Back to Journals » Journal of Inflammation Research » Volume 17
Nonlinear Relationship Between Systemic Immune-Inflammation and Hepatic Steatosis: A Population-Based Study in China
Authors Zhao J, Yu L, Sun K, Wang Y, Xie F
Received 18 September 2023
Accepted for publication 23 January 2024
Published 3 February 2024 Volume 2024:17 Pages 711—720
DOI https://doi.org/10.2147/JIR.S440430
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Jing Zhao,* Li Yu,* Kangyun Sun, Yun Wang, Fangfei Xie
Physical Examination Center, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital, Gusu School, Nanjing Medical University, Suzhou, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fangfei Xie; Yun Wang, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital, Gusu School, Nanjing Medical University, 242 Guangji Road, Suzhou, Jiangsu, 215008, People’s Republic of China, Tel +86 – 13913186601 ; +86 – 18913151605, Email [email protected]; [email protected]
Background: Studies on the associations between Systemic Immune-Inflammation (SII) and hepatic steatosis in China are still lacking. It is necessary to clarify the relationship between SII and hepatic steatosis in the Chinese population.
Methods: This study was conducted from January 2022 to December 2022. A total of 37,095 participants were enrolled, among them, with 20,709 (55.83%) being males, and 16,386 (44.17%) being females. Physical and biochemical indicators were measured during a morning health examination after the examinees had fasted overnight. Diagnoses of hepatic steatosis were determined using an ultrasound test in accordance with the Chinese Guideline. Analysis of variance and chi-square tests were used to analyze the association between SII and hepatic steatosis. Stratification analyses were conducted based on age, gender, and obese status. Restricted cubic spline regression was also performed to explore the shapes of associations between SII and hepatic steatosis.
Results: The average age of the 37,095 participants was 44.78 years old, with those with hepatic steatosis (11,599 (31.27%)) averaging 47.06 years old and those (25,496 (68.73%)) in the control group averaging 43.73 years old. SII was positively associated with hepatic steatosis. This association remained significant after conducting stratification analysis by age and gender. The inflection points in the inverted U-shaped curve for the relationship between SII and hepatic steatosis were 399.78 for gender (1000 cells /μL)(nonlinear P< 0.01, OR=1.31 (male), 1.00 (female)) and 385.79 for age (1000 cells /μL)(nonlinear P< 0.01, OR=1.35 (18~44 years old), 1.87 (45~59 years old), 1.93 (60~ years old)).
Conclusion: SII is an independent risk factor for hepatic steatosis, and this effect appears to be stronger in subjects with BMI < 28 kg/m2. The nonlinear relationship between SII and hepatic steatosis, characterized by an inverted U-shaped distribution, may serve as a reference for diagnosing and evaluating hepatic steatosis.
Keywords: hepatic steatosis, systemic immune-inflammation index, inflammation, association
Introduction
Liver disease affects millions of people worldwide, and fatty liver disease, especially non-alcoholic fatty liver disease, has became the most common liver disease worldwide, with a global prevalence of up to 25%.1,2 Due to the rapid development of China’s economy and the change in people’s lifestyles in recent decades, the prevalence rate of non-alcoholic fatty liver disease in China has increased from 17% in 2003 to 29.2% in 2018.3,4 It has been speculated that the prevalence of fatty liver in China will continue to increase in the future, even if obesity and diabetes remain at current and historical rates.5,6
Since the liver plays a central role in lipid metabolism, the accumulation of excessive lipids in the liver tissue may lead to inflammation, resulting in liver tissue damage liver fibrosis, and cirrhosis.7,8 Immune organ plays a significant role in causing liver inflammation, and any disruption in immune homeostasis can impact both innate and adaptive immunity, leading to a range of liver diseases.9–11 Therefore, it is necessary to have a clear understanding of the relationship between fatty liver disease and inflammation in order to develop effective treatment strategies for preventing and treating fatty liver disease. The liver is a crucial immune organ that houses a significant number of innate and adaptive immune cells. Innate T-cell populations, especially natural killer T cells, are particularly abundant in the livers of mice.12 Immunomonitoring of the liver also includes conventional CD4+ and CD8+ αβT cells, which are fewer in number compared to CD8+T cells. Neutrophils are the most abundant type of natural immune cells in the human body. After liver inflammation is triggered by pro-inflammatory cytokines and chemokines produced by liver adipocytes, neutrophils quickly gather and infiltrate the inflammation site, leading to the release of a large number of reactive oxygen species and inflammatory mediators, which worsen liver inflammation and fibrosis.13
The systemic immune-inflammation index (SII) is a novel index that measures systemic immune inflammation. It is based on lymphocyte, neutrophil, and platelet counts, and has been shown to be a powerful predictor for many prospective and retrospective inflammatory and cancer-related diseases.14–16 Several studies conducted on the American population have found that SII is not only associated with hepatic steatosis but also has diagnostic value for predicting the prognosis of non-alcoholic fatty liver disease.17–19 However, studies on the relationship between SII and hepatic steatosis in the Chinese population are still very limited. Therefore, this study aimed to explore the relationship between SII and hepatic steatosis in adults, utilizing a large sample of individuals over 18 years old from Southeast China.
Materials and Methods
Study Participants
The cross-sectional sample of participants was recruited from January 2022 to December 2022 at Suzhou physical examination center in southeastern China. This study was approved by the Ethics Committee of the Affiliated Suzhou Hospital of Nanjing Medical University (Ethical Approval Number: K-2023-022-K01). All participants received and signed written informed consent.
A total of 80,318 participants were included in this study. After excluding subjects who did not meet the inclusion criteria, 37,095 participants were enrolled in the study (Figure 1).
Data Measurements
A health examination was performed in the morning after the examinees had fasted overnight. Height, weight and waist circumference (WC) were measured while wearing light indoor clothing and without shoes or heavy clothes by an eligible physician. Serum related indicators, including total cholesterol (TC), triglycerides (TG), high density lipoprotein (HDL), low density lipoprotein (LDL), very low density lipoprotein (VLDL), fast blood glucose (FBG), alanine aminotransferase (ALT), glutamic oxalacetic transaminase (AST) and γ-glutamyltranspeptidase (GGT) were measured using the Beckman AU5800 autoanalyzer. Body mass index (BMI) was calculated as weight (kg)/(height (m))2 and SII was calculated as plate count × neutrophil count/lymphocyte count (1000 cells/µL)(blood related indictators were measured by SysmexXN9100autoanalyzer).20,21 Diagnoses of hepatic steatosis were determined through abdominal ultrasound scans (General Electric Company) conducted by experienced radiologists specializing in liver imaging. During the examination, the patient was lying on his back, breathing steadily and placing both hands behind the pillow. The examination includes the observation of the size, shape, edge, finish and continuity of the liver envelope, the uniformity of the echo in the liver parenchyma, whether there is diffuse or focal enhancement, attenuation, and increased or decreased sound transmission. Based on the Chinese Guideline, participants were diagnosed with hepatic steatosis if they exhibited two out of three following criteria: diffuse hyperechoic liver relative to kidney, ultrasound beam attenuation, and weakening visualization of intrahepatic structures).22
Statistical Analysis
Data distribution is presented using mean±standard deviation for numerical variables and count (%) for categorical variables. SII was divided equally into four parts ([0, 269.32], [269.33, 360.34], [360.35, 482.16], [482.18, 4669.56]), and an analysis of variance was performed for continuous variables, while chi-square tests were performed for categorical variables, respectively. The Cochran-Mantel-Haenszel test is used to test for stratified odds ratio (OR). Stratification analyses were further performed to explore the relationship between SII and hepatic steatosis in different subgroups, based on BMI and WC. Tests for nonlinear associations between SII and hepatic steatosis in different gender and age groups were performed using cubic spline regression. SPSS 23.0 and R 4.0 were used for all statistical analyses. All reported P-values were two-sided, and P<0.05 was considered statistically significant.
Results
Statistically Significant Differences Exist in SII between Individuals with Hepatic Steatosis and the Control group
A total of 37,095 participants were involved, with 20,709 (55.83%) beingmalesand 16,386 (44.17%) being females. And 11,599 (31.27%) participants were categorized as having hepatic steatosis. The average age of the 37,095 participants was 44.78 years old, with those with hepatic steatosis averaging 47.06 years old and those in the control group averaging 43.73 years old. As shown in Table 1, there were statistically significant differences in the gender and age composition between the hepatic steatosis and control groups (P<0.05). The prevalence of hepatic steatosis in males and females gradually converges with age (Supplementary Table 1). Furthermore, the differences of BMI, WC, TC, TG, LDL, HDL, FBG, ALT, AST, GGT, SII between the hepatic steatosis and the control groups were also statistically significant (P<0.01).
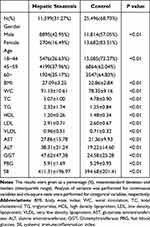 |
Table 1 Baseline Characteristics Between Hepatic Steatosis and Control Groups |
SII is an Independent Risk Factor for Hepatic Steatosis
Table 2 showed that SII was positively associated with hepatic steatosis. Compared to the lowest quartiles of SII, the OR (95%confidence interval (CI)) was 1.26 (1.18, 1.34), 1.31 (1.23, 1.40) and 1.39 (1.30, 1.48) for the second, third, and highest quartiles, respectively (P<0.05). The associations still existed after using the Cochran-Mantel-Haenszel test, stratifying by gender and age. Besides, there were significant differences in TC, TG, LDL, HDL, FBG, ALT, AST, and GGT (P<0.01) among the different quartiles of SII in the total populations (Table 3). And we also found that liver enzymes, including AST, ALT, and GGT, were significantly associated with hepatic steatosis (Supplementary Table 2).
 |
Table 2 OR for Hepatic Steatosis in Four Groups According to SII Quartile |
 |
Table 3 Variables Characteristics in Four Groups According to SII Quartile Among Total Population |
A Strong Positive Association Between SII and Hepatic Steatosis in Subjects with BMI<28 kg/m2
The associations between SII and hepatic steatosis were further evaluated by stratifying BMI and WC levels (Table 4). After adjustment, the positive association between SII and hepatic steatosis appeared stronger with BMI<28 kg/m2 (ORQ2vsQ1= 1.21 (1.12, 1.30), ORQ3vsQ1= 1.24 (1.15, 1.33), ORQ4vsQ1= 1.26 (1.17, 1.36))). When further stratified via sex according to obese status, the positive associations still existed in males with BMI <28 kg/m2 (ORQ2vsQ1= 1.31 (1.20, 1.44), ORQ3vsQ1= 1.38 (1.26, 1.51), ORQ4vsQ1= 1.52 (1.39, 1.67) and females with BMI<28 kg/m2 (ORQ2vsQ1= 1.10 (0.95, 1.27), ORQ3vsQ1= 1.23 (1.06, 1.41), ORQ4vsQ1= 1.35 (1.18, 1.55). Regarding stratification via WC, the positive associations were found in females with normal WC (ORQ2vsQ1= 1.17 (0.91, 1.49), ORQ3vsQ1= 1.46 (1.16, 1.84) and ORQ4vsQ1= 1.69 (1.32, 2.12)), and in males with both normal (ORQ2vsQ1=1.29 (1.06, 1.57), ORQ3vsQ1=1.46 (1.21, 1.78) and ORQ4vsQ1=1.68 (1.38, 2.04)) and abnormal WC (ORQ2vsQ1=1.28 (1.17, 1.41), ORQ3vsQ1=1.39 (1.26, 1.52) and ORQ4vsQ1=1.51 (1.38, 1.67)).
 |
Table 4 Stratification Analyses on the Obesity for Association Between Hepatic Steatosis and SII |
An Inverted U-Shaped Nonlinear Relationship Between SII and Hepatic Steatosis
A nonlinear relationship between SII and hepatic steatosis was explored using restricted cubic spline with a smooth curve fit in Figure 2. After adjusting for BMI, WC, TC, TG, LDL, HDL, FBG, ALT, AST and GGT, the curves representing the relationship between SII and hepatic steatosis showed an inverted U-shaped distribution. The inflection point of the curve was found to be 371.81 (1000 cells/µL) (P for nonlinear<0.01, OR= 1.29). When stratified by gender and age, there is still an inverted U-shaped curve. The inflection points for gender and age group were 399.78 (1000 cells/µL) (nonlinear P<0.01, OR=1.31, 1.00) and 385.79 (1000 cells/µL) (nonlinear P<0.01, OR=1.35, 1.87, 1.93), respectively.
Discussion
Since SII is an indicator of a comprehensive evaluation system composed of peripheral blood lymphocytes, neutrophils, and platelets, it may reflect three pathways: thrombosis, inflammation and adaptive immunity. Research supports an association between hepatic steatosis and thrombosis. Tissue factor-PAR2 signaling, which is associated with thrombosis, in adipocytes promotes diet-induced obesity by reducing metabolism and energy consumption.23 Additionally, in hematopoietic and bone marrow cells, it contributes to the development of hepatic steatosis.24,25 The pro-inflammatory cytokines and chemokines produced by hepatic adipocytes cause inflammation in the liver. These cells and chemokines further promote the activation of hepatic stellate cells, which are responsible for fibrosis7,8 At the same time, neutrophils, which play a central role in a variety of liver diseases, rapidly gather at the site of inflammation, leading to the recruitment of macrophages and the elicitation of immune responses.7,8,11 All of these evidences support SII as a good indicator for exploring the relationship between liver steatosis and inflammation.
The development of fatty liver is mainly divided into three stages: simple fatty liver, steatohepatitis, and fatty liver fibrosis (or cirrhosis).2 SII is an indicator related to inflammation that, when combined with the development of fatty liver, shows an initial increase followed by a decline. This pattern is consistent with the nonlinear relationship observed in the study results. In addition, ultrasound can only detect fatty liver only when the histological fat deposits in the liver exceed 20%.26 Therefore, it can be said that SII is more recognizable in the late stage of simple fatty liver, throughout the course of steatohepatitis, and in the early stage of fatty liver fibrosis (or cirrhosis). Compared to the diagnostic criteria for fatty liver proposed in previous epidemiological studies, such as the Fatty Liver Index, Hepatic Steatosis Index and Framingham Steatosis Index,27–30 the SII focuses more on the development of hepatic steatosis during the progression of fatty liver from an inflammatory perspective. Our study found that SII is an independent risk factor for hepatic steatosis. Considering that there are relatively few studies on the relationship between SII and hepatic steatosis in a Chinese population, this study can serve as a supplement and provide a reference for the diagnosis and evaluation of hepatic steatosis.
The protective effect of estrogen on fatty liver disease in women has been demonstrated in many studies. This is also the reason why the prevalence of fatty liver disease is significantly higher in men of childbearing age than in women.31,32 As shown in Supplementary Table 2, the prevalence of hepatic steatosis in men and women gradually converges with age. Immune cells are influenced by gender, and estrogen has been found to increase the quantity of T-cells and T-regulatory cells. This, in turn, impacts liver inflammation.33 A study of adult NAFLD found greater lobular inflammation and hepatocyte ballooning in premenopausal women, suggesting that sex hormones regulate liver damage/inflammation regardless of the level of metabolic stress.34 Animal models show that innate immune cells from male mice exhibit liver inflammation, while macrophages from female mice exhibit regulatory and anti-fibrotic functions.35,36 Animal studies also found that a high-fat diet induced steatohepatitis and inflammasome activation only in male mice.37 This may explain the male inflection point of 399.78 (1000 cells /µL) with OR>1 in this study. Studies have shown that chronic diseases are the result of an interaction between aging and inflammation.38 The innate immune system is activated in young adults and adults when the body senses danger signals.10 However, this immune response can become a detrimental health issue with age.39,40 The “garbage” theory, proposed in 2017, suggests that inflammation is primarily caused and sustained by age-related progressive damage during the “cleaning” of physiological or pathological cell death processes. This damage occurs as a result of dislocation and/or damage to the body’s own molecules (cell debris), in addition to persistent viral and bacterial infections.41,42 Research conducted by Montoliu et al found that long-lived individuals exhibited significant anti-inflammatory molecular characteristics.43 Additionally, Gonzalez-Covarrubias et al’s study demonstrated that subjects in the long-lived group displayed lower levels of lipid peroxidation and inflammation.44 These findings may explain why the OR of the relationship between SII and hepatic steatosis degeneration in this study increased with age.
Mechanism studies have shown that the imbalance between lipid uptake and utilization ultimately leads to oxidative stress and liver cell damage, which is the cause of hepatic steatosis, and thus hepatic steatosis is often accompanied by changes in blood lipids.7,10 The occurrence of hepatic fatty liver degeneration is often accompanied by elevated blood sugar and insulin resistance. This is consistent with our results in Table 1. Lymphocytes and neutrophils, as significant components of SII, are present and sustained throughout the entire process, along with stress response and cell damage, when there is an imbalance in liver synthesis and decomposition. These are also accompanied by abnormal blood lipids and blood sugar, as indicated in Table 3.
Obesity plays a significant role in the development of fatty liver. When lipid regulation in the liver is disrupted, lipids accumulate and form fatty liver. The buildup of lipids triggers inflammation and immune responses, which can result in liver fibrosis and cirrhosis.45–47 This regulatory mechanism gradually fails with the development of obesity, which is accompanied by liver fibrosis.8,48 In this study, a stratified analysis was conducted to examine the relationship between hepatic steatosis and SII among obese individuals. The analysis found that this relationship was more significant in non-obese individuals, suggesting that inflammation in obese individuals may be weakened due to the presence of fibrosis. The result is also consistent with Xie et al,18 who found that elevated levels of SII were associated with hepatic steatosis but not with hepatic fibrosis. A large multicenter cohort study of NAFLD patients from Italy and Finland found that when they analyzed biopsy specimens from patients with non-alcoholic fatty liver disease at a single point in time, one-third of patients with significant fibrosis showed no signs of steatohepatitis.49
The main strength of this study is the utilization of a relatively large sample size in China to investigate the role of SII in hepatic steatosis. Studies on the relationship between SII and hepatic steatosis in the Chinese population are still very limited. This founding could potentially be valuable for the future diagnosis of hepatic steatosis using SII. However, the study’s shortcomings call for caution in interpreting the findings and warrant further research. Firstly, cross-sectional studies can only provide correlation. Secondly, there is a lack of information regarding life style and comorbidities. Finally, hepatic steatosis can only be diagnosed by ultrasound, it is not possible to distinguish the three stages of hepatic steatosis, and the relationship between SII and the degree of adipose fibrosis cannot be analyzed.
Conclusions
In conclusion, SII is an independent risk factor for hepatic steatosis. This effect appears to be stronger in subjects with BMI<28 kg/m2. We have also discovered that there is an inverted U-shaped nonlinear relationship existed between SII and hepatic steatosis. This founding could be used as a reference for diagnosing and evaluating hepatic steatosis.
Abbreviations
SII, Systemic Immune-Inflammation; WC, waist circulation; TC, total cholesterol; TG, triglycerides; LDL, high density lipoprotein; HDL, low density lipoprotein; VLDL, very low density lipoprotein; FBG, glutamate aminotransferase; ALT, alanine aminotransferase; AST, glutamate aminotransferase; GGT, glutamyltransferase; BMI, Body mass index; OR, odds radio.
Acknowledgments
Jing Zhao and Li Yu are co-first authors for this study. This work was supported by Jiangsu senile health research project of Jiangsu Provincial Health Commission [grant NO. LKM2023037]. The study was approved the Ethics Committee of the Affiliated Suzhou Hospital of Nanjing Medical University (Ethical Approval Number: K-2023-022-K01) and was conducted in accordance with the ethical norms of the 1975 Helsinki Declaration.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stefan N, Cusi K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022;10(4):284–296. doi:10.1016/s2213-8587(22)00003-1
2. Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;158(7):1851–1864. doi:10.1053/j.gastro.2020.01.052
3. Xiao J, Wang F, Wong NK, et al. Global liver disease burdens and research trends: analysis from a Chinese perspective. J Hepatol. 2019;71(1):212–221. doi:10.1016/j.jhep.2019.03.004
4. Zhou F, Zhou J, Wang W, et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: a systematic review and meta-analysis. Hepatology. 2019;70(4):1119–1133. doi:10.1002/hep.30702
5. Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896–904. doi:10.1016/j.jhep.2018.05.036
6. Sanyal AJ. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2019;16(6):377–386. doi:10.1038/s41575-019-0144-8
7. Manne V, Handa P, Kowdley KV. Pathophysiology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Clin Liver Dis. 2018;22(1):23–37. doi:10.1016/j.cld.2017.08.007
8. Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Investig. 2017;127(1):55–64. doi:10.1172/jci88881
9. Heymann F, Tacke F. Immunology in the liver--from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13(2):88–110. doi:10.1038/nrgastro.2015.200
10. Meli R, Mattace Raso G, Calignano A. Role of innate immune response in non-alcoholic fatty liver disease: metabolic complications and therapeutic tools. Front Immunol. 2014;5:177. doi:10.3389/fimmu.2014.00177
11. Sutti S, Albano E. Adaptive immunity: an emerging player in the progression of NAFLD. Nat Rev Gastroenterol Hepatol. 2020;17(2):81–92. doi:10.1038/s41575-019-0210-2
12. Huby T, Gautier EL. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat Rev Immunol. 2022;22(7):429–443. doi:10.1038/s41577-021-00639-3
13. He Y, Rodrigues RM, Wang X, et al. Neutrophil-to-hepatocyte communication via LDLR-dependent miR-223-enriched extracellular vesicle transfer ameliorates nonalcoholic steatohepatitis. J Clin Invest. 2021;131(3). doi:10.1172/jci141513
14. Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23(34):6261–6272. doi:10.3748/wjg.v23.i34.6261
15. Li J, Cao D, Huang Y, et al. The prognostic and clinicopathological significance of systemic immune-inflammation index in bladder cancer. Front Immunol. 2022;13:865643. doi:10.3389/fimmu.2022.865643
16. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi:10.1158/1078-0432.Ccr-14-0442
17. Zhao E, Cheng Y, Yu C, et al. The systemic immune-inflammation index was non-linear associated with all-cause mortality in individuals with nonalcoholic fatty liver disease. Ann Med. 2023;55(1):2197652. doi:10.1080/07853890.2023.2197652
18. Xie R, Xiao M, Li L, et al. Association between SII and hepatic steatosis and liver fibrosis: a population-based study. Front Immunol. 2022;13:925690. doi:10.3389/fimmu.2022.925690
19. Song Y, Guo W, Li Z, et al. Systemic immune-inflammation index is associated with hepatic steatosis: evidence from NHANES 2015-2018. Front Immunol. 2022;13:1058779. doi:10.3389/fimmu.2022.1058779
20. Hong YM, Yoon KT, Cho M. Systemic immune-inflammation index predicts prognosis of sequential therapy with sorafenib and regorafenib in hepatocellular carcinoma. BMC Cancer. 2021;21(1):569. doi:10.1186/s12885-021-08124-9
21. Knott P, Mardjetko S, Tager D, et al. The influence of body mass index (BMI) on the reproducibility of surface topography measurements. Scoliosis. 2012;7(1):O18. doi:10.1186/1748-7161-7-S1-O18
22. Fan JG, Wei L, Zhuang H. Guidelines of prevention and treatment of nonalcoholic fatty liver disease (2018, China). J Dig Dis. 2019;20(4):163–173. doi:10.1111/1751-2980.12685
23. Badeanlou L, Furlan-Freguia C, Yang G, et al. Tissue factor-protease-activated receptor 2 signaling promotes diet-induced obesity and adipose inflammation. Nature Med. 2011;17(11):1490–1497. doi:10.1038/nm.2461
24. Samad F, Pandey M, Loskutoff DJ. Regulation of tissue factor gene expression in obesity. Blood. 2001;98(12):3353–3358. doi:10.1182/blood.v98.12.3353
25. Nagai N, Hoylaerts MF, Cleuren AC, et al. Obesity promotes injury induced femoral artery thrombosis in mice. Thromb Res. 2008;122(4):549–555. doi:10.1016/j.thromres.2007.12.017
26. Ballestri S, Romagnoli D, Nascimbeni F, et al. Role of ultrasound in the diagnosis and treatment of nonalcoholic fatty liver disease and its complications. Expert Rev Gastroenterol Hepatol. 2015;9(5):603–627. doi:10.1586/17474124.2015.1007955
27. Xie R, Zhang Y. Index-based calculation or transient elastography to assess the degree of hepatic steatosis and fibrosis. J Nutr. 2023;153(3):909. doi:10.1016/j.tjnut.2022.10.015
28. Xie R, Zhang Y. Associations between dietary flavonoid intake with hepatic steatosis and fibrosis quantified by VCTE: evidence from NHANES and FNDDS. Nutr Metab Cardiovasc Dis. 2023;33(6):1179–1189. doi:10.1016/j.numecd.2023.03.005
29. Xie R, Zhang Y. Is assessing the degree of hepatic steatosis and fibrosis based on index calculations the best choice for epidemiological studies? Environ Pollut. 2023;317:120783. doi:10.1016/j.envpol.2022.120783
30. Xie F, Pei Y, Zhou Q, et al. Comparison of obesity-related indices for identifying nonalcoholic fatty liver disease: a population-based cross-sectional study in China. Lipids Health Dis. 2021;20(1):132. doi:10.1186/s12944-021-01560-3
31. Long MT, Pedley A, Massaro JM, et al. A simple clinical model predicts incident hepatic steatosis in a community-based cohort: the Framingham Heart Study. Liver Int. 2018;38(8):1495–1503. doi:10.1111/liv.13709
32. Lonardo A, Nascimbeni F, Ballestri S, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70(4):1457–1469. doi:10.1002/hep.30626
33. Khorram O, Garthwaite M, Golos T. The influence of aging and sex hormones on expression of growth hormone-releasing hormone in the human immune system. J Clin Endocrinol Metab. 2001;86(7):3157–3161. doi:10.1210/jcem.86.7.7652
34. Yang JD, Abdelmalek MF, Guy CD, et al. Patient sex, reproductive status, and synthetic hormone use associate with histologic severity of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2017;15(1):127–31.e2. doi:10.1016/j.cgh.2016.07.034
35. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. doi:10.1038/nri.2016.90
36. Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Human Reproduction Update. 2005;11(4):411–423. doi:10.1093/humupd/dmi008
37. Ganz M, Csak T, Szabo G. High fat diet feeding results in gender specific steatohepatitis and inflammasome activation. World J Gastroenterol. 2014;20(26):8525–8534. doi:10.3748/wjg.v20.i26.8525
38. Franceschi C, Garagnani P, Parini P, et al. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–590. doi:10.1038/s41574-018-0059-4
39. Prattichizzo F, De Nigris V, Spiga R, et al. Inflammageing and metaflammation: the yin and yang of type 2 diabetes. Ageing Res Rev. 2018;41:1–17. doi:10.1016/j.arr.2017.10.003
40. Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. doi:10.1016/j.mad.2006.11.016
41. Ye J, Keller JN. Regulation of energy metabolism by inflammation: a feedback response in obesity and calorie restriction. Aging. 2010;2(6):361–368. doi:10.18632/aging.100155
42. Franceschi C, Garagnani P, Vitale G, et al. Inflammaging and ‘Garb-aging’. Trend Endocrinol Metabol. 2017;28(3):199–212. doi:10.1016/j.tem.2016.09.005
43. Montoliu I, Scherer M, Beguelin F, et al. Serum profiling of healthy aging identifies phospho- and sphingolipid species as markers of human longevity. Aging. 2014;6(1):9–25. doi:10.18632/aging.100630
44. Gonzalez-Covarrubias V, Beekman M, Uh HW, et al. Lipidomics of familial longevity. Aging Cell. 2013;12(3):426–434. doi:10.1111/acel.12064
45. Polyzos SA, Kountouras J, Mantzoros CS. Adipose tissue, obesity and non-alcoholic fatty liver disease. Minerva Endocrinol. 2017;42(2):92–108. doi:10.23736/s0391-1977.16.02563-3
46. Milic S, Lulic D, Stimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20(28):9330–9337. doi:10.3748/wjg.v20.i28.9330
47. Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 2019;92:82–97. doi:10.1016/j.metabol.2018.11.014
48. Caviglia GP, Rosso C, Fagoonee S, et al. Liver fibrosis: the 2017 state of art. Panminerva Med. 2017;59(4):320–331. doi:10.23736/s0031-0808.17.03359-6
49. Pelusi S, Cespiati A, Rametta R, et al. Prevalence and risk factors of significant fibrosis in patients with nonalcoholic fatty liver without steatohepatitis. Clin Gastroenterol Hepatol. 2019;17(11):2310–19.e6. doi:10.1016/j.cgh.2019.01.027
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


