Back to Journals » International Journal of Women's Health » Volume 15
Nonlinear Relationship Between Maternal and Cord Blood Vitamin B12 and Folate from a Chinese Population-Based Study
Authors Du Y, Li J, Qu P, Dang S
Received 6 May 2023
Accepted for publication 30 August 2023
Published 6 September 2023 Volume 2023:15 Pages 1405—1415
DOI https://doi.org/10.2147/IJWH.S420206
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Yujiao Du,1 Jing Li,1 Pengfei Qu,2 Shaonong Dang1
1Department of Epidemiology and Biostatistics, School of Public Health, Xi’an Jiaotong University Health Science Center, Xi’an, 710061, People’s Republic of China; 2Assisted Reproduction Center, Northwest Women’s and Children’s Hospital, Xi’an Jiaotong University Health Science Center, Xi’an, 710003, People’s Republic of China
Correspondence: Shaonong Dang, Department of Epidemiology and Biostatistics, School of Public Health, Xi’an Jiaotong University Health Science Center, Xi’an, 710061, People’s Republic of China, Tel +86-13468779736, Fax +86-2982655730, Email [email protected]
Purpose: There remains a data gap on vitamin B12 and folate level in maternal and child populations. This study aimed to assess the status of vitamin B12 and folate in maternal serum (MS) and umbilical cord serum (UCS).
Materials and Methods: This was a planned secondary analysis of a case-control study. A total of 858 pregnant women during late pregnancy and their newborns in the hospitals of China were included. Maternal peripheral venous blood and neonatal umbilical cord blood were collected to determine serum vitamin B12 and folate concentration. Relationship of vitamin B12 or folate concentration between MS and UCS was assessed by a quantile regression model and the non-linear relationship between them was examined.
Results: Nutritional status of serum folate was better than that of vitamin B12. Prevalence of deficiency in MS vitamin B12 and folate was 73.4% and 14.2%, respectively and these figures were about 17.8% and 0.1% in UCS. Both vitamin B12 and folate levels in UCS were significantly higher than those in MS (vitamin B12: 321.0 pg/mL vs 158.3 pg/mL, folate: 16.5 ng/mL vs 7.0 ng/mL, P < 0.001). The median UCS-MS ratio of vitamin B12 and folate was 2.0 (95% CI: 1.94– 2.06) and 2.4 (95% CI: 2.30– 2.53), respectively. The levels of folate and vitamin B12 in UCS increased nonlinearly with their increase in MS which presented an inverted U-shaped curve.
Conclusion: Deficiency in vitamin B12 and folate in the women during late pregnancy in China is prevalent. Nutritional status of the two vitamins in umbilical cord serum is correlated nonlinearly with that in maternal serum. Folic acid supplementation may be accompanied with vitamin B12 to improve status of vitamin B12 and folate during pregnancy.
Keywords: vitamin B12, folate, umbilical cord serum, maternal serum, pregnancy
Folate is a well-known B vitamin and participates in the synthesis of nucleic acids and amino acids in the form of tetrahydrofolate which is an indispensable substance in the development of nerve cells. Folic acid supplementation during pregnancy has been verified to reduce the incidence of neural tube defects and was also associated with a reduced risk of pre-eclampsia, congenital heart disease, and preterm birth.1,2 Many countries have adopted folic acid supplementation policies, which have played a significant role in the prevention of neural tube defects (NTDs). However, the prevalence of NTDs has not been continuously reduced. The reasons for this phenomenon are complex, except that the compliance with folic acid administration was low, and vitamin B12 deficiency might need to be considered.3–5 Vitamin B12 functions as a coenzyme in the body, and its lack may affect the folic acid metabolic pathway.6 A cohort study in Canada showed a 3-fold increase in the risk of NTDs in mothers who had vitamin B12 status in the lower quartile, regardless of folic acid fortification, suggesting that simultaneous fortification of vitamin B12 may reduce NTDs more than folic acid fortification alone.7 Moreover, poor maternal vitamin B12 status may increase the risk of adverse pregnancy outcomes such as NTDs, premature delivery, and low birth weight.8–10
Vitamin B12 and folate deficiency may exist in different populations. Studies on blood vitamin B12 and folate levels have been carried out in preschool-aged children, school-aged children, pregnant women or lactating women, elderly people and adults but most of the data were from adults.11,12 As a vulnerable population, the deficiency of such vitamins in pregnant women not only affects their own health but also influences fetal development. Maternal vitamin B12 and folate are transported into the fetus through the placenta to maintain the growth and development of the fetus. However, the data on maternal blood vitamin B12 and folate concentration at population level, especially umbilical cord blood, were still limited. Previous studies in the UK, South Korea, and Ireland showed higher concentrations of vitamin B12 and folate in cord blood than in maternal blood13–15 but the pattern of change has not been clear. Further, the data on blood vitamin B12 and folate from China are sparse. The available studies showed that adults in northern China had lower concentrations of vitamin B12 and folate than those in the south.16 Our previous survey also showed low vitamin B12 in women of childbearing age in Shaanxi, China and 45.5% of prevalence of deficiency.17 In order to fill in such an important data gap, this study was conducted to assess the status of vitamin B12 and folate in maternal peripheral venous blood during late pregnancy and umbilical cord blood of newborns by using data from a population-based study, and further investigated the relationship between folate and vitamin B12 in maternal blood and their levels in cord blood among the Chinese population.
Materials and Methods
Data and Participants
The data of this study were from a case-control study on risk factors of congenital heart disease, which was conducted in six tertiary grade A hospitals in Shaanxi province of China from 2014 to 2016, all of which were monitoring sites for birth defects. The study design and investigation has been described elsewhere.18 In present study, the newborns and their mothers in the control group (excluding birth defects) were selected. On this basis, the participants who lacked questionnaire key information, blood samples or the values of folate and vitamin B12 were excluded, and 858 mothers and their newborns were eventually included. The flow chart of participant selection is shown in Figure S1. There was no significant difference in the basic characteristics between the subjects included and those excluded (Table S1). Based on the previous folate deficiency rate of females (15%) in Shaanxi Province,17 level of α=0.05 and the relative difference of 20%, the estimated sample size was 567. Considering the non-response of the survey, the sample size was expanded by 20% and at least 680 subjects were required. Finally, 858 participants were included in present study, which provided enough power for statistical analysis.
A self-administered questionnaire was developed to collect information and investigators conducted face-to-face surveys with the participants. The questionnaire covered the information on socio-demographic status, exposure to environmental risk factors, nutrient supplementation and medication during pregnancy, diseases during pregnancy, family history, reproductive history, and health care during pregnancy. Both maternal and umbilical cord blood samples were collected in the hospital for biomarker detection. Personnel involved in the questionnaire survey, blood samples collection, transportation and detection have received unified training to ensure standard operation. The study was performed in accordance with the Declaration of Helsinki and approved by the ethics committee of Xi’an Jiaotong University Health Science Center (No. 2012008). All participants signed written informed consent.
Measurement of Vitamin B12 and Folate
Three milliliter peripheral venous blood was collected when pregnant women were waiting for delivery in the hospitals, and 3 mL neonatal umbilical cord blood was collected immediately from umbilical cords after delivery. All blood specimens were transported on dry ice to the laboratory center of School of Public Health, Xi’an Jiaotong University and the samples were centrifuged and serum was stored at −70°C. Serum vitamin B12 and folic acid were detected by chemiluminescence method, and the instrument used was an Abbott luminometer and its matching reagents (7k61.35 vitamin B12 test kit, 1p74.35 folate test kit). All measurements were performed by Xi’an Jinyu Medical Assay Co., Ltd. Key biomarkers in this study were serum vitamin B12 and folate. The serum folate concentration of <3 ng/mL was used to define folate deficiency and 3–6 ng/mL for marginal folate status. Vitamin B12 status was defined as deficiency based on serum vitamin B12 concentration of <200 pg/mL and 200–300 pg/mL for marginal vitamin B12 status.19
Assessment of Covariates
According to the literature13,14,20 and the characteristics of this study, the covariates considered in the study were socio-demographic characteristics including maternal residence, maternal age, maternal educational level, neonatal gender, gestational age, and parity and maternal health-related factors from 3 months before pregnancy to delivery covering passive smoking, having had a cold, and folic acid supplementation. Gestational age was calculated based on the date of last menstruation and the date of delivery. This study grouped the participants into primiparous and multiparous women. Passive smoking was defined as exposure to tobacco smoke from someone nearby for more than 15 minutes at least one day a week. The women were determined as having had a cold when they reported the symptoms of upper respiratory tract infections such as nasal congestion and runny nose. Folic acid supplementation referred to taking folic acid supplements (0.4 mg/day) for more than 30 days during pregnancy.
Statistical Analysis
Right-skewed distributions of serum vitamin B12 and folate concentration were observed according to the Shapiro–Wilk test. Consequently the median and quartile spacing were used to describe vitamin B12 and folate concentration in maternal serum (MS) and umbilical cord serum (UCS). The Mann–Whitney U-test was applied to compare difference in folate and vitamin B12 between the groups of interest. In order to explore the relationship of vitamin B12 or folate concentration between MS and UCS, a quantile regression model was established, which would allow the impact of the vitamin status in UCS to vary along the whole range of vitamin status in MS. The coefficients unadjusted and adjusted for covariates and their 95% confidence interval (CIs) for selected quantiles (10, 20, 30, …, 90 quantile) were estimated and a positive coefficient at each quantile indicated an increasing amount of UCS vitamin B12 or folate concentration with increase of MS vitamin B12 or folate concentration. Accordingly the coefficients were also estimated from a typical regression using ordinary least squares (OLS) to provide a basis for comparison with the quantile regression. Further, the curve estimation regression model was also established to reveal a non-linear relationship between MS and UCS vitamin B12 and folate concentration. Finally, a quadratic equation was tried to fit the non-linear relationship. The expression of the model was 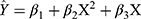 , where
, where 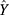 was the estimation value of micronutrient concentration in UCS, X was the value of micronutrient concentration in MS, and β1 was a constant and β2/β3 were corresponding coefficients. The vertex coordinates of the quadratic equation were calculated from the expressions
was the estimation value of micronutrient concentration in UCS, X was the value of micronutrient concentration in MS, and β1 was a constant and β2/β3 were corresponding coefficients. The vertex coordinates of the quadratic equation were calculated from the expressions 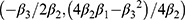 . Moreover, UCS-MS ratio of micronutrient concentration was determined as the value of UCS concentration divided by MS micronutrient concentration, reflecting the relative micronutrient concentration of UCS concentration to MS concentration. The 95% CI of the median of ratio was estimated by the Bootstrap method. In addition, the subgroup analysis on the ratio was also conducted by main covariates to explore the robustness of the relationship in the concentrations of the two vitamins between UCS and MS. All analyses were performed using SPSS Statistics version 21 and R version 3.5.2, with P < 0.05 as the difference being statistically significant.
. Moreover, UCS-MS ratio of micronutrient concentration was determined as the value of UCS concentration divided by MS micronutrient concentration, reflecting the relative micronutrient concentration of UCS concentration to MS concentration. The 95% CI of the median of ratio was estimated by the Bootstrap method. In addition, the subgroup analysis on the ratio was also conducted by main covariates to explore the robustness of the relationship in the concentrations of the two vitamins between UCS and MS. All analyses were performed using SPSS Statistics version 21 and R version 3.5.2, with P < 0.05 as the difference being statistically significant.
Results
Characteristics of the Participants
In total 858 mothers were included in this study, with an average age of 28.91±3.94 years old. A majority of the mothers (78.0%) had college education or above, and 69.3% of them lived in cities. Male newborns accounted for 50.2%. Primiparous mothers accounted for 75.9% and mothers with gestational age less than 37 weeks accounted for 5.7%; 44.8% of mothers had a cold from 3 months before pregnancy to delivery, 10.6% experienced passive smoking during pregnancy, and 89.0% had supplemented with folic acid during pregnancy (Table 1).
 |
Table 1 The Concentration of Maternal Serum Vitamin B12 and Folate by Socio-Demographic Characteristics and Maternal Health-Related Factors |
Status of Vitamin B12 and Folate in MS and UCS
Table 1 shows that the urban mothers had higher MS vitamin B12 and folate concentrations, and the mothers who were older or more educated had higher vitamin B12 and folate concentrations. The folate concentration of mothers who received folic acid supplementation was higher than those who did not (P <0.05). As shown in Table 2, the concentration of UCS vitamin B12 was significantly higher in the mothers who lived in cities, were more educated, and received folic acid supplementation (P <0.05). UCS vitamin B12 concentration was significantly lower in the primiparous mothers and the mothers who had been exposed to passive smoke, as well as in newborns whose gestational age was less than 37 weeks (P <0.05). UCS folate concentration was not statistically significant among the groups. The median was 158.3 pg/mL for MS vitamin B12 and 321.0 pg/mL for UCS vitamin B12. The median of MS folate was 7.0 ng/mL and 16.5 ng/mL UCS folate. The prevalence of MS vitamin B12 deficiency was 73.4%, while that of UCS vitamin B12 was 17.8%. The status of serum folate was better than that of vitamin B12 and the prevalence of MS folate deficiency was 14.2%, while that of UCS folate was only 0.1%.
 |
Table 2 The Concentration of Vitamin B12 and Folate in UCS by Socio-Demographic Characteristics and Maternal Health-Related Factors |
Relationship Between MS and UCS in Vitamin B12 and Folate
Figure 1 shows a box plot of UCS concentration according to MS concentration quantiles. The median of UCS vitamin B12 concentration increased from 234.2 pg/mL at the 20th percentile group to 404.9 pg/mL at the 80th percentile group, and the median UCS folate concentration increased from 13.8 ng/mL at the 20th percentile group to 17.9 ng/mL at the 80th percentile group. In general, as the concentration of MS increased from low to high quantiles, the median concentrations of vitamin B12 and folate in UCS also increased, and the concentration of UCS at any quantile groups was higher than the corresponding MS concentration.
 |
Figure 1 The UCS concentrations at quantiles of MS vitamin B12 and folate. |
The concentrations of both vitamin B12 and folate in UCS were significantly higher than those in MS (vitamin B12: 321.0 pg/mL vs 158.3 pg/mL, folate: 16.5 ng/mL vs 7.0 ng/mL, both P <0.001). The median UCS-MS ratio of vitamin B12 and folate was 2.0 (95% CI: 1.94–2.06) and 2.4 (95% CI: 2.30–2.53), respectively. Further subgroup analysis indicated that the concentration ratio of UCS and MS was consistent regardless of main covariates, suggesting a robust relationship of folate and vitamin B12 between MS and UCS (Figure S2).
Quantile Regression Analysis
Quantile regression was used to further investigate the relationship in folate and vitamin B12 between MS and UCS. Table 3 shows that the regression coefficients were statistically significant (P <0.001) even controlling for potential covariates, indicating the significantly positive relationship between MS and UCS folate and vitamin B12. OLS results found that the concentration of UCS vitamin B12 increased by 1.68 pg/mL on average for every 1 pg/mL increase in MS concentration, and the concentration of UCS folate increased by 0.38 ng/mL on average for every 1 ng/mL increase in MS concentration. Regarding MS vitamin concentration as independent variable and UCS vitamin concentration as dependent variable, a quadratic relationship between them was found to be statistically significant and showed an inverted U-shaped curve. The vertex coordinates of the curve could be calculated (vitamin B12: (515.83, 739.40 pg/mL), folate: (29.55, 22.83 ng/mL)). It meant that UCS vitamin B12 increased with the increase of MS vitamin B12 and reached peak value (739.40 pg/mL) at the 515.83 pg/mL of MS vitamin B12. Also, it was observed that UCS vitamin B12 increasingly slowed down after 300 pg/mL of MS B12. For folate, a similar pattern was found. The increasing extent of UCS folate diminished after the MS folate reached approximately 15 ng/mL (Figure 2).
 |
Table 3 Quantile Regression on the Relationship Between MS and UCS Vitamin B12 and Folate Levels (β, 95% CI) |
Discussion
Vitamin B12 and folate are vital during embryonic development. In order to prevent the adverse effects caused by insufficiency or deficiency in vitamin B12 and folate during pregnancy, various interventions including micronutrient supplementation have been taken worldwide.3–5 However, more epidemiological profiles on these two vitamins in maternal and child populations are required, especially for vitamin B12. This study filled in this evidence gap to some extent by evaluating the status of vitamin B12 and folate in maternal and umbilical cord blood.
The main finding from this study was that deficiency in vitamin B12 and folate in women during late pregnancy was still prevalent in China. The prevalence of deficiency in maternal vitamin B12 and folate was 73.4% and 14.2%, respectively. The prevalence of folate deficiency in adults was about 0–25% worldwide,11 and about 6–37% in other regions of China.16 Consequently, the women in late pregnancy in our study may be at a moderate level of folate deficiency. The vitamin B12 deficiency rate of pregnant women varied from 0 to 74.1% around the world.12 Obviously, this study found a higher deficiency in maternal vitamin B12 which implies a salient public health issue for Chinese pregnant women. The human body cannot actively synthesize vitamin B12 and folate, so it needs to be supplied from an external source. If pregnant women do not pay attention to increase the intake of such vitamins from diet or supplements, it was very likely they will be insufficient in those vitamins in the body, which could affect the health of themselves and the fetus. Such lower vitamin B12 may be largely due to female poor dietary patterns. According to our previous investigation, the dietary pattern of pregnant women in Shaanxi province was not balanced, and the intake of foods derived from animals was insufficient,21 which partly accounted for deficiency in maternal folate and vitamin B12. Therefore, a balanced dietary pattern and an increase in the intake of animal-derived food may be an important way to improve the vitamin B12 status of Chinese women. In addition, consumption of processed foods and reheating of cooked foods can reduce the bioavailability of vitamins in food products and may reduce the vitamin levels including vitamin B of the population.22–24 A Brazilian cohort study has shown that lower maternal B12 was associated with lower levels of the methyl donor (S-adenosyl methionine) in the cord blood, which could affect the metabolic pathway of folate, leading to folate deficiency or aggravating the symptoms of folate deficiency.25 Lower maternal vitamin B12 and folate could increase the concentration of homocysteine, which may be related to NTDs, cardiovascular diseases, kidney diseases, and other diseases.8,20,26 The symptoms due to vitamin B12 deficiency were largely similar to those of folate deficiency, and increased folic acid intake could interfere with the clinical diagnosis of vitamin B12 deficiency,6 which means that folic acid supplementation may cover up the lack of vitamin B12, so it is suggested that attention should also be paid to replenishment of vitamin B12 in the practice of maternal folic acid supplementation.
Socio-demographic factors could affect dietary intake of micronutrients, among them residence, age, and educational level of women could be key variables.16,27–29 In our study, the rural pregnant women presented lower folate and vitamin B12 than the urban women, which was also similar to the urban-rural differences found in other areas of China.16 The difference in economic development and lifestyle between rural and urban areas in China could account for the variation of these two vitamin levels during pregnancy. Studies indicated that low folate concentration of pregnant women was related to young age, lower education level, and annual income of the mothers.29 The serum vitamin B12 and folate concentration of women over 30 years old were significantly higher than those of women under 30 years old. This may be due to young women’s insufficient awareness of pregnancy nutrition supplements and the high rate of unwanted pregnancy, most of which are unplanned pregnancies, or lack of preparation before pregnancy,28 while older women have higher awareness of pregnancy related knowledge and adequate pregnancy planning due to their higher risk of pregnancy and childbirth, so vitamin B12 and folate levels would increase to varying degrees.27 Interestingly, these factors seemed not to affect vitamin B12 level in umbilical cord blood.
A significantly positive relationship was found between MS and UCS in term of vitamin B12 and folate in that the two vitamins of UCS increased with increase of those in MS. Even after controlling for potential confounders, this close relationship persisted. It should be noted that UCS vitamins increased slowly and even showed a downward trend when the vitamins of MS increased to a certain level, which clearly presented an inverted U-shaped curve indicating a non-linear relationship. Generally, the concentration of UCS was significantly higher than that of MS. The concentration of vitamin B12 in UCS was about 2 times that of MS, and the concentration of folate in UCS was about 2.4 times that of MS in the Chinese population. Molloy’s study showed that the concentration of vitamin B12 in umbilical cord plasma was nearly 70% higher than that in maternal plasma, and the concentration of folate in umbilical cord plasma was nearly 80% higher than in maternal plasma in pregnant women in Ireland.15 Ahn found that folate concentration in UCS was 2.1 times that of MS in Korean pregnant women.14 Our results further confirmed these studies but implied that such differences between maternal and cord blood could vary slightly across different populations. This study supported the fact of fetal priority in pregnancy that the transport of vitamin B12 and folate from mother to fetus was the result of the active transport of the placenta, regardless of socio-demographic characteristics and maternal health-related factors from 3 months before pregnancy to delivery. The transport of vitamin B12 and folate from mother to fetus was closely related to the activity of folate transporters (FOLR1, RFC1, and HCP1/PCFT).30–32 Vitamin B12 and folate may be accumulated in the placenta or umbilical cord of the mother and then transported to the fetus.14 This mechanism may be the reason for the fetal concentration staying higher than the maternal concentration, and may mitigate some of the adverse pregnancy outcomes associated with maternal vitamin B12 and folate deficiencies. Previous studies showed that zinc concentration was significantly higher in umbilical cord blood than in maternal blood, whereas maternal cadmium, lead, selenium, and cuprum levels were significantly higher than those in umbilical cord blood, suggesting that the placental barrier may have some protective effects, which could reduce the adverse consequences of heavy metal exposure and nutrient deficiency in the fetus.33,34
This study evaluated the status of vitamin B12 and folate in MS and UCS using a large sample, which could provide practical data support for a micronutrient supplementation program. An inverted U-shaped curve was found between MS and UCS in vitamin B12 and folate, which quantitatively evaluated the relationship of the two vitamins between MS and UCS. However, several limitations should be considered when interpreting the results. First, the participants came from urban hospitals in northwest China, and the proportion of urban women with higher education was relatively high, which affected the representativeness of the sample to some extent thus the generalization of results to the whole Chinese population should be cautious. Second, the serum folate concentration measured in this study was a short-term folate indicator while the red blood cell folate concentration can be a better indicator. Unfortunately, this indicator was unavailable due to limited conditions. Finally, so far there have been no rational reference values of vitamin B12 and folate concentration for maternal peripheral venous blood and umbilical cord blood. The physiological state of pregnant women has changed, so using the standard of the general population may not estimate correctly the prevalence of deficiency. In addition, the thresholds for folate deficiency or marginal deficiency used in this study were established to prevent megaloblastic anemia, but there has been no accepted standard for the prevention of NTDs. A recent study proposed 11.26 ng/mL as the preventive threshold of NTDs but it may be inappropriate for populations with high prevalence of vitamin B-12 deficiency or marginal deficiency.35 According to this threshold, the estimated prevalence of folate deficiency in our sample was 72.4% in maternal blood and 8.4% in umbilical cord blood, which was far higher than those from the current standard. Vitamin B12 is involved in folate metabolism and its internal level may affect the level of this threshold.35 There was high deficiency in vitamin B12 among our participants, which could affect the application of the threshold. Further, it would be worth measuring additionally the concentration of either homocysteine, methylmalonic acid or transcobalamin as additional indicators of vitamin B12 deficiency.36 In view of the importance of the prevention of NTDs, how to define the standard of folate deficiency in groups with a high proportion of vitamin B12 deficiency needs further study. Moreover, the association between maternal folate and vitamin B12 requires further investigation.
In conclusion, both vitamin B12 and folate are deficient to some extent in Chinese parturient women and the nutritional status of maternal vitamin B12 is worse. On average the concentration of vitamin B12 and folate in umbilical cord blood is about twice as high as that in maternal blood but the relationship is nonlinear, presenting an inverted U-shaped curve. Improving maternal dietary nutrition and increasing supplementation with folate and vitamin B12 during pregnancy require particular attention in maternal and child care. Given the high risk of vitamin B12 deficiency, vitamin B12 supplementation should be combined with folic acid supplementation to further benefit fetal growth. In addition, more evidence linking vitamin B12 deficiency to adverse pregnancy outcomes is needed in future research.
Data Sharing Management
All data used in this study will be available from the corresponding author upon reasonable request.
Ethical Approval and Informed Consent
The study was performed in accordance with the Declaration of Helsinki and approved by the ethics committee of Xi’an Jiaotong University Health Science Center (No. 2012008). All participants signed written informed consent.
Acknowledgments
We would like to express sincere thanks to all participants and the investigators and staff contributing to this project. Especially, we are grateful for the support of relevant hospitals and Shaanxi Commission of Health of China.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was supported by Scientific Research Project Xi’an Municipal Health Commission (20221223), the National Natural Science Foundation of China (grant number 81230016) and National Key Research and Development Program of China (grant number 2017YFC0907200, 2017YFC0907201).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Liu C, Liu C, Wang Q, Zhang Z. Supplementation of folic acid in pregnancy and the risk of preeclampsia and gestational hypertension: a meta-analysis. Arch Gynecol Obstet. 2018;298(4):697–704. doi:10.1007/s00404-018-4823-4
2. Liu X, Lv L, Zhang H, et al. Folic acid supplementation, dietary folate intake and risk of preterm birth in China. Eur J Nutr. 2016;55(4):1411–1422. doi:10.1007/s00394-015-0959-1
3. Caffrey A, McNulty H, Irwin RE, Walsh CP, Pentieva K. Maternal folate nutrition and offspring health: evidence and current controversies. Proc Nutr Soc. 2018;78(2):208–220. doi:10.1017/S0029665118002689
4. Estevez-Ordonez D, Davis MC, Hopson B, et al. Reducing inequities in preventable neural tube defects: the critical and underutilized role of neurosurgical advocacy for folate fortification. Neurosurg Focus. 2018;45(4):E20. doi:10.3171/2018.7.FOCUS18231
5. Laharwal MA, Sarmast AH, Ramzan AU, et al. Epidemiology of the neural tube defects in Kashmir Valley. J Pediatr Neurosci. 2016;11(3):213–218. doi:10.4103/1817-1745.193368
6. Stover PJ. Physiology of folate and vitamin B12 in health and disease. Nutr Rev. 2004;62(6 Pt 2):S3–S12; discussion S13. doi:10.1111/j.1753-4887.2004.tb00070.x
7. Thompson MD, Cole DE, Ray JG. Vitamin B-12 and neural tube defects: the Canadian experience. Am J Clin Nutr. 2009;89(2):697S–701S. doi:10.3945/ajcn.2008.26947B
8. Peker E, Demire N, Tuncer O, et al. The levels of vitamin B12, folate and homocysteine in mothers and their babies with neural tube defects. J Matern Fetal Neonatal Med. 2016;29(18):2944–2948. doi:10.3109/14767058.2015.1109620
9. Salcedo-Bellido I, Martinez-Galiano JM, Olmedo-Requena R, et al. Association between vitamin intake during pregnancy and risk of small for gestational age. Nutrients. 2017;9(12):1277. doi:10.3390/nu9121277
10. Senousy SM, Farag MK, Gouda AS, El Noury MA, Dabbous OA, Gaber KR. Association between biomarkers of vitamin B12 status and the risk of neural tube defects. J Obstet Gynaecol Res. 2018;44(10):1902–1908. doi:10.1111/jog.13751
11. McLean E, de Benoist B, Allen LH. Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr Bul. 2008;29(2 Suppl):S38–S51. doi:10.1177/15648265080292S107
12. Sukumar N, Rafnsson SB, Kandala NB, Bhopal R, Yajnik CS, Saravanan P. Prevalence of vitamin B-12 insufficiency during pregnancy and its effect on offspring birth weight: a systematic review and meta-analysis. Am J Clin Nutr. 2016;103(5):1232–1251. doi:10.3945/ajcn.115.123083
13. Adaikalakoteswari A, Vatish M, Lawson A, et al. Low maternal vitamin B12 status is associated with lower cord blood HDL cholesterol in white Caucasians living in the UK. Nutrients. 2015;7(4):2401–2414. doi:10.3390/nu7042401
14. Ahn HS. Relation between folate levels of maternal-umbilical cord blood, placenta tissue and pregnancy outcomes. J Community Nutr. 2004;6(2):91–96.
15. Molloy AM, Mills JL, McPartlin J, Kirke PN, Scott JM, Daly S. Maternal and fetal plasma homocysteine concentrations at birth: the influence of folate, vitamin B12, and the 5,10-methylenetetrahydrofolate reductase 677C–>T variant. Am J Obstet Gynecol. 2002;186(3):499–503. doi:10.1067/mob.2002.121105
16. Hao L, Ma J, Zhu J, et al. High prevalence of hyperhomocysteinemia in Chinese adults is associated with low folate, vitamin B-12, and vitamin B-6 status. J Nutr. 2007;137(2):407–413. doi:10.1093/jn/137.2.407
17. Dang S, Yan H, Zeng L, et al. The status of vitamin B12 and folate among Chinese women: a population-based cross-sectional study in northwest China. PLoS One. 2014;9(11):e112586. doi:10.1371/journal.pone.0112586
18. Yang J, Kang Y, Cheng Y, Zeng L, Yan H, Dang S. Maternal dietary patterns during pregnancy and congenital heart defects: a Case-Control Study. Int J Environ Res Public Health. 2019;16(16):2957. doi:10.3390/ijperph16162957
19. Allen LH. Folate and vitamin B12 status in the Americas. Nutr Rev. 2004;62(6 Pt 2):S29–S33; discussion S34. doi:10.1111/j.1753-4887.2004.tb00069.x
20. Miliku K, Mesu A, Franco OH, Hofman A, Steegers EAP, Jaddoe VWV. Maternal and fetal folate, vitamin B12, and homocysteine concentrations and childhood kidney outcomes. Am J Kidney Dis. 2017;69(4):521–530. doi:10.1053/j.ajkd.2016.11.014
21. Yang J, Dang S, Cheng Y, et al. Dietary intakes and dietary patterns among pregnant women in Northwest China. Public Health Nutr. 2017;20(2):282–293. doi:10.1017/S1368980016002159
22. Ling B, Tang J, Kong F, Mitcham EJ, Wang S. Kinetics of food quality changes during thermal processing: a review. Food Bioprocess Technol. 2015;8(2):343–358. doi:10.1007/s11947-014-1398-3
23. Oghbaei M, Prakash J. Effect of compositional alteration of food matrices and processing on availability of selected nutrients and bioactive components in rice products. Int J Food Sci Nutr. 2011;62(3):250–261. doi:10.3109/09637486.2010.527322
24. van Boekel M, Fogliano V, Pellegrini N, et al. A review on the beneficial aspects of food processing. Mol Nutr Food Res. 2010;54(9):1215–1247. doi:10.1002/mnfr.200900608
25. Guerra-Shinohara EM, Morita OE, Peres S, et al. Low ratio of S-adenosylmethionine to S-adenosylhomocysteine is associated with vitamin deficiency in Brazilian pregnant women and newborns. Am J Clin Nutr. 2004;80(5):1312–1321. doi:10.1093/ajcn/80.5.1312
26. Cianciolo G, De Pascalis A, Di Lullo L, Ronco C, Zannini C, La Manna G. Folic acid and homocysteine in chronic kidney disease and cardiovascular disease progression: which comes first? Cardiorenal Med. 2017;7(4):255–266. doi:10.1159/000471813
27. Branum AM, Bailey R, Singere BJ. Dietary supplement use and folate status during pregnancy in the United States. J Nutr. 2013;143(4):486–492. doi:10.3945/jn.112.169987
28. Wellings K, Jones KG, Mercer CH, et al. The prevalence of unplanned pregnancy and associated factors in Britain: findings from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Lancet. 2013;382(9907):1807–1816. doi:10.1016/S0140-6736(13)62071-1
29. Yila TA, Araki A, Sasaki S, et al. Predictors of folate status among pregnant Japanese women: the Hokkaido Study on environment and children’s health, 2002–2012. Br J Nutr. 2016;115(12):2227–2235. doi:10.1017/S0007114516001628
30. Castano E, Caviedes L, Hirsch S, Llanos M, Iniguez G, Ronco AM. Folate transporters in placentas from preterm newborns and their relation to cord blood folate and vitamin B12 levels. PLoS One. 2017;12(1):e0170389. doi:10.1371/journal.pone.0170389
31. Caviedes L, Iniguez G, Hidalgo P, Castro JJ, Castano E, Llanos M. Relationship between folate transporters expression in human placentas at term and birth weights. Placenta. 2016;38:24–28. doi:10.1016/j.placenta.2015.12.007
32. Yasuda S, Hasui S, Yamamoto C, et al. Placental folate transport during pregnancy. Biosci Biotechnol Biochem. 2008;72(9):2277–2284. doi:10.1271/bbb.80112
33. Butler Walker J, Houseman J, Seddon L, et al. Maternal and umbilical cord blood levels of mercury, lead, cadmium, and essential trace elements in Arctic Canada. Environ Res. 2006;100(3):295–318. doi:10.1016/j.envres.2005.05.006
34. Kim YM, Chung JY, An HS, et al. Biomonitoring of lead, cadmium, total mercury, and methylmercury levels in maternal blood and in umbilical cord blood at birth in South Korea. Int J Environ Res Public Health. 2015;12(10):13482–13493. doi:10.3390/ijerph121013482
35. Chen MY, Rose CE, Qi YP, et al. Defining the plasma folate concentration associated with the red blood cell folate concentration threshold for optimal neural tube defects prevention: a population-based, randomized trial of folic acid supplementation. Am J Clin Nutr. 2019;109(5):1452–1461. doi:10.1093/ajcn/nqz027
36. Vashi P, Edwin P, Popiel B, Lammersfeld C, Gupta D, Sengupta S. Methylmalonic acid and homocysteine as indicators of vitamin B-12 deficiency in cancer. PLoS One. 2016;11(1):e0147843. doi:10.1371/journal.pone.0147843
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

