Back to Journals » Journal of Inflammation Research » Volume 16
Nomogram Based on Preoperative Fibrinogen and Systemic Immune-Inflammation Index Predicting Recurrence and Prognosis of Patients with Borrmann Type III Advanced Gastric Cancer
Authors Wang H , Yin X , Ma K, Wang Y , Fang T , Zhang Y , Xue Y
Received 12 January 2023
Accepted for publication 8 March 2023
Published 12 March 2023 Volume 2023:16 Pages 1059—1075
DOI https://doi.org/10.2147/JIR.S404585
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Hao Wang,1,* Xin Yin,1,* Keru Ma,2,* Yufei Wang,1,* Tianyi Fang,1 Yao Zhang,1 Yingwei Xue1
1Department of Gastroenterological Surgery, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China; 2Department of Thoracic Surgery, Esophagus and Mediastinum, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yingwei Xue, Department of Gastrointestinal Surgery, Harbin Medical University Cancer Hospital, Harbin Medical University, Harbin, 150081, People’s Republic of China, Tel +86-13304646901, Email [email protected]
Background and Objectives: The prognosis is known to differ significantly among advanced gastric cancer (AGC) with Borrmann type III. This study aimed to evaluate the prognosis of these patients more individually.
Methods: We selected 542 AGC patients with Borrmann type III. We used the receiver operating characteristic curve to analyze the cutoff values of inflammation indexes, and used Kaplan–Meier and Log rank tests to analyze recurrence-free survival (RFS) and overall survival (OS). The independent risk factors for recurrence and prognosis were analyzed by Cox proportional hazards regression model. The nomogram models were constructed by R studio.
Results: Patients with high preoperative fibrinogen (F) and systemic immune-inflammation index (SII) levels had worse RFS and OS and higher risk of postoperative locoregional recurrence, hematogenous metastasis and lymph node metastasis. F and SII can combine with different clinicopathological features (all P< 0.05) to construct nomograms to predict 5-year recurrence and prognosis, which both were superior to pTNM stage alone.
Conclusion: The nomogram models based on F and SII can evaluate AGC with Borrmann type III postoperative recurrence and prognosis.
Keywords: gastric cancer, Borrmann classification, fibrinogen, systemic immune-inflammation index, recurrence, nomogram
Introduction
Gastric cancer (GC) is the third malignant tumor that causes cancer death, with about 865,000 deaths annually.1 There were of 60% early GC patients in Korea and Japan, while more than 80% advanced GC (AGC) in China.2,3 In order to evaluate the AGC patients prognosis, experts have proposed a variety of classification methods according to tumor biological characteristics.4,5 Since 1926, the Borrmann classification according to tumor macroscopic characteristics,6 and the most common macro type of AGC is Borrmann III,7,8 which has significant prognostic differences according to different clinicopathological features. Hosoda et al9 considered that tumor size was related to the prognosis of AGC with Borrmann III. The five -year survival rate of patients with preoperative tumor diameter ≥10 cm was lower than those with tumor size <10 cm (27.9% vs 49.9%). Zhai et al10 found that the presence or absence of vascular infiltration had a significant impact on the survival of AGC with Borrmann type III (16.4% vs 29.1%). Therefore, combining the clinicopathological features to build prognostic models to evaluate prognosis of AGC patients with Borrmann III will contribute to individualized treatment.
Tumor immunity plays a crucial role in immunotherapy, because clinical experts can select the specific immune trigger point.11 Galon proposed the pTNM-I (Immunology) stage, which suggests that the tumor immunity can provide comprehensive prognosis information.12,13 However, the selection of tissue section sites limits immunohistochemical staining. Then, peripheral blood immune also plays an important role in the occurrence of tumors and progress. Fibrinogen (F) plays an important role in the coagulation process and inflammatory response, and elevated levels suggest a poor prognosis in patients with GC.14 Our research team found that for early diagnosis of GC, the predicted performance of neutrophil–lymphocyte ratio (NLR) and platelet–lymphocyte ratio (PLR) were significantly better than traditional tumor markers.15 Furthermore, there are significant differences in the peripheral immune status of patients with different Lauren type patients.16 Borrmann type III AGC as an infiltrative ulcerative carcinoma usually has a large ulcer surface, and may be associated with a stronger inflammatory response.17,18 However, there is no research to analyze its relationship with the peripheral immune inflammatory response. Thus, whether peripheral inflammation indexes and clinicopathological features can assess Borrmann III AGC patients’ prognosis needs in-depth research.
Hence, we compared the values of different inflammation indexes in predicting the postoperative recurrence and prognosis of Borrmann III AGC. After that, build a prediction model based on inflammation indexes to predict patients’ prognosis.
Methods
Patients
This study retrospectively analyzed 542 patients diagnosed with Borrmann type III AGC between January 4, 2013 and December 30, 2014. All GC patients underwent radical gastrectomy according to their respective conditions.19 The diagnosis of GC was based on tissue samples obtained during gastroscopy and confirmation by pathologists through examination of postoperative tissue specimens.
Exclusion criteria were: (1) preoperative chemotherapy; (2) severe heart disease; (3) platelet therapy within 3 months before surgery; (4) active bleeding; (5) intravascular coagulation; (6) severe infection; (7) hematological malignancies; (8) steroid drug treatment.
Postoperative chemotherapy regimens were based on the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology.20 To ensure that the research is accurate, we included 220 patients who received complete postoperative chemotherapy in our institution. We did not include patients who have not received chemotherapy in our institution, or patients have returned to the local hospital chemotherapy without complete chemotherapy records.
Datasets
The datasets were saved in the Harbin Medical University Cancer Hospital, including gender, age, body mass index (BMI), tumor size, tumor location, metastatic lymph node ratio (mLNR), pTNM stage, vascular infiltration, nerve infiltration, postoperative chemotherapy and laboratory examination. The pTNM staging conforms to the eighth edition of American Joint Commission on Cancer (AJCC). The tumor invasion exceeds the muscular propria (T2), and without considering lymph nodes status, which was defined as AGC.21 All patients were discharged by phone, email or at the outpatient department of Harbin Medical University Cancer Hospital for regular follow-up examination, including hematological analysis, tumor markers, gastroscopy, abdominal ultrasound, abdominal CT, and PET-CT according to the condition of some patients. Stage I patients are followed every 12 months, stage II patients are followed up every 6 months, and stage III patients are followed every 3–6 months. According to the medical history, imaging examination, cytological examination, and the organization biopsy determine recurrence and metastasis. Chest or abdominal CT is performed for suspected tumor recurrence or tumor marker levels higher than pathological levels, and bone scan for suspected bone metastases. The types of recurrence and metastasis can be divided into locoregional recurrence (anastomotic or gastric remnant), hematogenous recurrence (liver, bone or other sites of metastatic disease), peritoneal recurrence and lymph node recurrence.22,23
Inflammation Indexes
Calculate the inflammatory indexes based on the results of the peripheral blood test. The calculation formula is as follows, neutrophil–lymphocyte ratio (NLR) = N/L, platelet–lymphocyte ratio (PLR) = P/L, systemic immune-inflammation index (SII) = N×P/L (N = Neutrophil count, L = Lymphocyte count, and P = Platelet count).
Statistical methods
Recurrence-free survival (RFS) was defined as the time from surgery to recurrence or the last recurrence-free follow-up. OS was defined as the time from surgery to death or the last surviving follow-up. The Receiver operating characteristic curve (ROC) was used to compare the predictive performance of the inflammatory indexes and compare the area under the curve (AUC). Judging the cutoff values of inflammatory indexes according to the Youden index. The relationship between clinical pathological characteristics and inflammation indexes was judged by chi-square test. The Log rank test and Kaplan–Meier method were used to analyze survival curves. Cox regression model analysis recurrence and independent risk factors related to prognosis. Hazard ratios (HRs) and 95% CIs were estimated for each factor. The nomogram models were drawn through the R studio by “SvyNom” and “rms” packages. SPSS version 25.0 was used for analysis and P<0.05 was considered statistically significant.
Results
Clinical Characteristics
In this study, the median white blood cell count was 6.10±1.95×109/L, the median age was 58 (range 23–87). There were 408 men (75.3%) and 134 women (24.7%). Patients with pTNM stage I, II and III were 52 (9.6%), 184 (34.3%) and 306 (56.1%), respectively (Table 1). Two hundred and six patients (38.0%) had tumor recurrence or metastasis, and 197 (36.3%) died within 5 years after radical gastrectomy. The number of patients with recurrence patterns of locoregional recurrence, peritoneal metastasis, hematogenous metastasis and lymph node metastasis were 32 (5.9%), 64 (11.8%), 68 (12.5%) and 41 (7.6%), respectively. At least two types of recurrence occurred in 16 patients, and no specific recurrence patterns were observed in 19 patients.
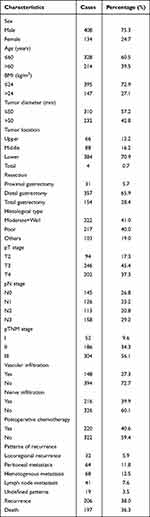 |
Table 1 Baseline Characteristics of the Patients |
NLR, PLR, Fibrinogen and SII
For preoperative NLR, PLR, Fibrinogen (F) and SII of patients, 1.94, 133.56, 3.24 and 489.90, respectively, were found to be the optimal cutoff values for assessing the risk of recurrence. For these values, AUC was 0.633 (95% CI: 0.584–0.681), 0.648 (95% CI: 0.601–0.696), 0.652 (95% CI: 0.604–0.699) and 0.666 (95% CI: 0.619–0.714), respectively (Figure 1A). The optimal cutoff values for assessing patient’s prognosis were 1.94, 133.56, 3.27 and 489.90. The AUC was 0.638 (95% CI: 0.589–0.687), 0.651 (95% CI: 0.603–0.700), 0.656 (95% CI: 0.608–0.704) and 0.672 (95% CI: 0.624–0.720), respectively (Figure 1B). Therefore, F and SII (F-SII) scoring system was constructed according to the optimal cutoff values. Patients who were both lower than the optimal values were 0 points, patients who were both higher than the optimal values were 2 points, and only one index higher than the optimal values was 1 point.
 |
Figure 1 ROC of different inflammation indexes of patients. (A) Assessing the risk of recurrence. (B) Assessing prognosis. |
F-SII and RFS
Chi-square found that F-SII was significantly correlated with clinicopathological characteristics, including tumor diameter and pTNM stage (Table 2). RFS of patients with F-SII=0, 1 and 2 was 50.88 (95% CI: 48.739–53.017), 42.37 (95% CI: 39.252–45.479) and 31.34 (95% CI: 26.602–36.082) months. The 5-year RFS rate was 74.1%, 56.8% and 24.7%, respectively (P<0.001) (Figure 2A).
 |
Table 2 Chi-Square Test Analysis of the Connection Between F-SII and Clinicopathological Features for RFS and OS |
 |
Figure 2 Kaplan–Meier curves analyses for RFS based on F-SII score. (A) All patients. (B) Stage I/II. (C) Stage III. (D) Tumor diameter ≤50 mm. (E) Tumor diameter >50 mm. |
For patients with stage I/II and III, there were significant differences in RFS based on F-SII score. For patients with stage I/II, RFS of patients with F-SII=0, 1 and 2 was 56.08 (95% CI: 54.148–58.003), 52.68 (95% CI: 48.967–56.399) and 43.85 (95% CI: 34.854–52.841) months, respectively. The 5-year RFS rate was 86.5%, 81.8% and 53.8%, respectively (P<0.001) (Figure 2B). For patients with stage III, RFS of patients with F-SII=0, 1 and 2 was 45.11 (95% CI: 41.405–48.816), 35.45 (95% CI: 31.336–39.570) and 26.63 (95% CI: 21.557–31.697) months, respectively. The 5-year RFS rate was 60.0%, 39.9% and 13.1%, respectively (P<0.001) (Figure 2C). RFS of patients based on F-SII differed significantly with tumor diameter (P<0.001) (Figure 2D and E).
There were 146 (146/459, 31.8%) patients in the F-SII=0-1 group and 60 (60/83, 72.3%) in the F-SII=2 group with tumor recurrence (P<0.001). We also found that the incidence of locoregional recurrence, hematogenous metastasis and lymph node metastasis in the F-SII=2 group was significantly higher than that in the F-SII=0-1 group (14.5% vs 4.4%, 21.7% vs 10.9%, 15.7 vs 6.1%, respectively) (P<0.05), while peritoneal metastasis had no significant difference (15.7% vs 11.1%) (P=0.915) (Figure 3).
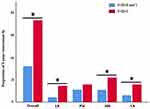 |
Figure 3 Recurrence patterns based on F-SII score. LR: locoregional recurrence, PM: peritoneal metastasis, HM: hematogenous metastasis, LN: lymph node metastasis. (★: P<0.05). |
Univariate analysis showed that age, tumor diameter, metastatic lymph node ratio (mLNR), pTNM stage, body mass index (BMI), F, SII, alanine aminotransferase (ALT), carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 19–9, vascular infiltration and nerve infiltration were statistically significant. Multivariate analysis showed that age, mLNR, pTNM stage, BMI, F and SII were independent risk factors associated with recurrence (Table 3).
 |
Table 3 Univariate and Multivariate Analyses of Factors Associated with RFS |
We combined the independent risk factors associated with recurrence to construct a nomogram that was used to evaluate recurrence (Figure 4a). For predicting recurrence within 3 and 5 years after radical resection, the AUC of the nomogram models both were greater than that of pTNM stage alone, 0.793 (95% CI: 0.753–0.832) and 0.805 (95% CI: 0.767–0.842) vs 0.706 (95% CI: 0.662–0.750) and 0.703 (95% CI: 0.660–0.747). The sensitivity were 74.4% and 71.7%, respectively, and the specificity were 73.7% and 77.4% (Figure 4b and c).
F-SII and OS
Chi-square analysis showed that F-SII was significantly correlated with clinicopathological characteristics, including tumor diameter and pTNM stage (both P<0.001) (Table 2). OS of patients with F-SII=0, 1 and 2 was 52.51 (95% CI: 50.640–54.376), 45.02 (95% CI: 42.220–47.826) and 35.35 (95% CI: 30.901–39.804) months, respectively. The 5-year OS rate was 75.9%, 56.9% and 25.3%, respectively (P<0.001) (Figure 5A).
 |
Figure 5 Kaplan–Meier curves analyses for OS based on F-SII score. (A) All patients. (B) Stage I/II. (C) Stage III. (D) Tumor diameter ≤50 mm. (E) Tumor diameter >50 mm. |
For patients with stage I/II and III, there were significant differences in OS based on F-SII score. For patients with stage I/II, the OS of patients with F-SII=0, 1 and 2 was 56.60 (95% CI: 54.816–58.374), 54.75 (95% CI: 51.628–57.872) and 45.87 (95% CI: 37.981–53.762) months, respectively. The 5-year OS rate was 86.6%, 83.6% and 50.0%, respectively (P<0.001) (Figure 5B). For patients with stage III, the OS of patients with F-SII=0, 1 and 2 was 47.94 (95% CI: 44.717–51.171), 38.49 (95% CI: 34.742–42.232) and 31.75 (95% CI: 26.757–36.747) months. The 5-year OS rate was 63.8%, 38.8% and 16.0% (P<0.001) (Figure 5C). OS of patients based on F-SII differed significantly with tumor diameter (P<0.001) (Figure 5D and E).
Univariate analysis showed that age, tumor diameter, mLNR (P<0.001), pTNM stage, BMI, F, SII, ALT, CEA, CA19-9, vascular infiltration and nerve infiltration were significant. In multivariate analysis, age, mLNR, pTNM stage, BMI, F, SII and nerve infiltration were independent risk factors associated with prognosis (Table 4).
 |
Table 4 Univariate and Multivariate Analyses of Factors Associated with OS |
We combined the independent risk factors associated with prognosis to construct a nomogram that was used to evaluate prognosis (Figure 6a). The results showed that the AUC of the nomogram models both were greater than that of the pTNM stage alone, 0.797 (95% CI: 0.757–0.837) and 0.806 (95% CI: 0.769–0.844) vs 0.701 (95% CI: 0.656–0.746) and 0.701 (95% CI: 0.657–0.745). The sensitivity were 75.0% and 71.6%, respectively, and the specificity were 72.6% and 76.8% (Figure 6b and c).
Discussion
Borrmann classification was proposed based on the macro characteristics of GC, and has played an important role clinically since 1926.6,24 Borrmann type IV GC has unique clinicopathological characteristics25 and has attracted a lot of clinical research. The clinical characteristics of Borrmann III are indirectly reflected by the research of other types. The proportion of Borrmann III accounts for 40.6%–58.9% of AGC and the five-year survival rate was 46.0%-51.6%.7,8 When the diameter of the tumor is greater than 8cm or the combined vascular infiltration, the prognosis of Borrmann III is similar to Borrmann IV.10,26,27 Thus, we still need to develop a new biomarker evaluation Borrmann III AGC patients’ prognosis.
Some inflammation indexes, such as NLR, PLR can evaluate the prognosis of patients and be used for individualized adjuvant treatment.28–30 The proliferation of tumor cells will make it break through mechanical pressure and escape immune monitoring, and enter the peripheral vein to become circulating tumor cells (CTCs).31 In this process, interleukin (IL)-1β and IL-6 released by neutrophils play a crucial part in promoting the proliferation of tumor cells. Neutrophils can also promote distant metastasis of CTCs in peripheral blood, and elevated level of CTCs and neutrophil clusters is associated with worse RFS.32 However, lymphocytes can secrete interferon-γ to inhibit CTCs migrating.33 Besides, platelets can induce epithelial–mesenchymal transformation by transforming growth factor β to promote distant metastasis and form aggregates with CTCs to promote migration.34 Wang et al35 found that preoperative SII level was an independent prognostic factor for OS in GC patients. Our study found that preoperative SII level was an independent prognostic factor for RFS and OS in Borrmann type III AGC patients. Similarly, as an important component of peripheral blood immunity, F acts as a scaffold to support the adhesion of CTCs, promote the proliferation and migration of CTCs, and ultimately affect the whole process of tumor growth and metastasis.36 Yamamoto et al37 believed that F level was an independent prognostic factor of RFS and could be used to predict postoperative GC recurrence. Our study not only found that F level was an independent risk factor for postoperative recurrence in patients with Borrmann III AGC, but also further investigated the patterns of recurrence.
Inflammation indexes also predict different recurrence patterns of GC. Yamashita et al38 retrospectively analyzed the preoperative plasma F level of 405 patients with AGC undergoing radical surgery, and found that high plasma F level (310 mg/dl) was independently associated with lymph node and liver metastases. Our study found that F-SII score predicted the recurrence risk of AGC with Borrmann type III, and had clinical significance for predicting different postoperative recurrence patterns. Patients with F-SII=2 were more likely to have locoregional recurrence, hematogenous metastasis and lymph node metastasis. Peripheral blood inflammatory cytokines play an important role in tumor metastasis by interacting with CTCs. Borrmann type III AGC with a higher level of preoperative F and SII may activate adhesion, proliferation and migration of CTCs, so as to promote hematogenous and distant lymph node metastases. In addition, inflammation in the surrounding tissues of GC is considered an important factor in the locoregional recurrence of cancer.39 Although Chen et al40 showed that the inflammatory index PLR is an independent predictor of GC peritoneal metastasis, cancer cells penetrating the serosa are considered the main source of peritoneal seeding or metastasis, and further studies have proved that serosal infiltration is the strongest indicator of GC peritoneal metastasis.41,42 Therefore, whether peripheral blood inflammatory markers could be used as an independent indicator to predict GC peritoneal metastasis needs verification.
In various studies, predictive models have been shown to provide quick and accurate preoperative prognostic information. However, GC is a highly heterogeneous gastrointestinal malignancy, and different tumor characteristics can greatly influence the immune status of the patient, which in turn affects the predictive performance of various predictors. Therefore, considering their clinical utility, the selection of appropriate predictors can help in a comprehensive assessment of tumor biology and facilitate personalized predictions. Some retrospective studies have provided evidence that different inflammation indices have varying predictive abilities for different biological behaviors of GC. For instance, Ye et al reported that NLR had better predictive performance than PLR in predicting peritoneal metastases,43 while Fang et al found that NLR had higher sensitivity than PLR in early diagnosis of GC.15 Yin et al found that PLR had better predictive performance than NLR and SII for diffuse/mixed GC.16 Moreover, Wang et al observed a significant correlation between SII and tumor size and Borrmann type.35 We also found that F-SII was significantly associated with tumor diameter. Therefore, we speculate that SII may be more appropriate for assessing the prognosis of patients with Borrmann type III and larger tumor size. These findings provide a theoretical basis for individualizing the assessment of tumor biology using different inflammation indices in clinical practice.
Immunotherapy has demonstrated remarkable success in clinical practice. However, despite a higher response rate compared to chemotherapy alone, there is still a need to identify biomarkers that can predict immune response. Previous studies have revealed a correlation between immune infiltration in the TME and GC immunotherapy response.44,45 However, the clinical utility of immunohistochemistry-based detection methods is limited due to the heterogeneity of GC. Yuang et al discovered a significant correlation between immune infiltration in the TME and peripheral blood levels of NLR, PLR, and SII. These inflammatory indices accurately predicted the prognosis of GC patients receiving anti-PD-1/PD-L1 immunotherapy.45 These findings suggest that inflammatory indices offer insights into the effectiveness of immunotherapy and the heterogeneity of the immune environment.
We found that F and SII both had higher AUCs, and were independent risk factors associated with recurrence and prognosis, which suggested that peripheral blood neutrophils, lymphocytes, platelets and fibrinogen were the main components of peripheral immunity of AGC patients with Borrmann III, and F-II was significantly correlated with tumor diameter and pTNM stage. Tumor cells can secrete various cytokines, and chemokines cause an increased inflammatory response,46 and often lead to an elevated inflammatory response when tumor aggressiveness increases.47 We found that Boremann type III GC, especially in patients with a higher pTNM stage and a larger tumor diameter, tends to lead to a significantly elevated inflammatory response, which in turn leads to a poor prognosis. It confirmed our previous suspicion that a larger ulcer surface indicates a stronger inflammatory response in AGC with Borrmann III. In this study, we also found that the risk of recurrence and metastasis in the F-SII score 2 group was significantly higher than in the 0–1 group, and F-SII score predicted different recurrence patterns. As a result, the F-SII score could not only evaluate Borrmann type III AGC patients prognosis, but also assess the risk of recurrence and metastasis. For patients with high F-SII, we suggest that the corresponding treatment should be improved, and regular review and close follow-up should be carried out.
Tumor immunity complements the pTNM stage with comprehensive prognostic information.48,49 For example, analysis of the ratio of cytokines to immune cells in body fluids can predict the prognosis of patients with metastatic GC.50 Liu et al51 constructed a nomogram based on inflammation indexes, which can better predict the prognosis of GC patients. We found that age, mLNR, pTNM stage, BMI, F and SII were independent risk factors associated with recurrence, and age, mLNR, pTNM stage, BMI, F, SII and nerve infiltration were independent risk factors associated with prognosis. After that, the above factors are integrated and constructed a nomogram. ROC showed that the AUC of predicting 3 and 5 years recurrence were 0.793 and 0.805. The AUC of predicting 3 and 5 years prognosis were 0.797 and 0.806. This indicates that the prediction performance of the nomogram is better than the pTNM stage, which is worthy of clinical application.
This study has some limitations. Firstly, this was a single center study, focusing only on Asian populations. The results need to be further verified. Secondly, we only include blood samples before surgery, and the dynamic changes in inflammation before and after surgery are also worth studying.
Conclusions
Preoperative F and SII were independent risk factors for recurrence and prognosis of Borrmann type III AGC. The nomogram models based on these two inflammatory biomarkers and clinicopathological features were used to evaluate the recurrence and prognosis of Borrmann type III AGC, and its predictive ability is superior to traditional pTNM stage alone.
Data Sharing Statement
The datasets generated and/or analysed during the current study are available in the [Gastric Cancer Information Management System v1.2 of Harbin Medical University Cancer Hospital] repository, [Copyright No. 2013SR087424, http:www.sgihmu.com].
Ethics Approval and Consent to Participate
All programs followed were according to the ethical standards of the Human Subjects Responsibility Committee (institutions and countries), as well as the 1964 Helsinki Declaration and subsequent editions. This research was approved by the Ethics Committee of the Harbin Medical University Cancer Hospital (Approval Number: 2019-57-IIT). All patients provided written informed consent to the use and publication of their information.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Nn10 program of Harbin Medical University Cancer Hospital, China (No. Nn10 PY 2017-03).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Fitzmaurice C, Fitzmaurice C, Abate D, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–1768. doi:10.1001/jamaoncol.2019.2996
2. Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends--an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
3. Zhao JK, Wu M, Kim CH, et al. Jiangsu four cancers study: a large case-control study of lung, liver, stomach, and esophageal cancers in Jiangsu Province, China. Eur J Cancer Prev. 2017;26(4):357–364. doi:10.1097/CEJ.0000000000000262
4. Saito H, Miyatani K, Takaya S, et al. Tumor infiltration pattern into the surrounding tissue has prognostic significance in advanced gastric cancer. Virchows Arch. 2015;467(5):519–523. doi:10.1007/s00428-015-1811-y
5. Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi:10.1111/apm.1965.64.1.31
6. Association, N.J.J.O.T.A.M. Handbuch der speziellen pathologischen Anatomie und Histologie. Springer. 1166; 1958.
7. Song XH, Zhang W-H, Chen K-L, et al. Prognostic impact of Borrmann classification on advanced gastric cancer: a retrospective cohort from a single institution in western China. World J Surg Oncol. 2020;18(1):204. doi:10.1186/s12957-020-01987-5
8. Hosoda K, Watanabe M, Yamashita K. Re-emerging role of macroscopic appearance in treatment strategy for gastric cancer. Ann Gastroenterol Surg. 2019;3(2):122–129. doi:10.1002/ags3.12218
9. Hosoda K, Yamashita K, Katada N, et al. Preoperative tumor size is a critical prognostic factor for patients with Borrmann type III gastric cancer. Surg Today. 2015;45(1):68–77. doi:10.1007/s00595-014-1060-8
10. Zhai Z, Zhu Z-Y, Zhang Y, et al. Prognostic significance of Borrmann type combined with vessel invasion status in advanced gastric cancer. World J Gastrointest Oncol. 2020;12(9):992–1004. doi:10.4251/wjgo.v12.i9.992
11. Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287. doi:10.1038/nrc.2016.36
12. Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi:10.1126/science.1129139
13. Pagès F, Mlecnik B, Marliot F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391(10135):2128–2139. doi:10.1016/S0140-6736(18)30789-X
14. Arigami T, Uenosono Y, Matsushita D, et al. Combined fibrinogen concentration and neutrophil-lymphocyte ratio as a prognostic marker of gastric cancer. Oncol Lett. 2016;11(2):1537–1544. doi:10.3892/ol.2015.4049
15. Fang T, Wang Y, Yin X, et al. Diagnostic sensitivity of NLR and PLR in early diagnosis of gastric cancer. J Immunol Res. 2020;2020:9146042. doi:10.1155/2020/9146042
16. Yin X, Fang T, Wang Y, et al. Prognostic significance of serum inflammation indexes in different Lauren classification of gastric cancer. Cancer Med. 2021;10(3):1103–1119. doi:10.1002/cam4.3706
17. Xu CY, Shen JG, Shen JY, et al. Ulcer size as a novel indicator marker is correlated with prognosis of ulcerative gastric cancer. Dig Surg. 2009;26(4):312–316. doi:10.1159/000231881
18. Yao D, Dong M, Dai C, et al. Inflammation and inflammatory cytokine contribute to the initiation and development of ulcerative colitis and its associated cancer. Inflamm Bowel Dis. 2019;25(10):1595–1602. doi:10.1093/ibd/izz149
19. Japanese Gastric Cancer Association jgca@ koto. kpu-m. ac. jp. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19. doi:10.1007/s10120-016-0622-4
20. Ajani JA, D’Amico TA, Bentrem DJ, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(2):167–192.
21. Japanese Gastric Cancer Association jgca@ koto. kpu-m. ac. jp. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14(2):101–112. doi:10.1007/s10120-011-0041-5
22. Lee D, Son S-Y, Kim Y-B, et al. Neural invasion is a significant contributor to peritoneal recurrence in signet ring cell gastric carcinoma. Ann Surg Oncol. 2018;25(5):1167–1175. doi:10.1245/s10434-018-6371-3
23. Lu J, Xu -B-B, Zheng Z-F, et al. CRP/prealbumin, a novel inflammatory index for predicting recurrence after radical resection in gastric cancer patients: post hoc analysis of a randomized Phase III trial. Gastric Cancer. 2019;22(3):536–545. doi:10.1007/s10120-018-0892-0
24. Yamashita K, Sakuramoto S, Katada N, et al. Simple prognostic indicators using macroscopic features and age in advanced gastric cancer. Hepatogastroenterology. 2014;61(130):512–517.
25. An JY, Kang TH, Choi MG, et al. Borrmann type IV: an independent prognostic factor for survival in gastric cancer. J Gastrointest Surg. 2008;12(8):1364–1369. doi:10.1007/s11605-008-0516-9
26. Yamashita K, Ema A, Hosoda K, et al. Macroscopic appearance of Type IV and giant Type III is a high risk for a poor prognosis in pathological stage II/III advanced gastric cancer with postoperative adjuvant chemotherapy. World J Gastrointest Oncol. 2017;9(4):166–175. doi:10.4251/wjgo.v9.i4.166
27. Gao S, Cao G-H, Ding P, et al. Retrospective evaluation of lymphatic and blood vessel invasion and Borrmann types in advanced proximal gastric cancer. World J Gastrointest Oncol. 2019;11(8):642–651. doi:10.4251/wjgo.v11.i8.642
28. Zhang X, Hu D, Lin X, et al. Prognostic value of an inflammation-related index in 6865 Chinese patients with postoperative digestive tract cancers: the Fiesta study. Front Oncol. 2019;9:427. doi:10.3389/fonc.2019.00427
29. Li Z, Li S, Ying X, et al. The clinical value and usage of inflammatory and nutritional markers in survival prediction for gastric cancer patients with neoadjuvant chemotherapy and D2 lymphadenectomy. Gastric Cancer. 2020;23(3):540–549. doi:10.1007/s10120-019-01027-6
30. Bilen MA, Martini DJ, Liu Y, et al. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced-stage cancer treated with immunotherapy. Cancer. 2019;125(1):127–134. doi:10.1002/cncr.31778
31. Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells - mechanisms of immune surveillance and escape. Nat Rev Clin Oncol. 2017;14(3):155–167. doi:10.1038/nrclinonc.2016.144
32. Szczerba BM, Castro-Giner F, Vetter M, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566(7745):553–557. doi:10.1038/s41586-019-0915-y
33. Ferrone C, Dranoff G. Dual roles for immunity in gastrointestinal cancers. J Clin Oncol. 2010;28(26):4045–4051. doi:10.1200/JCO.2010.27.9992
34. Kanikarla-Marie P, Lam M, Menter DG, et al. Platelets, circulating tumor cells, and the circulome. Cancer Metastasis Rev. 2017;36(2):235–248. doi:10.1007/s10555-017-9681-1
35. Wang K, Diao F, Ye Z, et al. Prognostic value of systemic immune-inflammation index in patients with gastric cancer. Chin J Cancer. 2017;36(1):75. doi:10.1186/s40880-017-0243-2
36. Repetto O, De Re V. Coagulation and fibrinolysis in gastric cancer. Ann N Y Acad Sci. 2017;1404(1):27–48. doi:10.1111/nyas.13454
37. Yamamoto M, Kurokawa Y, Miyazaki Y, et al. Usefulness of preoperative plasma fibrinogen versus other prognostic markers for predicting gastric cancer recurrence. World J Surg. 2016;40(8):1904–1909. doi:10.1007/s00268-016-3474-5
38. Yamashita H, Kitayama J, Kanno N, et al. Hyperfibrinogenemia is associated with lymphatic as well as hematogenous metastasis and worse clinical outcome in T2 gastric cancer. BMC Cancer. 2006;6:147. doi:10.1186/1471-2407-6-147
39. Bednarz-Misa I, Fortuna P, Diakowska D, et al. Distinct local and systemic molecular signatures in the esophageal and gastric cancers: possible therapy targets and biomarkers for gastric cancer. Int J Mol Sci. 2020;21(12):21. doi:10.3390/ijms21124509
40. Chen XD, Mao C-C, Wu R-S, et al. Use of the combination of the preoperative platelet-to-lymphocyte ratio and tumor characteristics to predict peritoneal metastasis in patients with gastric cancer. PLoS One. 2017;12(4):e0175074. doi:10.1371/journal.pone.0175074
41. Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi:10.1016/S0140-6736(16)30354-3
42. Boku T, Nakane Y, Minoura T, et al. Prognostic significance of serosal invasion and free intraperitoneal cancer cells in gastric cancer. Br J Surg. 1990;77(4):436–439. doi:10.1002/bjs.1800770425
43. Ye Z, Yu P, Cao Y, et al. Prediction of peritoneal cancer index and prognosis in peritoneal metastasis of gastric cancer using NLR-PLR-DDI score: a retrospective study. Cancer Manag Res. 2022;14:177–187. doi:10.2147/CMAR.S343467
44. Liu C, Wang Y, Li S, et al. Early change in peripheral CD4 + T cells associated with clinical outcomes of immunotherapy in gastrointestinal cancer. Immunotherapy. 2021;13(1):55–66. doi:10.2217/imt-2020-0068
45. Yuan J, Zhao X, Li Y, et al. The association between blood indexes and immune cell concentrations in the primary tumor microenvironment predicting survival of immunotherapy in gastric cancer. Cancers. 2022;14(15):1.
46. Pęczek P, Gajda M, Rutkowski K, et al. Cancer-associated inflammation: pathophysiology and clinical significance. J Cancer Res Clin Oncol;2022:1–16. doi:10.1007/s00432-022-04399-y
47. Lin JX, Lin J-P, Xie J-W, et al. Prognostic value and association of sarcopenia and systemic inflammation for patients with gastric cancer following radical gastrectomy. Oncologist. 2019;24(11):e1091–e1101. doi:10.1634/theoncologist.2018-0651
48. Kim SM, Min B-H, Ahn JH, et al. Nomogram to predict lymph node metastasis in patients with early gastric cancer: a useful clinical tool to reduce gastrectomy after endoscopic resection. Endoscopy. 2020;52(6):435–443. doi:10.1055/a-1117-3059
49. Cheung KS, Leung WK, Seto WK. Application of big data analysis in gastrointestinal research. World J Gastroenterol. 2019;25(24):2990–3008. doi:10.3748/wjg.v25.i24.2990
50. Park HS, Kwon WS, Park S, et al. Comprehensive immune profiling and immune-monitoring using body fluid of patients with metastatic gastric cancer. J Immunother Cancer. 2019;7(1):268. doi:10.1186/s40425-019-0708-8
51. Liu J, Geng Q, Chen S, et al. Nomogram based on systemic inflammatory response markers predicting the survival of patients with resectable gastric cancer after D2 gastrectomy. Oncotarget. 2016;7(25):37556–37565. doi:10.18632/oncotarget.8788
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


