Back to Journals » Risk Management and Healthcare Policy » Volume 16
Prognostic Model of D2 Radical Gastrectomy Combined with Neoadjuvant Chemotherapy for Gastric Cancer
Authors Wang G, Tan Y, Jiang Y, Liu J, Su Y, Sun Z, Liu B
Received 2 April 2023
Accepted for publication 21 June 2023
Published 10 July 2023 Volume 2023:16 Pages 1259—1271
DOI https://doi.org/10.2147/RMHP.S413052
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Mecit Can Emre Simsekler
Guangjun Wang,1,* Yinghua Tan,2,* Yongjie Jiang,1 Jia Liu,1 Yuanhui Su,3 Zhengang Sun,4 Bo Liu3,5
1Department of General Surgery, Qingdao West Coast New Area Central Hospital, Qingdao, Shandong, People’s Republic of China; 2Department of Paediatrics, Qingdao West Coast New Area Central Hospital, Qingdao, Shandong, People’s Republic of China; 3Department of General Surgery, Lanzhou University Second Hospital, Lanzhou, Gansu, People’s Republic of China; 4Department of Spine Surgery, Qingdao West Coast New Area Central Hospital, Qingdao, Shandong, People’s Republic of China; 5Department of Gastrointestinal Surgery, the Affiliated Hospital of Qingdao University, Qingdao, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bo Liu, Email [email protected]
Purpose: The AJCC (the American Joint Committee on Cancer) ypTNM (post-neoadjuvant pathologic stage group) staging was established based on patients with lymphadenectomy scope less than D2 and did not include ypT0N0 patients with pathologically complete response (PCR). The purpose of this study was to construct a survival predictive model for gastric cancer patients after neoadjuvant chemotherapy and gastrectomy combined with D2 lymphadenectomy.
Patients and Methods: The multicenter data of 838 gastric cancer patients who received neoadjuvant chemotherapy and gastrectomy combined with D2 lymphadenectomy were analyzed retrospectively. These dual center patients were divided into training (n = 671, the Affiliated Hospital of Qingdao University) and validation (n = 167, Qingdao West Coast New Area Central Hospital) cohorts. Based on training cohort, univariate and multivariable COX regression analyses were performed to select the clinicopathological characteristics significantly correlating with overall survival and construct a nomogram. Based on training and validation cohorts, the distinguishing and calibrating capabilities of nomogram was evaluated by the receiver operating characteristic (ROC) curve, Harrell’s concordance index (C-index), decision curve analysis (DCA) curve and calibration curve.
Results: Platelet-to-lymphocyte ratio (PLR), pathologic stage after neoadjuvant treatment: ypT and ypN stage, tumor regression grade (TRG) became independent variables intimately related to the prognosis and was used to construct nomograms of 3/5-year prognosis. The nomograms showed an accuracy in predicting OS (overall survival) rate, with area under the ROC curve (AUC) of 0.818 (95% CI = 0.753~0.883) and C-index of 0.801 (95% CI = 0.744~0.858) in validation cohort. Calibration curves showed satisfactory agreement between nomogram prediction and actual result, and DCA curves indicated the large positive net benefit and excellent clinical usefulness of nomogram.
Conclusion: This study successfully developed a nomogram to predict overall survival of gastric cancer patients after neoadjuvant chemotherapy and gastrectomy combined with D2 lymphadenectomy, which might have excellent predictive performance and clinical application value.
Keywords: gastric cancer, neoadjuvant therapy, gastrectomy, nomogram, survival analysis
Introduction
Nowadays, neoadjuvant chemotherapy and gastrectomy combined with D2 lymphadenectomy has been widely used in the treatment of advanced gastric cancer.1–3 Compared with surgery merely, combined neoadjuvant chemotherapy can prolong survival rate, reduce tumor staging, and improve radical resection (R0 resection) rate. The timing and method of surgery can be selected according to the reaction degree after neoadjuvant chemotherapy, so as to achieve D2 lymphadenectomy as far as possible.4,5 However, patients have different prognosis due to individual differences, and affect postoperative treatment decisions.6–8 Therefore, patients receiving neoadjuvant chemotherapy combined with D2 radical gastrectomy for gastric cancer need more accurate prognosis prediction to guide treatment. Many previous studies have utilized clinicopathological factors as prognostic factors, but most prognostic factors are still limited and controversial.9–14 AJCC (the American Joint Committee on Cancer) ypTNM (post-neoadjuvant pathologic stage group) staging is the most authoritative guideline prognostic system for gastric cancer patients receiving neoadjuvant chemotherapy.2,15 However, this system was established based on patients with lymphadenectomy scope less than D2 and did not include ypT0N0 patients with pathologically complete response (PCR), so it may not be suitable for patients with D2 radical gastrectomy or pathologically complete response. In addition, other clinicopathological prognostic factors were not incorporated.16,17 Therefore, more generalized models are needed to predict the prognosis of these patients. Nowadays, nomogram can contain a variety of prognostic factors, and can be quantified and visualized according to the proportion of each factor, which has been widely used in the study of gastric cancer.7,13–16 In this study, we retrospectively analyzed the dual center data of 838 gastric cancer patients who received neoadjuvant chemotherapy combined with D2 radical gastrectomy, and established and confirmed a prognostic model.
Materials and Methods
Case Selection
From January 2010 to January 2023, dual center of 838 patients with gastric cancer who received neoadjuvant chemotherapy and D2 radical gastrectomy were selected. These patients were divided into the training cohort (671 patients, the Affiliated Hospital of Qingdao University), and validation cohort (167 patients, Qingdao West Coast New Area Central Hospital). Inclusion criteria: 1) Preoperative pathological diagnosis of gastric cancer (gastric adenocarcinoma); 2) The AJCC clinical stage of T3-4aN1-3M0 or T4bN0-3M0 as evaluated by preoperative examination were included in the study. 3) Patients were evaluated as tolerable for neoadjuvant chemotherapy after Multi-Disciplinary Team (MDT), and were treated with two-drug combination (SOX (Tegafur Timeracil Oteracil Potassium Capsule (S-1) plus oxaliplatin)/XELOX (Oxaliplatin plus Capecitabine) regimen). 4) According to the patient’s requirements and preoperative conditions, D2 radical gastrectomy is taken as the standard treatment mode, and R0 resection is performed. Exclusion criteria: 1) Patients with a history of other malignant tumors and who cannot tolerate neoadjuvant chemotherapy and surgery; 2) Clinical data are incomplete. 3) There was previous history of abdominal or gastric surgery. This is a retrospective study that follows the Declaration of Helsinki and good clinical practice guidelines, and was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University and the Qingdao West Coast New Area Central Hospital The committee waived the need for informed consent to be obtained because this case series is a retrospective study. The medical records and data accessed in the study contained no personal or identifying information.
Data Collection
Patient data are collected by two independent researchers. The collected data included clinicopathological information before neoadjuvant therapy: gender, age, age-adjusted Charlson Comorbidity Index (aCCI), body mass index (BMI), Borrmann classification, carcinoembryonic antigen (CEA), inflammatory indicators (neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR)), gastric surgery type and pathological date after surgery: pathologic type, lymph node positive rate, neurovascular invasion, AJCC ypTNM stage, histological differentiated type. We apply the AJCC criteria in the National Comprehensive Cancer Network (NCCN) and CSCO gastric cancer guidelines to evaluate the tumor regression grade (TRG) score.2,3 The TRG score was divided into 4 categories based on the tumor response in pathological sections after treatment. Among them, TRG0 is defined as complete regression of the tumor; TRG1 is defined as very few tumor cell residues; TRG2 is defined as partial regression of the tumor and TRG3 is defined as basically no response in the tumor. For the Lymph node positive rate, NLR and PLR without obvious grouping criteria, the OS is used as the overall endpoint to create an ROC curve, with the cut-off value of the ROC curve as its critical value.
The first outpatient review will begin at 3 weeks after the end of treatment. The patients are followed up until January 2023 or death. From 0~2 years after the operation, follow-up will occur every 3 months. From 3~5 years after the operation, follow-up will occur every 6 months. Patients’ overall survival (OS) is recorded, which is defined as the interval between diagnosis and death or the last follow-up.
Construction and Performance Evaluation of the Nomogram
Statistical analysis is performed using SPSS 24.0 software (Chicago, IL, USA) and R software (www.r-project.org, version 3.63). 1) Construction of nomogram: Based on the data of the training cohort, univariate and multivariate COX regression analyses were performed with SPSS software and R software package “RMS”, “Foreign” and “Survival” to calculate the Hazard Rate (HR) and 95% confidence interval (CI) of the corresponding prognostic factors, select the independent prognostic factors and construct the nomogram. Kaplan–Meier survival curves are drawn to test the difference of each prognostic factor. 2) Evaluation of the performance: ROC curve, DCA curve and calibration curve are plotted by using R software package “rms” “foreign” “survival” “survivalROC” “stdca” based on the data of training cohort and validation cohort. The area under ROC curve (AUC) and consistency index (C-index) are calculated. DCA curve is used to evaluate the clinical utility clinical application value and net benefit of the model, and calibration curve is utilized to evaluate the consistency between actual and predicted results. P < 0.05 is considered statistically significant.
Result
Patient Data
Patients in this study are followed for 0–84 months, with a median follow-up of 58 months. The clinicopathological characteristics of patients in the training cohort and the validation cohort are shown in Table 1. According to Table 1, there are no statistically significant differences in other clinicopathological characteristics between the two cohorts (P > 0.05).
 |
Table 1 Comparison of Clinicopathological Features Between the Two Cohorts (Cases (X/%)) |
Univariate and Multivariate COX Regression Analysis
The univariate COX regression analysis results show that aCCI, Borrmann type, PLR, ypT stage, ypN stage, lymph node positive rate and TRG are all related to the survival of patients in the training cohort, as shown in Table 2. Multivariate COX regression analysis results show that PLR, ypT and ypN staging, TRG are closely related to prognosis. The higher the value of PLR, patients have worse prognosis. Early ypT and ypN staging, low TRG suggest a good prognosis, as shown in Table 3. Kaplan-Meier method is used to plot the survival curve of prognostic factors. The results show that the overall survival rate of patients with higher PLR values, later ypT and ypN staging and higher TRG have poor prognosis. The differences are statistically significant (P < 0.05), as shown in Figure 1.
 |
Table 2 Univariate Cox Regression Analysis of Clinicopathological Characteristics |
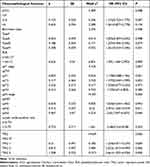 |
Table 3 Multivariate Cox Regression Analysis of Clinicopathological Characteristics |
Construction of Prognostic Nomogram
The nomogram included the independent prognostic factors built by multivariate COX regression analysis, which can be used to calculate the individual scores of each prognostic factor and add them together to obtain the total score, as shown in Figure 2.
 |
Figure 2 Prediction model of 3-year and 5-year overall survival rate based on the nomogram. |
Evaluation of Nomogram
Based on the data of the training cohort and validation cohort, the differentiation and calibration of the nomogram are calculated. The results show that the C-index of the nomogram in the training cohort and validation cohort are 0.815 (95% CI = 0.750~0.880) and 0.801 (95% CI = 0.744~0.858), respectively. In the training cohort, the AUC for predicting 3-year and 5-year overall survival is 0.863 (95% CI = 0.777~0.949) and 0.84 (95% CI = 0.775~0.905). In the validation cohort, the AUC of 3 - and 5-year overall survival is 0.818 (95% CI = 0.753~0.883) and 0.796 (95% CI = 0.793~0.873), indicating that the model has good predictive value, as shown in Figure 3. The calibration curves of the 3 - and 5-year overall survival predicted by the nomogram in the training cohort and the validation cohort are shown in Figure 4. It can be seen from Figure 4 that the calibration curve is in good agreement with the 45° ideal oblique line, indicating a high consistency between the prediction and the actual results. In this study, the DCA curve shows that the nomogram showed a large positive net benefit across a wide range of death risks in both cohorts, indicating that the model has good clinical practicability, as shown in Figure 5.
Discussion
Nowadays, D2 radical resection and perioperative comprehensive therapy are the main treatment methods for advanced gastric cancer. A large number of evidence-based medical studies have confirmed that neoadjuvant chemotherapy can significantly improve the prognosis of patients. However, there are individual differences in the impact of neoadjuvant chemotherapy on the prognosis of patients,5,6,18 Therefore, it is necessary to find relevant prognostic factors to assist in the selection of treatment. The accurate prediction of nomograms has been proved to be significant for clinical decision-making in previous studies.10,19–21 In this multicenter study, the prognostic factors included in the nomograms, which proved to have good predictive ability.
Ever since Vichow22 proposed the “tumor-inflammation” theory in 1863, many studies have confirmed that inflammatory indicators such as NLR and PLR are related to tumor occurrence, development and prognosis. Neutrophilia is an inflammatory response factor, which starts the immune system by inhibiting the cytolysis of immune cells. Multiple previous studies have shown that NLR is an independent predictor of the prognosis of gastric cancer patients.23,24 Both lymphocytopenia and thrombocytopenia are associated with poor prognosis of tumor.25 Studies have shown that the increase of PLR in gastric cancer patients before treatment is significantly correlated with pathological types and affects the prognosis of gastric cancer.9,20 Serum levels of various tumor markers may be elevated in patients with gastric cancer. It has been reported that the serum levels of various tumor markers (such as CEA and CA199, etc.) might rise. However, the prognostic value of these tumor markers remains controversial.13,26 The results of this study showed that high levels of PLR before neoadjuvant chemotherapy were independent adverse prognostic factors, and the analysis of data showed that patients with low degree of pathological response were mostly accompanied by high levels of PLR. Relevant literature found that high level of PLR was also independent risk factors affecting the remission rate of neoadjuvant chemotherapy, and was related to tumor load and growth rate.4,17,18 Therefore, the clinical application value of PLR and other inflammatory indicators should be explored in a large sample study.
Nowadays, in recent years, due to the increase in human life expectancy, the proportion of elderly patients with gastric cancer has been increasing. There are many concomitant diseases in elderly patients with gastric cancer, with high mortality. Charlson comorbidities Index (CCI) was proposed by Charlson et al in 1987 and has been widely used to assess the impact of comorbidities.27 Previous studies have shown that aCCI corrects the patient’s age and improves accuracy, and aCCI has been a prognostic factor.12,14,28 In recent years, aCCI has also been gradually applied in the study of gastric cancer.14 In this study, Univariate COX analysis showed that high aCCI was a prognostic risk factor, but multivariate COX analysis showed that it is not statistically significant, and high aCCI was not an independent prognostic factor. Since there are relatively few studies on aCCI for gastric cancer, the results of this study still remind clinicians to pay attention to the basic situation of patients, attach importance to the use of clinically relevant indexes, assist in the selection of treatment options.
AJCC ypTNM staging system is still the most authoritative guideline prediction system, although there have been many studies on the prognosis of gastric cancer.13,24,25 The system was established based on patients undergoing lymphadenectomy (less than D2) and did not include patients with pathological complete response.16 The results of this study showed that ypT and ypN stages were independent prognostic factors of neoadjuvant chemotherapy combined with D2 radical resection for gastric cancer. The impact of ypT and ypN staging on prognosis has been widely discussed, and a consensus has been reached that later staging is associated with poorer prognosis.21,28 Therefore, this study can be complimentary to ypTNM staging system. Recent studies have found that lymph node positive rate can also be an independent risk factor for the prognosis of gastric cancer.11,29 However, in the multivariate COX analysis of this study, lymph node positive rate was not included in the nomogram. The reasons may be that thoroughness of lymph node dissection and sampling determines the accuracy of positive number of pathological lymph nodes,30 and the number of positive lymph nodes obtained may be affected by lymph node fragmentation or confluence.31 For advanced gastric cancer, several previous studies have confirmed that TRG after neoadjuvant therapy is significantly correlated with survival prognosis.32,33 In this study, TRG was also included in the nomogram. Multivariate COX analysis showed that HR changes in the TRG group were significantly higher than other prognostic factors, suggesting that patients with poor pathological reactions had a worse prognosis, which reflected that TRG had a greater impact on survival.
Based on these prognostic factors, a nomogram was constructed to predict the overall survival rate of patients. In the validation cohort, the AUC of the 3 and 5-year survival rate predicted by the nomogram was 0.818 and 0.796, respectively, and a good calibration curve was obtained, indicating that the nomogram had a good predictive value. A satisfactory DCA curve indicated that the nomogram had a high clinical value. The nomogram constructed in this study provides a more accurate personalized visual tool that can be flexibly combined with clinicopathological factors, which conform to the current concept of precision medicine and can provide a reference for evaluating the prognosis and selection of gastric cancer patients.
This study also has some limitations. As a retrospective multicenter study, due to the limited total sample size, data offset is inevitable. This study is validated only for Chinese patients and the underlying biological differences might make their applicability in other populations difficult unless also validated there. In addition, the rapid development of studies on marker genes of gastric cancer (such as human epidermal growth factor receptor 2 (HER-2)) in recent years34,35 has diversified the studies on prognosis of gastric cancer. Although some novel factors were included in this study, most of the variables were clinical routine factors. At present, the XELOX regimen and the SOX regimen are mostly chosen for gastric cancer patients in China, and adjuvant SOX chemotherapy has similar survival benefits compared to XELOX chemotherapy in Chinese patients.36 These two chemotherapy regimens are not comprehensively applied in some western countries, limiting the external application value of this study.3 Therefore, further multi-center studies are needed.
Conclusions
In conclusion, this study innovatively constructed a nomogram to predict the overall survival rate of patients with advanced gastric cancer treated with neoadjuvant chemotherapy combined with D2 radical gastrectomy, and external validation showed that the model had good predictive performance and clinical application value.
Abbreviations
ROC, receiver operating characteristic; C-index, Harrell’s concordance index; DCA, decision curve analysis curve; PLR, platelet-to-lymphocyte ratio; TRG, tumor regression grade; OS, overall survival; AUC, area under the ROC curve; R0 resection, radical resection; AJCC, the American Joint Committee on Cancer; ypTNM, post-neoadjuvant pathologic stage group staging; PCR, pathologically complete response; MDT, Multi-Disciplinary Team; SOX regimen, tegafur gimeracil oteracil potassium capsule (S-1) + oxaliplatin regimen; XELOX regimen, Oxaliplatin +Capecitabine; aCCI, age-adjusted Charlson Comorbidity Index; BMI, body mass index; NLR, neutrophil-lymphocyte ratio; NCCN, National Comprehensive Cancer Network; HR, Hazard Rate; CI, confidence interval; CCI, Charlson comorbidities Index; HER-2, human epidermal growth factor receptor 2.
Language Editing Services
The authors would like to thank MJEditor for providing English editing services during the preparation of this manuscript.
Data Sharing Statement
The data of this article can be obtained with the consent of the corresponding author.
Ethics Approval and Informed Consent
This is a retrospective study that follows the Declaration of Helsinki and good clinical practice guidelines, and was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University and the Qingdao West Coast New Area Central Hospital. The committee waived the need for informed consent to be obtained because this case series is a retrospective study. The medical records and data accessed in the study contained no personal or identifying information.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Qingdao Municipal Health Science and Technology Project (grant number 2019-YJZD196) and the Scientific Research Project of Qingdao University Medical Group in 2021..
Disclosure
The authors report no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24(1):1–21. doi:10.1007/s10120-020-01042-y
2. Ajani JA, D’Amico TA, Bentrem DJ, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Comprehens Cancer Ntwk. 2022;20(2):167–192. doi:10.6004/jnccn.2022.0008
3. Wang FH, Zhang XT, Li YF, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. 2021;41(8):747–795. doi:10.1002/cac2.12193
4. Zheng XH, Zhang W, Yang L, et al. Role of D2 gastrectomy in gastric cancer with clinical para-aortic lymph node metastasis. World J Gastroenterol. 2019;25(19):2338–2353. doi:10.3748/wjg.v25.i19.2338
5. Tokunaga M, Sato Y, Nakagawa M, et al. Perioperative chemotherapy for locally advanced gastric cancer in Japan: current and future perspectives. Surg Today. 2020;50(1):30–37. doi:10.1007/s00595-019-01896-5
6. Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28(35):5210–5218. doi:10.1200/jco.2009.26.6114
7. D’Ugo D, Rausei S, Biondi A, Persiani R. Preoperative treatment and surgery in gastric cancer: friends or foes? Lancet Oncol. 2009;10(2):191–195. doi:10.1016/s1470-2045(09)70021-x
8. Chen D, Liu Z, Liu W, et al. Predicting postoperative peritoneal metastasis in gastric cancer with serosal invasion using a collagen nomogram. Nat Commun. 2021;12(1):179. doi:10.1038/s41467-020-20429-0
9. Qu JL, Qu XJ, Li Z, et al. Prognostic model based on systemic inflammatory response and clinicopathological factors to predict outcome of patients with node-negative gastric cancer. PLoS One. 2015;10(6):e0128540. doi:10.1371/journal.pone.0128540
10. Aliustaoglu M, Bilici A, Ustaalioglu BB, et al. The effect of peripheral blood values on prognosis of patients with locally advanced gastric cancer before treatment. Med Oncol. 2010;27(4):1060–1065. doi:10.1007/s12032-009-9335-4
11. Lauricella S, Caricato M, Mascianà G, et al. Topographic lymph node staging system shows prognostic superiority compared to the 8th edition of AJCC TNM in gastric cancer. A western monocentric experience. Surg Oncol. 2020;34:223–233. doi:10.1016/j.suronc.2020.04.022
12. Qiu XT, Song YC, Liu J, Wang ZM, Niu X, He J. Identification of an immune-related gene-based signature to predict prognosis of patients with gastric cancer. World J Gastrointest Oncol. 2020;12(8):857–876. doi:10.4251/wjgo.v12.i8.857
13. Sun Z, Zhang N. Clinical evaluation of CEA, CA19-9, CA72-4 and CA125 in gastric cancer patients with neoadjuvant chemotherapy. World J Surg Oncol. 2014;12:397. doi:10.1186/1477-7819-12-397
14. Lin JX, Huang YQ, Xie JW, et al. Association of the age-adjusted Charlson Comorbidity Index and systemic inflammation with survival in gastric cancer patients after radical gastrectomy. Eur J Surg Oncol. 2019;45(12):2465–2472. doi:10.1016/j.ejso.2019.07.010
15. Zhu MH, Zhang KC, Yang ZL, Qiao Z, Chen L. Comparing prognostic values of the 7th and 8th editions of the American Joint Committee on Cancer TNM staging system for gastric cancer. Int J Biol Markers. 2020;35(1):26–32. doi:10.1177/1724600819891585
16. Li Z, Wang Y, Shan F, et al. ypTNM staging after neoadjuvant chemotherapy in the Chinese gastric cancer population: an evaluation on the prognostic value of the AJCC eighth edition cancer staging system. Gastric Cancer. 2018;21(6):977–987. doi:10.1007/s10120-018-0830-1
17. Wang HH, Li K, Xu H, Sun Z, Wang ZN, Xu HM. Improvement of T stage precision by integration of surgical and pathological staging in radically resected stage pT3-pT4b gastric cancer. Oncotarget. 2017;8(28):46506–46513. doi:10.18632/oncotarget.14828
18. Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71(3):264–279. doi:10.3322/caac.21657
19. Meng XY, Yang G, Dong CJ, Zheng RY. Gastric adenocarcinoma of the fundic gland: a review of clinicopathological characteristics, treatment and prognosis. Rare Tumors. 2021;13:20363613211060171. doi:10.1177/20363613211060171
20. Zhou X, Dong Z, Zhang C, et al. A novel nomogram for predicting survival of patients with poorly differentiated gastric adenocarcinoma. Transl Cancer Res. 2021;10(2):886–898. doi:10.21037/tcr-20-2794
21. Li Z, Xiao Q, Wang Y, et al. A modified ypTNM staging system-development and external validation of a nomogram predicting the overall survival of gastric cancer patients received neoadjuvant chemotherapy. Cancer Manag Res. 2020;12:2047–2055. doi:10.2147/cmar.S236696
22. Virchow R. Cellular pathology. As based upon physiological and pathological histology. Lecture XVI--Atheromatous affection of arteries. 1858. Nutr Rev. 1989;47(1):23–25. doi:10.1111/j.1753-4887.1989.tb02747.x
23. Sun J, Chen X, Gao P, et al. Can the neutrophil to lymphocyte ratio be used to determine gastric cancer treatment outcomes? A systematic review and meta-analysis. Dis Markers. 2016;2016:7862469. doi:10.1155/2016/7862469
24. Li Z, Li S, Ying X, et al. The clinical value and usage of inflammatory and nutritional markers in survival prediction for gastric cancer patients with neoadjuvant chemotherapy and D2 lymphadenectomy. Gastric Cancer. 2020;23(3):540–549. doi:10.1007/s10120-019-01027-6
25. Yin X, Fang T, Wang Y, et al. Prognostic significance of serum inflammation indexes in different Lauren classification of gastric cancer. Cancer Med. 2021;10(3):1103–1119. doi:10.1002/cam4.3706
26. Lin JP, Lin JX, Ma YB, et al. Prognostic significance of pre- and post-operative tumour markers for patients with gastric cancer. Br J Cancer. 2020;123(3):418–425. doi:10.1038/s41416-020-0901-z
27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
28. Zeng X, Zhu S, Xu C, et al. Effect of comorbidity on outcomes of patients with advanced non-small cell lung cancer undergoing anti-PD1 immunotherapy. Med Sci Monit. 2020;26:e922576. doi:10.12659/msm.922576
29. Xiao LB, Yu JX, Wu WH, Xu FF, Yang SB. Superiority of metastatic lymph node ratio to the 7th edition UICC N staging in gastric cancer. World J Gastroenterol. 2011;17(46):5123–5130. doi:10.3748/wjg.v17.i46.5123
30. Hung YS, Chang SC, Liu KH, et al. A prognostic model based on lymph node metastatic ratio for predicting survival outcome in gastric cancer patients with N3b subclassification. Asian J Surg. 2019;42(1):85–92. doi:10.1016/j.asjsur.2017.10.001
31. Wu YC, Lin CF, Hsu WH, Huang BS, Huang MH, Wang LS. Long-term results of pathological stage I non-small cell lung cancer: validation of using the number of totally removed lymph nodes as a staging control. Eur J Cardiothorac Surg. 2003;24(6):994–1001. doi:10.1016/s1010-7940(03)00567-0
32. Tong Y, Liu D, Zhang J. Connection and distinction of tumor regression grading systems of gastrointestinal cancer. Pathol Res Pract. 2020;216(9):153073. doi:10.1016/j.prp.2020.153073
33. Achilli P, De Martini P, Ceresoli M, et al. Tumor response evaluation after neoadjuvant chemotherapy in locally advanced gastric adenocarcinoma: a prospective, multi-center cohort study. J Gastrointest Oncol. 2017;8(6):1018–1025. doi:10.21037/jgo.2017.08.13
34. Yagi S, Wakatsuki T, Yamamoto N, et al. Clinical significance of intratumoral HER2 heterogeneity on trastuzumab efficacy using endoscopic biopsy specimens in patients with advanced HER2 positive gastric cancer. Gastric Cancer. 2019;22(3):518–525. doi:10.1007/s10120-018-0887-x
35. Seeneevassen L, Bessède E, Mégraud F, Lehours P, Dubus P, Varon C. Gastric cancer: advances in carcinogenesis research and new therapeutic strategies. Int J Mol Sci. 2021;22(7):3418. doi:10.3390/ijms22073418
36. Yu S, Wang Y, Cheng X, et al. Prognosis of adjuvant SOX vs XELOX chemotherapy for gastric cancer after D2 gastrectomy in Chinese patients. Cancer Manag Res. 2020;12:10091–10101. doi:10.2147/cmar.S270387
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




