH1-mutant subset was significantly superior than that of NOTCH1-WT subset among NSCLC patients receiving ICB therapy in the Samstein et al16 cohort. Dot lines indicate 95% confidence intervals. |
In the Samstein et al cohort, 329 patients were administrated with anti-PD-1/PD-L1 antibody, and 21 patients with a combination of anti-PD-1/PD-L1 antibody and anti-CTLA-4 antibody. The 3-year OS of NOTCH1-mutant and -WT patients were 57.1% (95% confidence interval [CI], 22.4%-81.1%) and 26.9% (95% CI, 20.3%-33.9%), respectively (HR, 0.40; 95% CI, 0.16–0.96; log-rank P = 0.041; Figure 1B).16 Cox regression analyses were performed to further confirm the independent predictive value of NOTCH1 mutation status. On univariate analysis, NOTCH1 mutational status, TMB and drug type were predictors of survival, and were included in the multivariate analysis. On multivariate analysis, the value of NOTCH1 mutational status reached marginal statistical significance (HR, 0.42; 95% CI, 0.17–1.04; P = 0.059; Table 2).
 |
Table 2 Univariate and Multivariate Cox Regression Analysis of NSCLC Patients Receiving ICB Therapy in the Samstein et al Cohort |
NOTCH1 Mutations Correlated With Higher TMB
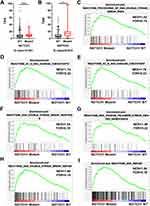
The vital significance of TMB in the setting of ICB therapy has been recently demonstrated by cohort studies and clinical trials across multiple cancer types. We next sought to elucidate the influences of NOTCH1 mutation status on the tumor mutational load. In the TCGA cohort, the median of TMB for NOTCH1-mutant tumors (8.9; IQR, 6.3–12.6) was significantly higher than that for NOTCH1-WT tumors (6.6; IQR, 3.5–10.7; Wilcoxon P < 0.001, Q value < 0.001; Figure 2A). In the Samstein et al cohort, NOTCH1-mutant tumors also displayed significantly higher TMB (median, 14; IQR, 6.8–20.2) than that of NOTCH1-WT tumors (median, 6.9; IQR, 3.9–11.7; Wilcoxon P = 0.02, Q value = 0.073; Figure 2B).
We speculated whether alterations in DNA damage and repair-related genes resulted from NOTCH1 mutations could account for the differential tumor mutational load. GSEA revealed prominent enrichment of signatures relating to PROCESSING OF DNA DOUBLE STRAND BREAK ENDS (Figure 2C), DNA DOUBLE STRAND BREAK REPAIR (Figure 2D) and RESPONSE (Figure 2E), DNA REPAIR (Figure 2F), DNA DAMAGE TELOMERE STRESS INDUCED SENESCENCE (Figure 2G), G1/S DAN DAMAGE CHECKPOINTS (Figure 2H), and G2/M DAN DAMAGE CHECKPOINTS (Figure 2I). NOTCH1 mutations manifested various defects in the repair of DNA damage that greatly enhanced point mutation.
NOTCH1 Mutations Were Associated with an Inflamed Microenvironment
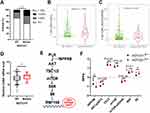
We subsequently investigate whether NOTCH1 mutations and mutational load could lead to the remodeling of the immune microenvironment of NSCLC. The proportion for PD-L1 strong positive (TPS ≥ 50%) and weak positive (1% < TPS ≤ 49%) of NOTCH1-mutant NSCLC were 19.5% and 26.8%, respectively, which were significantly higher than those of NOTCH1-WT tumors (strong positive, 8.0%; weak positive, 19.3%; P < 0.01, Figure 3A). The TIMER 2.0 was used to evaluate the association between NOTCH1 mutation status and the immune cell subtypes. Notably, NOTCH1-mutant tumors had significantly higher level of CD8+ T cell than NOTCH1-WT tumors by XCELL algorithm (P = 0.0085, Figure 3B) and MCP-COUNTER algorithm (P = 0.029, Figure 3C). Besides, NOTCH1-mutant tumors also exhibited remarkable increases in the mRNA level of CD8A in the TCGA database (P = 0.029, Figure 3D). Collectively, NOTCH1-mutant tumors displayed an inflamed TME, manifesting as remarkably increase of PD-L1 expression and tumor-infiltrating CD8+ T cells, which had been demonstrated to be associated with benefits from ICB therapy.
To identify the potential signaling pathways that mediated the increased mutation load and the inflamed immune microenvironment, we compared the protein levels between NOTCH1-mutant and -WT tumors in the TCGA database. Overall, expression levels of 208 proteins were assessed by RPPA, and the levels of eight proteins (PARP1, CDKN1B, SMAD4, PRDX1, INPP4B, S6, BCL2, CHEK2) were significantly different between two groups (all P < 0.05). Of note, S6, the downstream protein of PI3K-AKT-mTOR signaling, was significantly activated by NOTCH1 mutations, and INPP4B, suppressor of PI3K-AKT signaling, was significantly decreased (Figure 3E and F). Besides, other key proteins of the signaling, including AKT-pS473, mTOR, mTOR-pS2448 and S6K, were higher in the mutant group, and TSC2, suppressor of mTOR, was lower in the mutant group, although statistical significance was not reached (Figure 3E and F). Our25 and Lastwika’s26 previous studies demonstrated that AKT-mTOR signaling could promote the expression of PD-L1 and the infiltration of CD8+ T cells in NSCLC, and Xie et al27 found that hyperactivation of mTOR-S6 signaling could impair the DNA damage response via inhibiting RNF168. These findings hinted that NOTCH1 mutations increased TMB and reprogrammed the TME probably via activating the PI3K-AKT-mTOR signaling.
Discussion
The present study comprehensively analyzed three cohorts of NSCLC patients with different background, and identified NOTCH1-mutant NSCLC as a unique subtype with distinct clinicopathological characteristics and treatment response. Patients with NOTCH1-mutant NSCLC were more likely to be smokers and squamous cell carcinoma in histology. Moreover, NOTCH1 mutations intrinsically increased tumor mutation load, promoted PD-L1 expression and facilitated T cell infiltration, and thus enhanced sensitivity to ICB therapy.
In the present study, we comprehensively analyzed the NOTCH1 mutational status of three NSCLC cohorts with different treatment modalities and ethnic background, and revealed that the mutation rates ranged from 4% to 6.8%. Considering the relatively high prevalence of NSCLC worldwide, there would be a huge number of patients harboring NOTCH1 mutations. Moreover, NOTCH1 is a poor therapeutic target, and there has been no chemical inhibitors or antibodies which are proven to be clinically effective. Our analyses highlighted not only the distinct clinicopathological characteristics, but also the superior efficacy of ICB therapy of these patients.
From a biological perspective, GSEA showed prominent enrichment of signatures of DNA double-strand break response and repair, indicating the defects in repairing DNA damage of NOTCH1-mutant NSCLC. These defects resulted in significantly increased mutational burden and increased number of neoantigens presented on major histocompatibility complex molecules that facilitate immune recognition as foreign, thus promote immunogenicity and render NOTCH1-mutant tumors susceptible to antitumor immunity.
Different from chemotherapy or molecular-targeted therapies which attack cancer cells via a single oncogenic variants or other autonomous features, immunotherapy enhances the antitumor response to eliminate cancer cells. Hence, tumor immune microenvironment is the basis of ICB therapies.28 Previous studies have classified the TME as four categories according to the level of tumor infiltrating T cells and expression level of PD-L1. Of note, an inflamed TME, considered as a paradigm of adaptive resistance of tumor mediated by PD-1/PD-L1 pathway, is characterized as increased level of PD-L1 expression and T cells infiltration.29,30 It has been demonstrated that an inflamed TME is associated with clinical benefits from immunotherapy.29,30 The current study used a cohort of patients undergoing complete resection and TIMER 2.0 platform to reveale that NOTCH1-mutant NSCLC displayed an inflamed TME.
These findings have significant clinical and research implications. As the current study demonstrated NOTCH1 mutational status as a predictor of the outcomes of NSCLC treated with ICB, more attention should be paid to the mutation status of NOTCH1 in clinical practice and trials. Further studies should obtain the mutation status of NOTCH1 if possible, and consider it as a potential confounding factor of survival.
The major limitation of the current study derives from the nature of retrospective design, which potentially introduce selection bias. Our study applied three NSCLC cohorts with large sample size, diagnosed with different stages, and from different ethnic backgrounds. Combinational analyses of the data from these three cohorts highlighted the distinct characteristics and treatment response of NOTCH1-mutant NSCLC. Additionally, other members of NOTCH family except NOTCH1 were not included in the study, and we could not figure out whether mutations of other NOTCH genes had similar effects as NOTCH1. Lastly, the results mainly derived from analyses of clinicopathologic data of NSCLC patients. We would perform laboratory experiments in the future to further explore the mechanisms of NOTCH mutations.
In summary, the current study identifies NOTCH1-mutant NSCLC as a distinct clinical subtype, and reveals the potential interplay between NOTCH1 mutations of tumor cells and tumor immune microenvironments. NOTCH1 mutations intrinsically increase the mutational load of NSCLC cells, reprogrammed the immune microenvironment as an inflamed subtype, and thus lead to significant survival benefits among patients receiving ICB therapies.
Funding
This work was supported by National Natural Science Foundation of China (82203504) and Shanghai Sailing program (20YF1409100).
Disclosure
Qingyuan Huang, Hang Cao, and Qianlan Yao are co-first authors in this study. The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Yu Y, Mao L, Cheng Z, et al. A novel regQTL-SNP and the risk of lung cancer: a multi-dimensional study. Arch Toxicol. 2021;95(12):3815–3827. doi:10.1007/s00204-021-03170-5
3. Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214 8. doi:10.1038/nature12213
4. Puri M, Gawri K, Dawar R. Therapeutic strategies for BRAF mutation in non-small cell lung cancer: a review. Front Oncol. 2023;13:1141876. doi:10.3389/fonc.2023.1141876
5. Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137(2):216–233. doi:10.1016/j.cell.2009.03.045
6. Mutvei AP, Fredlund E, Lendahl U. Frequency and distribution of Notch mutations in tumor cell lines. BMC Cancer. 2015;15(1):311. doi:10.1186/s12885-015-1278-x
7. Grego-Bessa J, Diez J, Timmerman L, de la Pompa JL. Notch and epithelial-mesenchyme transition in development and tumor progression: another turn of the screw. Cell Cycle. 2004;3(6):718–721. doi:10.4161/cc.3.6.949
8. Espinoza I, Miele L. Deadly crosstalk: notch signaling at the intersection of EMT and cancer stem cells. Cancer Lett. 2013;341(1):41–45. doi:10.1016/j.canlet.2013.08.027
9. Weng AP, Ferrando AA, Lee W, et al. Activating Mutations of NOTCH1 in Human T Cell Acute Lymphoblastic Leukemia. Science. 2004; 306(5964):269–271. doi:10.1126/science.1102160
10. Klinakis A, Lobry C, Abdel-Wahab O, et al. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature. 2011;473(7346):230–233. doi:10.1038/nature09999
11. Wang NJ, Sanborn Z, Arnett KL, et al. Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proc Natl Acad Sci U S A. 2011;108(43):17761–17766. doi:10.1073/pnas.1114669108
12. Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333(6046):1154–1157. doi:10.1126/science.1206923
13. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774
14. Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi:10.1056/NEJMoa1801005
15. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. doi:10.1056/NEJMoa1801946
16. Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–206. doi:10.1038/s41588-018-0312-8
17. Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4. doi:10.1126/scitranslmed.aad7118
18. Huang Q, Zhang H, Hai J, et al. Impact of PD-L1 expression, driver mutations and clinical characteristics on survival after anti-PD-1/PD-L1 immunotherapy versus chemotherapy in non-small-cell lung cancer: a meta-analysis of randomized trials. Oncoimmunol. 2018;7(12):e1396403. doi:10.1080/2162402X.2017.1396403
19. Dong ZY, Zhong WZ, Zhang XC, et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2017;23(12):3012–3024. doi:10.1158/1078-0432.CCR-16-2554
20. Coelho MA, de Carne Trecesson S, Rana S, et al. Oncogenic RAS signaling promotes tumor immunoresistance by stabilizing PD-L1 mRNA. Immunity. 2017;47(6):1083–99 e6. doi:10.1016/j.immuni.2017.11.016
21. Cortez MA, Ivan C, Valdecanas D, et al. PDL1 regulation by p53 via miR-34. J Natl Cancer Inst. 2016;108(1). doi:10.1093/jnci/djv303.
22. Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585–4593. doi:10.1158/1078-0432.CCR-15-3101
23. Yao Q, Liu Y, Zhang L, et al. Mutation landscape of homologous recombination repair genes in epithelial ovarian cancer in china and its relationship with clinicopathlological characteristics. Front Oncol. 2022;12:709645. doi:10.3389/fonc.2022.709645
24. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi:10.1073/pnas.0506580102
25. Huang Q, Li F, Hu H, et al. Loss of TSC1/TSC2 sensitizes immune checkpoint blockade in non-small cell lung cancer. Sci Adv. 2022;8(5):eabi9533. doi:10.1126/sciadv.abi9533
26. Lastwika KJ, Wilson W 3rd, Li QK, et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 2016;76(2):227–238. doi:10.1158/0008-5472.CAN-14-3362
27. Xie X, Hu H, Tong X, et al. The mTOR-S6K pathway links growth signalling to DNA damage response by targeting RNF168. Nat Cell Biol. 2018;20(3):320–331. doi:10.1038/s41556-017-0033-8
28. Kudling TV, Clubb JHA, Pakola S, et al. Effective intravenous delivery of adenovirus armed with TNFα and IL-2 improves anti-PD-1 checkpoint blockade in non-small cell lung cancer. Oncoimmunol. 2023;12(1):2241710. doi:10.1080/2162402X.2023.2241710
29. Sanmamed MF, Chen L. Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation. Cancer J. 2014;20(4):256–261. doi:10.1097/PPO.0000000000000061
30. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. doi:10.1038/nature21349
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.