Back to Journals » Cancer Management and Research » Volume 11
NIMA-related kinase 2 overexpression is associated with poor survival in cancer patients: a systematic review and meta-analysis
Authors Yao Y , Su J, Zhao L, Luo N, Long L , Zhu X
Received 21 September 2018
Accepted for publication 19 November 2018
Published 3 January 2019 Volume 2019:11 Pages 455—465
DOI https://doi.org/10.2147/CMAR.S188347
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Yang Yao,1,* Jie Su,1,* Lei Zhao,2 Na Luo,3 Lihui Long,4 Xingmei Zhu5
1Department of Central Laboratory, The First Affiliated Hospital, Xi’an Medical University, Xi’an, Shaanxi 710077, PR China; 2Department of Molecular Physiology and Biophysics, Holden Comprehensive Cancer Center, University of Iowa Carver College of Medicine, Iowa City, IA 52242, USA; 3Department of Clinical Medicine (Four-Year Program) of Grade 2014, Xi’an Medical University, Xi’an, Shaanxi 710021, PR China; 4Department of Pharmacy, The First Affiliated Hospital of Xi’an Medical University, Xi’an, Shaanxi 710077, PR China; 5Department of Pharmacy, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi 712046, PR China
*These authors contributed equally to this work
Objective: NIMA-related kinase 2 (NEK2) has been reported to be overexpressed in various types of cancer and correlated with poor prognosis. The role(s) of NEK2 in cancer, however, is still uncertain. The aim of this study was to evaluate the prognostic value of NEK2 in human tumors.
Methods: A comprehensive literature search was performed for PubMed, Embase, Web of Science, and CNKI databases, and eligible studies were included based on the inclusion and exclusion criteria. A meta-analysis of the included studies was then carried out.
Results: Fifteen studies with 3,280 cancer patients were included in the present meta-analysis. All publications were of moderate to high quality, and had no significant heterogeneity (I2=46%, P=0.03) or publication bias was discovered. The results showed that a high NEK2 level was associated with shorter overall survival (OS) in patients with various types of cancers (pooled HR=1.72, 95% CI 1.49–2.00, P<0.00001). However, the disease-free survival (DFS) had no significant association with NEK2 level (HR=1.13, 95% CI: 0.29–4.38, P=0.85). In the subgroup analyses, high NEK2 level was correlated with an increased risk of poor OS in patients with hepatocellular carcinoma (HR=1.62, 95% CI: 1.25–2.10, P=0.02) and lung cancer (HR=2.18, 95% CI: 1.40–3.38, P=0.0005). However, other factors, including sample size, follow-up period, HR estimation method, and country, also affect the association between NEK2 expression and OS. Analysis of clinicopathological parameters further showed that increased NEK2 level was correlated with younger age, male gender, better tumor differentiation, and lower number of tumor nodules.
Conclusion: The results of this study indicated that increased expression of NEK2 was associated with unfavorable survival of cancer patients and that NEK2 could be used as a prognostic predictor for cancers.
Keywords: NEK2, prognosis, cancer, diagnosis, meta-analysis, clinical characteristics
Introduction
Cancer is one of the main causes of death and is a challenge to healthcare systems around the world.1 Epidemiological studies show that 14.1 million new cancer cases and 8.2 million cancer-related deaths occur annually worldwide, and these numbers are on the rise.2,3 Early diagnosis and prognosis prediction are helpful for choosing treatments for cancer patients, but traditional detection methods such as imaging techniques and biopsy have their limitations.4,5 Therefore, it is necessary to identify new biomarkers for early diagnosis and for predicting patients’ prognosis to improve the efficacy of cancer treatment.
NIMA-related kinase 2 (NEK2), which is a member of the cell cycle-related kinase (CCRK) family of proteins, is a serine/threonine kinase located in the centrosome.6 In recent years, determining the role(s) of NEK2 in tumor pathogenesis and progression has become a hotspot of research.7,8 Elevation in NEK2 level contributes to the formation of centrosomal abnormalities and monopolar spindles and promotes aneuploidy by disrupting the control of mitotic checkpoints.9,10 An increasing number of studies have reported that expression of NEK2 is increased in cancer and that up-regulation of NEK2 is associated with tumor progression and poor prognosis in various types of cancer, including breast cancer,11,12 colorectal cancer,13,14 cervical cancer,15 and cholangiocarcinoma.16 Notably, the number of studies on NEK2 is still small and the role, or roles, of NEK2 in cancer remains unclear. Overall, the reported results are inconclusive, and no consensus has been reached. Thus, we performed this meta-analysis to summarize the existing data and evaluate the prognostic value of NEK2 in cancer patients.
Methods
Literature search strategy
A comprehensive literature search of online databases, including PubMed, Web of Science, Embase, and CNKI, was carried out to retrieve studies evaluating the association between NEK2 expression and clinical outcome in any type of tumor up to August 2017. The keywords for the literature search included: “NIMA-related kinase 2”, “NEK2”, “cancer”, “tumor”, “carcinoma”, “neoplasm”, “survival”, or “prognosis”. The references of eligible literature were also manually screened to further retrieve potentially eligible publications. Conflicts were solved through group discussion.
Inclusion and exclusion criteria
To be eligible for inclusion, a study had to meet the following criteria: 1) the association of NEK2 level with the clinicopathological features or prognosis of cancer patients was described; 2) patients were categorized into two groups based on high and low expression levels of NEK2; and 3) the study provided the data for estimating the HRs and 95% CIs of survival outcomes. The exclusion criteria included: 1) studies that did not provide the correlation between NEK2 level and the clinicopathological features or prognosis of cancer patients; 2) duplicated publications; and 3) letters, reviews, case reports, and expert opinions.
Data extraction and quality assessment
Two investigators independently evaluated the included studies for the number of patients, name of the first author, year of publication, cancer type, TNM stage, follow-up period, outcome measures, methods for measuring NEK2 expression, HR and its 95% CI, and clinicopathological parameters from each study. The quality of included studies was assessed with the Newcastle–Ottawa Scale (NOS), which is composed of three domains: selection, comparability, and exposure. The NOS is a semi-quantitative scale, from which a score of 0–9 was assigned to each study. A total score of ≤3 was considered poor quality, 4–6 was considered moderate qualify, and 7–9 was deemed high quality.
Statistical methods
The meta-analysis was performed with RevMan 5.3 software. The HR with corresponding 95% CI was used to estimate the strength of the relationship between NEK2 expression and prognosis of patients. Heterogeneity among studies was quantified using the chi-squared test and I2 statistics. A chi-squared test with P<0.05 or I2>50% indicated significant heterogeneity across the studies. If the heterogeneity was not significant, a fixed effects model was used to investigate the pooled HRs. A random effects model was used to summarize the statistical synthesis if among-study heterogeneity was substantial. Sensitivity analysis was also carried out to assess the stability of the results. Publication bias was estimated qualitatively using funnel plots with the standard error.
Results
Study characteristics
The initial literature search retrieved 322 relevant studies. According to the inclusion and exclusion criteria, 14 eligible studies with 3,280 enrolled patients were included in the present meta-analysis (Figure 1). The characteristics of the included studies are summarized in Table 1. All studies were published in the past 4 years, suggesting that the value of NEK2 in predicting prognosis is a novel field of research. Regarding the type of cancer, four studies investigated hepatocellular carcinoma (HCC),18,20,26,28 three studies were focused on colorectal cancer,23,25,29 two studies were focused on non-small cell lung cancer,22,24 and one each evaluated prostate cancer,17 pancreatic ductal adenocarcinoma,19 malignant glioma,21 lung cancer,27 multiple myeloma,30 and breast cancer.31 The maximum and minimum sample sizes of the included studies were 594 and 50, respectively. The follow-up time ranged from 40 to 240 months. All included studies were carried out in four countries (PR China, Japan, the UK, and the USA) and published prior to August 2017. The NOS method was applied to evaluate the quality of each study, and the mean score of the included studies was 8.6 (range 6–9).
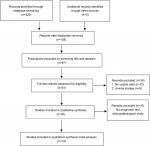  | Figure 1 Flow diagram of literature search and selection. |
Association between NEK2 and overall survival (OS)
Fourteen included studies assessed the HRs for poor OS. As shown in Figure 2, no obvious heterogeneity among these studies was found (I2=46%, P=0.03). Thus, a fixed effects model was employed to evaluate the pooled association between NEK2 expression and OS of different types of malignancies. The HR of the high NEK2 level group vs the low NEK2 expression group was 1.72 (95% CI: 1.49–2.00, P<0.00001), suggesting that up-regulated NEK2 expression was correlated with inferior OS in cancer patients (Figure 2).
  | Figure 2 Forest plot of HRs for the association between high NIMA-related kinase 2 (NEK2) expression and overall survival in cancer patients. |
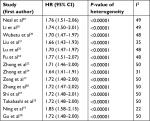
Subsequent stratified analyses were performed according to cancer type, sample size, follow-up period, HR estimation method, and country (Figures 3 and 4 and Table 2). Increased NEK2 expression was associated with poor OS in patients with HCC (HR=1.62, 95% CI: 1.25–2.10, P=0.0002) or lung cancer (HR=2.18, 95% CI: 1.40–3.38, P=0.0005) (Figure 3). In the analysis stratified by sample size, NEK2 expression was found to be significantly correlated with patient survival in studies with a sample size <300 (HR=1.88, 95% CI: 1.43–2.46, P<0.00001) or more than 300 (HR=1.59, 95% CI: 1.20–2.10, P=0.001) (Figure 4A). There was a significant correlation between NEK2 expression level and patient survival in studies with follow-up time ≤100 months (HR=1.68, 95% CI: 1.09–2.59, P=0.02) or >100 months (HR=1.95, 95% CI: 1.56–2.44, P<0.00001) (Figure 4B). Regarding the location of the study, the stratified analysis revealed a significant association between NEK2 level and OS in both Asian countries (HR=1.86, 95% CI: 1.45–2.38, P<0.0001) and other countries (HR=1.55, 95% CI: 1.08–2.23, P=0.02) (Figure 4C). In addition, the association was significant in studies with both direct (HR=2.01, 95% CI: 1.18–3.43, P=0.01) and indirect (HR=1.75, 95% CI: 1.37–2.23, P<0.00001) HR estimation methods (Figure 4D). Altogether, the subgroup analyses indicated that sample size, follow-up period, country, and HR estimation method did not have any significant influence on the correlation between NEK2 level and patient OS, suggesting that the association is stable and therefore highlighting the value of NEK2 as a prognostic biomarker for cancer patients.
  | Figure 3 Forest plot of HRs for the association between high NIMA-related kinase 2 (NEK2) expression and overall survival stratified by cancer type. |
  | Table 2 Subgroup analysis of the pooled HRs for OS Abbreviations: CC, colon cancer; DFS, disease-free survival; HCC, hepatocellular carcinoma; LC, lung cancer; OS, overall survival. |
Association between NEK2 and disease-free survival (DFS)
As shown in Figure 5, two studies with 697 patients investigated the role of NEK2 expression in DFS. However, the pooled analysis failed to show a significant correlation between NEK2 level and DFS of patients (HR=1.13, 95% CI: 0.29–4.38, P=0.85) (Figure 5).
  | Figure 5 Forest plot of HRs for the association between high NIMA-related kinase 2 (NEK2) expression and disease-free survival in cancer patients by different cancer types. |
Correlation between NEK2 expression and clinicopathological parameters
To examine whether the NEK2 level had an association with the clinicopathological parameters of patients, we pooled the clinicopathological data to conduct a meta-analysis. As shown in Table 3, increased NEK2 expression was negatively associated with age (OR=0.45, 95% CI: 0.11–1.84, P<0.00001), but positively correlated with male gender (OR=3.02, 95% CI: 1.30–7.02, P=0.01), better tumor differentiation (OR=4.23, 95% CI: 1.30–13.77, P<0.00001), and lower tumor nodule number (OR=5.88, 95% CI: 2.19–5.80, P=0.0004). However, NEK2 expression was not correlated with other clinicopathological features, including disease stage and tumor size (Table 3).
  | Table 3 Association between NEK2 expression and clinicopathological features Abbreviation: NEK2, NIMA-related kinase 2. |
Sensitivity analysis
A sensitivity analysis was then performed by removing each study in turn from the pooled analysis for the association between NEK2 level and OS. This analysis enabled us to examine the impact of the removed study on the overall HRs. The results revealed that removing any of the included studies had no significant influence on the final results, which suggested the robustness of the association (Table 4).
  | Table 4 Sensitivity analysis |
Publication bias
No significant publication bias was discovered in the included studies from the funnel plots (Figure 6).
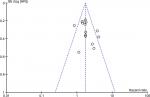  | Figure 6 Funnel plot analysis for the potential publication bias among included studies. |
Discussion
NEK2 belongs to the CCRK family of cell cycle-regulating proteins and it is a serine/threonine kinase located in the centrosome.32–34 Previous studies have demonstrated that elevation in NEK2 contributes to the regulation of centrosome separation and spindle formation, and to ensuring that the chromosome structure is more stable and complete.35,36 In cancer-related studies, NEK2 was found to be up-regulated in various types of cancer tissues and cell lines, suggesting the involvement of NEK2 in tumorigenesis. NEK2 has also been shown to be involved in abnormal cell differentiation, tumor proliferation, drug resistance, and poor prognosis in several kinds of tumors.37–39
A lot of effort has been made to understand the functional role of NEK2 in cancer. Zhang et al found that NEK2 significantly enhanced the invasive ability of HCC cells, and epithelial–mesenchymal transition played a pivotal role in the NEK2-mediated promotion of HCC cell invasion.28 Zhang et al demonstrated that NEK2 may regulate proliferation, apoptosis, and other biological behaviors of HepG2 cells via the mitogen-activated protein kinase signaling pathway.40 Tsunoda et al showed that interfering with or silencing NEK2 expression could inhibit the invasive capacity of breast cancer cells.41 Two groups reported that elevation in NEK2 contributed to the activation of the PI3K/AKT signaling pathway, a potent and critical oncogenic pathway for a variety of malignancies.41,42 Li et al reported that overexpression of NEK2 resulted in high expression of phospho-AKT and matrix metalloproteinase-2 proteins in HCC, which are key factors in HCC invasion and metastasis.43 These results suggest that targeting NEK2 may be beneficial in the treatment of human cancers. However, the role of NEK2 in other non-studied types of cancer needs to be further investigated to fully understand the functions of NEK2 in cancer.
This meta-analysis is the first systematic review to comprehensively investigate the relationship between NEK2 expression and the OS of patients with different types of cancers. Survival data of 3,280 patients from 15 included studies were systematically analyzed. The pooled HRs suggested the value of NEK2 in predicting the OS in cancer patients. Regarding tumors originating from different tissues, high NEK2 expression was relevant to poor OS in HCC and lung cancer. Further subgroup analyses for sample size, follow-up time, HR estimation method, and country showed that the significance of NEK2 in OS was not affected by these factors. The meta-analysis for the association between increased NEK2 expression and clinicopathological parameters was also analyzed in this study. Our results showed that increased NEK2 expression was significantly associated with younger age, male gender, better tumor differentiation, and solitary tumor nodule. Overall, this study highlighted the potential of NEK2 as a prognostic biomarker for cancer patients.
However, this meta-analysis had some deficiencies and limitations. First, the total sample size was relatively small, and most of the patients included in the meta-analysis were from PR China, raising concerns when applying the results of this study to different ethnicities and regions. Therefore, more large-scale studies are warranted to further verify the prognostic value of NEK2 in different ethnicities and regions. Second, some included studies did not offer HRs directly, and we calculated the HRs using the Kaplan–Meier curves from these studies. This may compromise the validity of the conclusions. Third, publication bias may exist, as studies with small sample size or negative results are less likely to be reported, despite the fact that no significant publication bias was observed for the included studies upon sensitivity and funnel plot analyses. Therefore, larger, multicenter, and higher-quality studies with unified criteria for determining NEK2 expression are necessary to validate the conclusions of this study.
Conclusion
In summary, our meta-analysis has demonstrated that high NEK2 levels in different types of solid tumors are significantly associated with poor prognosis. Accordingly, NEK2 has potential value as a prognostic biomarker for tumors. Nevertheless, this conclusion needs to be confirmed by larger-scale prospective studies in the future.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81402186) and the third batch of the key disciplines construction project of Xi’an Medical University.
Disclosure
The authors report no conflicts of interest in this work.
References
Ren L, Liu J, Gou K, Xing C. Copy number variation and high expression of DNA topoisomerase II alpha predict worse prognosis of cancer: a meta-analysis. J Cancer. 2018;9(12):2082–2092. | ||
Wang A, Wang S, Zhu C, et al. Coffee and cancer risk: a meta-analysis of prospective observational studies. Sci Rep. 2016;6:33711. | ||
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. | ||
Wang Y, Huang Y, Xiang P, Tian W. LncRNA expression and implication in osteosaroma: a systematic review and meta-analysis. OncoTargets Ther. 2017;10:5355–5361. | ||
Nagano K, Higashisaka K, Tsunoda SI, Tsutsumi Y. Development of a fundamental technology to seek drug targets, and its application to cancer targeting therapy. Yakugaku Zasshi. 2018;138(7):903–909. | ||
Hao M, Franqui-Machin R, Xu H, et al. NEK2 induces osteoclast differentiation and bone destruction via heparanase in multiple myeloma. Leukemia. 2017;31(7):1648–1650. | ||
Huang J, Sun SG, Hou S. Aberrant NEK2 expression might be an independent predictor for poor recurrence-free survival and overall survival of skin cutaneous melanoma. Eur Rev Med Pharmacol Sci. 2018;22(12):3694–3702. | ||
Fang Y, Zhang X. Targeting NEK2 as a promising therapeutic approach for cancer treatment. Cell Cycle. 2016;15(7):895–907. | ||
Prigent C, Glover DM, Giet R. Drosophila Nek2 protein kinase knockdown leads to centrosome maturation defects while overexpression causes centrosome fragmentation and cytokinesis failure. Exp Cell Res. 2005;303(1):1–13. | ||
Naro C, Barbagallo F, Chieffi P, Bourgeois CF, Paronetto MP, Sette C. The centrosomal kinase NEK2 is a novel splicing factor kinase involved in cell survival. Nucleic Acids Res. 2014;42(5):3218–3227. | ||
Lee J, Gollahon L. Mitotic perturbations induced by Nek2 overexpression require interaction with TRF1 in breast cancer cells. Cell Cycle. 2013;12(23):3599–3614. | ||
Nuncia-Cantarero M, Martinez-Canales S, Andrés-Pretel F, Santpere G, Ocaña A, Galan-Moya EM. Functional transcriptomic annotation and protein-protein interaction network analysis identify NEK2, BIRC5, and TOP2A as potential targets in obese patients with luminal A breast cancer. Breast Cancer Res Treat. 2018;168(3):613–623. | ||
Zhao D, Han W, Liu X, Cui D, Chen Y. MicroRNA-128 promotes apoptosis in lung cancer by directly targeting NIMA-related kinase 2. Thorac Cancer. 2017;8(4):304–311. | ||
Takahashi Y, Iwaya T, Sawada G, et al. Up-regulation of NEK2 by microRNA-128 methylation is associated with poor prognosis in colorectal cancer. Ann Surg Oncol. 2014;21(1):205–212. | ||
Koch M, Wiese M. Gene expression signatures of angiocidin and darapladib treatment connect to therapy options in cervical cancer. J Cancer Res Clin Oncol. 2013;139(2):259–267. | ||
Kokuryo T, Senga T, Yokoyama Y. Nek2 as an effective target for inhibition of tumorigenic growth and peritoneal dissemination of cholangiocarcinoma.NEK2 as an effective target for inhibition of tumorigenic growth and peritoneal dissemination of cholangiocarcinoma. Cancer Res. 2007;67(20):9637–9642. | ||
Zeng YR, Han ZD, Wang C, et al. Overexpression of NIMA-related kinase 2 is associated with progression and poor prognosis of prostate cancer. BMC Urol. 2015;15:90–98. | ||
Fu L, Liu S, Wang H, et al. Low expression of NEK2 is associated with hepatocellular carcinoma progression and poor prognosis. Cancer Biomark. 2017;20(1):101–106. | ||
Ning Z, Wang A, Liang J, et al. Abnormal expression of Nek2 in pancreatic ductal adenocarcinoma: a novel marker for prognosis. Int J Clin Exp Pathol. 2014;7(5):2462–2469. | ||
Li G, Zhong Y, Shen Q, et al. NEK2 serves as a prognostic biomarker for hepatocellular carcinoma. Int J Oncol. 2017;50(2):405–413. | ||
Liu H, Liu B, Hou X, et al. Overexpression of NIMA-related kinase 2 is associated with poor prognoses in malignant glioma. J Neurooncol. 2017;132(3):409–417. | ||
Zhong X, Guan X, Liu W, Zhang L. Aberrant expression of NEK2 and its clinical significance in non-small cell lung cancer. Oncol Lett. 2014;8(4):1470–1476. | ||
Takahashi Y, Iwaya T, Sawada G, et al. Up-regulation of NEK2 by microRNA-128 methylation is associated with poor prognosis in colorectal cancer. Ann Surg Oncol. 2014;21(1):205–212. | ||
Zhong X, Guan X, Dong Q, Yang S, Liu W, Zhang L. Examining Nek2 as a better proliferation marker in non-small cell lung cancer prognosis. Tumour Biol. 2014;35(7):7155–7162. | ||
Lu L, Zhai X, Yuan R. Examining Nek2 as a better proliferation marker in non-small cell lung cancer prognosis. Int J Clin Exp Pathol. 2015;8:15467–15473. | ||
Wubetu GY, Morine Y, Teraoku H, et al. High NEK2 expression is a predictor of tumor recurrence in hepatocellular carcinoma patients after hepatectomy. Anticancer Res. 2016;36(2):757–762. | ||
Shi YX, Yin JY, Shen Y, Zhang W, Zhou HH, Liu ZQ. Genome-scale analysis identifies NEK2, DLGAP5 and ECT2 as promising diagnostic and prognostic biomarkers in human lung cancer. Sci Rep. 2017;7(1):8072. | ||
Zhang Y, Wang W, Wang Y, et al. NEK2 promotes hepatocellular carcinoma migration and invasion through modulation of the epithelial-mesenchymal transition. Oncol Rep. 2018;39(3):1023–1033. | ||
Neal CP, Fry AM, Moreman C, et al. Overexpression of the Nek2 kinase in colorectal cancer correlates with beta-catenin relocalization and shortened cancer-specific survival. J Surg Oncol. 2014;110(7):828–838. | ||
Gu Z, Xia J, Xu H. NEK2 promotes aerobic glycolysis in multiple myeloma through regulating splicing of pyruvate kinase. J Hematol Oncol. 2017;10(1):17–28. | ||
Marina M, Saavedra HI. Nek2 and PIk4: prognostic markers, drivers of breast tumorigenesis and drug resistance. Front Biosci (Landmark Ed). 2014;19:352–365. | ||
Barbagallo F, Paronetto MP, Franco R, et al. Increased expression and nuclear localization of the centrosomal kinase Nek2 in human testicular seminomas. J Pathol. 2009;217(3):431–441. | ||
Cappello P, Blaser H, Gorrini C, et al. Role of Nek2 on centrosome duplication and aneuploidy in breast cancer cells. Oncogene. 2014;33(18):2375–2384. | ||
Chen C, Tian F, Lu L, et al. Characterization of Cep85 - a new antagonist of Nek2A that is involved in the regulation of centrosome disjunction. J Cell Sci. 2015;128(20):3837–3303. | ||
Nuncia-Cantarero M, Martinez-Canales S, Andrés-Pretel F, Santpere G, Ocaña A, Galan-Moya EM. Functional transcriptomic annotation and protein-protein interaction network analysis identify NEK2, BIRC5, and TOP2A as potential targets in obese patients with luminal A breast cancer. Breast Cancer Res Treat. 2018;168(3):613–623. | ||
Wang J, Cheng P, Pavlyukov MS, et al. Targeting NEK2 attenuates glioblastoma growth and radioresistance by destabilizing histone methyltransferase EZH2. J Clin Invest. 2017;127(8):3075–3089. | ||
Hayward DG, Fry AM. Nek2 kinase in chromosome instability and cancer. Cancer Lett. 2006;237(2):155–166. | ||
Zhao D, Han W, Liu X, Cui D, Chen Y. Deguelin inhibits epithelial-to-mesenchymal transition and metastasis of human non-small cell lung cancer cells by regulating NIMA-related kinase 2. Thorac Cancer. 2017;8(4):320–327. | ||
Lai XB, Nie YQ, Huang HL, et al. NIMA-related kinase 2 regulates hepatocellular carcinoma cell growth and proliferation. Oncol Lett. 2017;13(3):1587–1594. | ||
Zhang MX, Xu XM, Zhang P, et al. Effect of silencing NEK2 on biological behaviors of HepG2 in human hepatoma cells and MAPK signal pathway. Tumour Biol. 2016;37(2):2023–2035. | ||
Tsunoda N, Kokuryo T, Oda K, et al. Nek2 as a novel molecular target for the treatment of breast carcinoma. Cancer Sci. 2009;100(1):111–116. | ||
Zhou W, Yang Y, Xia J, et al. NEK2 induces drug resistance mainly through activation of efflux drug pumps and is associated with poor prognosis in myeloma and other cancers. Cancer Cell. 2013;23(1):48–62. | ||
Li G, Zhong Y, Shen Q, et al. NEK2 serves as a prognostic biomarker for hepatocellular carcinoma. Int J Oncol. 2017;50(2):405–413. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


