Back to Journals » International Journal of Nanomedicine » Volume 14
New Approach For Simvastatin As An Antibacterial: Synergistic Effect With Bio-Synthesized Silver Nanoparticles Against Multidrug-Resistant Bacteria
Authors Figueiredo EP, Ribeiro JM, Nishio EK, Scandorieiro S, Costa AF, Cardozo VF, Oliveira AG , Durán N, Panagio LA, Kobayashi RKT , Nakazato G
Received 10 April 2019
Accepted for publication 29 July 2019
Published 3 October 2019 Volume 2019:14 Pages 7975—7985
DOI https://doi.org/10.2147/IJN.S211756
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
EP Figueiredo,1 JM Ribeiro,1 EK Nishio,1 S Scandorieiro,1 AF Costa,2 VF Cardozo,1 AG Oliveira,1 N Durán,2–4 LA Panagio,1 RKT Kobayashi,1 G Nakazato1
1Department of Microbiology, Center of Biological Sciences, Universidade Estadual de Londrina, Londrina, Paraná, Brazil; 2NanoBioss, Institute of Chemistry, Universidade Estadual de Campinas, Campinas, São Paulo, Brazil; 3Institute of Chemistry, Universidade Estadual de Campinas, Campinas, São Paulo, Brazil; 4LNNano (National Laboratory of Nanotecnology), CNPEM, Campinas, São Paulo, Brazil
Correspondence: G Nakazato
Department of Microbiology, Center of Biological Sciences, Universidade Estadual de Londrina, Campus Universitário, CEP, Londrina 86055-990, Paraná, Brazil
Tel +55 43 3371 4396
Fax +55 43 3371 4788
Email [email protected]
Background: Multidrug-resistant bacteria such as extended-spectrum beta-lactamase (ESBL), Enterobacteriaceae, and methicillin-resistant Staphylococcus aureus (MRSA) pose a challenge to the human health care system. MRSA is among the major causes of hospital-acquired and community infections.
Methods: Therefore, in the present study, we evaluated the antibacterial activity of silver nanoparticles synthesized by Fusarium oxysporum (AgNPbio) in combination with simvastatin against reference and multidrug-resistant bacterial strains.
Results: Simvastatin showed a minimal inhibitory concentration (MIC) ranging from 0.062 to 0.25 mg mL−1 against MRSA. AgNPbio with a size of 77.68± 33.95 nm and zeta potential −34.6 ± 12.7 mV showed an MIC of 0.212 mg mL−1 against S. aureus including MRSA strains. The checkerboard assay and time-kill curves exhibited a synergistic effect of the simvastatin-AgNPbio combination on antibacterial activity against MRSA strains. The combination of simvastatin and AgNPbio demonstrated antibacterial activity against Escherichia coli producing ESBL. Scanning electron microscopy showed the formation of cell surface protrusions after treatment with AgNPbio and the formation of a large amorphous mass after treatment with simvastatin, both in MRSA.
Conclusion: Our results indicate that the combination of AgNPbio and simvastatin could be a great future alternative in the control of bacterial infections, where, when combined with simvastatin, smaller doses of AgNPbio are required, with the same antibacterial activity.
Keywords: antibacterial, metallic nanoparticles, multidrug-resistant bacterial, statins, synergism
Introduction
Antibiotics are the most commonly prescribed drugs in hospitals. This intensive and frequent use favors the selection of resistant strains, which can cause serious infections in patients. Resistance leads to ineffective clinical treatment in the case of some bacterial species, increasing the problem of microbial resistance to antimicrobials.1 Resistance such as extended-spectrum β-lactamase producing (ESBL), Klebsiella pneumoniae carbapenemase (KPC) producing, and methicillin-resistant Staphylococcus aureus (MRSA) lead to therapeutic failure, high treatment cost, and patient death (high mortality).2,3 MRSA strains were the most prevalent pathogens, contributed to 56% of nosocomial and community infections, and were the most common multidrug-resistant microorganisms in hospitals.4 A study published in 2016 estimated a rate of 10 million deaths due to antimicrobial-resistant microorganisms in 2050.1 Therefore, there is a necessity to discover new treatment options.
Statins are known for their antihyperlipidemic effects by competitively inhibiting the enzyme HMG-CoA reductase, decreasing cholesterol biosynthesis.5 This drug is also known to present pleiotropic effects, such as anti-inflammatory and antithrombotic.6,7 Furthermore, in 2008 a study that described the antibacterial effects of statins found that simvastatin and fluvastatin were active against MRSA and Vancomycin-resistant Enterococcus (VRE) strains.8
Metals have been used since ancient times as antibacterial agents and present different properties and spectra of action. Among the metals, silver is one of the most commonly used due to its efficiency as an antimicrobial and low toxicity, being impregnated in utensils and materials used in medicine.9
With the advent of nanotechnology in the medical area, silver nanoparticles (AgNP) have become widely studied for their antimicrobial action, including against multidrug-resistant bacteria.10–15 AgNP are interesting when compared to silver ions due to their small size and high superficial area, which, in turn, improves their ability to react with multiple molecules. This feature leads to an ultra-large surface area per volume, where a large proportion of atoms are in immediate contact with the environment and readily available for reactions.16–20 Biological synthesis is interesting when compared with chemical and physical syntheses because it not uses toxic solvents, being an environmentally friendly technology and low-cost.21,22 Therefore, metallic nanoparticles produced by biogenic synthesis with biomolecules and proteins hold up the stabilization of nanosystems.21,23,24
Despite the well-known antibacterial activity of silver, silver-resistant Escherichia coli was isolated and identified from a burn wound treated with silver nitrate.25 In addition, resistant microorganisms were isolated from different environments with a natural occurrence of silver, such as in mines and marine water,19,26,27 and, a recent study showed how fast E. coli develops resistance after contact with AgNP for several generations.28
To avoid this problem, according to the literature, combining AgNPbio with other antibiotic compounds is a promising new strategy to control resistant bacterial infections, since it is effective against multidrug-resistant bacteria and the combination decreases the emergence of new antimicrobial resistance.12,14,29
The combination of nanoparticles with antibiotics could have great potential in the control of multidrug-resistant microorganisms. This combination results in an improved bactericidal effect compared to drugs alone.20 AgNP has demonstrated different antimicrobial interactions depending on the microorganism strain and compound tested.30 Ampicillin, kanamycin, chloramphenicol, and erythromycin showed increased antibacterial activity when combined with AgNP against Gram-positive and Gram-negative bacteria.29 The combination of amoxicillin and AgNP resulted in a synergistic effect against E. coli.18 Phenazine-1-carboxamide combined with silver nanoparticles synthetized by Fusarium oxysporum (AgNPbio) resulted in a synergistic effect against MRSA.12 Eugenol with AgNPbio showed a synergistic effect against Streptococcus agalactiae.31 AgNP in combination with cinnamaldehyde exhibited a synergistic effect against spore-forming bacterial strains.32 A recent study demonstrated the antibacterial activity of oregano essential oil combined with AgNPbio. These combinations demonstrate antibacterial activity against multidrug-resistant bacteria.14 Besides, a recent report showed antifungal activity of simvastatin combined with AgNPbio against toxigenic species of Aspergillus.33
In the present study, we evaluated, for the first time, the antibacterial activity of simvastatin combined with AgNPbio against reference and multidrug-resistant bacterial strains and analyzed the bacterial morphological alterations through electronic microscopy. This combination is under patent BR1020140323759 (INPI – Brazil).
Materials And Methods
Simvastatin
Simvastatin was obtained commercially (Henan Topfond Pharmaceutical Co. Ltd, China) and dissolved in dimethyl sulfoxide (DMSO) 100% vv−1 at a stock concentration of 5 mg mL−1.
Synthesis Of The Silver Nanoparticles
AgNPbio was obtained biologically by fungus-mediated synthesis as previously described.34 This methodology of AgNPbio production has been patented (Patent, 2006, PI 0605681-4A2). The F. oxysporum strain 551 used was obtained from the culture collection of the Molecular Genetics Laboratory ESALQ-USP, Piracicaba-SP, Brazil. We cultured F. oxysporum in malt agar (Difco®) containing 0.5% yeast extract, 2% malt extract, 2% agar, and distilled water for 7 days at 28ºC. We then added 10 g of fungal biomass (previously washed) from the culture medium to 100 mL of sterile distilled water and incubated for 72 hrs at 28ºC. Subsequently, the supernatant was separated from the fungal biomass by vacuum filtration and AgNO3 (Sigma-Aldrich®) was added to the supernatant to a final concentration of 10 mM; the system solution was kept incubated at 28ºC in the absence of light until formation of AgNPbio. Observation of AgNPbio formation was performed visually and by absorptions until the formation of nanoparticles. We measured absorptions using ultraviolet-visible spectrophotometry (Varian Cary 50 Probe) to verify the formation of silver nanoparticles that presented surface plasmon resonance at 420 nm. After purification, the AgNPbio was characterized.
Characterization Of The AgNPbio And Simvastatin
Morphological and size of AgNPbio was determined by photon correlation spectroscopy using ZetaSizer NanoZS (Malvern®), the same instrument was used to perform the zeta potential measurement and polydispersity index (PDI). Transmission Electron Microscopy (TEM) was performed to confirm morphological and size of AgNPbio. UV-vis was made to detect wavelength corresponding to AgNPbio. Size of simvastatin particles was analyzed using Dynamic Light Scattering (DLS).
Bacterial Strains
Two reference methicillin-sensitive St. aureus (MSSA) strains (ATCC 25923 and ATCC 29213), two reference MRSA strains (MRSA N315 and MRSA BEC 9393), E. coli ATCC 25922, and extended-spectrum beta-lactamases E. coli-producing (ESBL 176) were used in this study. MRSA N315 strain was provided by Dr. Elsa Masae Mamizuka (Universidade de São Paulo, São Paulo-SP, Brazil) and BEC 9393 strain by Dr. Agnes Marie Sá Figueiredo (Universidade Federal do Rio de Janeiro, Rio de Janeiro-RJ, Brazil). E. coli ESBL 176 strain was provided by Dra. Eliana Carolina Vespero (University Hospital – HU, Universidade Estadual de Londrina, Londrina-PR, Brazil). Bacterial strains were stored in brain heart infusion (BHI) broth containing 20% (vv−1) glycerol and maintained at −80°C.
Antimicrobial Disk Susceptibility Test – Disk Diffusion
We performed the antimicrobial disk susceptibility test according to previously described procedures.35 Previously grown bacteria were suspended in saline according to 0.5 McFarland turbidity (corresponding to approximately 1 x 108 CFU mL−1) and the bacterial suspension was inoculated on a plate with Muller-Hinton agar (MHA) using a cotton swab according to the Clinical and Laboratory Standards Institute.36 The disks containing 10 μL of simvastatin and AgNPbio (corresponding to 0.115 mg and 169.86 mg, respectively) were placed on the surface of an inoculated agar plate. A negative control of DMSO was added to the test. The plates were incubated for 24 hrs at 37°C and the growth inhibition halo was measured.35
Minimal Inhibitory Concentration Of Simvastatin And AgNPbio
We determined minimal inhibitory concentration (MIC) by broth microdilution assay in 96-well microplates (Corning®) according to the Clinical and Laboratory Standards Institute guidance.37 In brief, we added different concentrations of simvastatin (from 0.015 mg mL−1 to 0.250 mg mL−1) and AgNPbio (from 0.013 mg mL−1 to 0.212 mg mL−1) diluted in Mueller-Hinton broth (MHB). Bacteria were grown in MHA medium and suspended according to 0.5 McFarland as previously described. This bacterial suspension was diluted in MHB to a ratio of 1:100 and inoculated in 96-well microplates at a density of 5.0 × 105 CFU mL−1 per well. We added DMSO as a negative control at equal concentrations (1.25 to 5% vv−1) to those used to dilute simvastatin. MHB medium was used for sterility control, and the positive control was performed by adding MHB medium and bacteria. The microplates were incubated at 37°C for 24 hrs. MIC was read visually and defined as the minimal concentration that inhibits bacterial growth visually according to turbidity. The assay was performed in triplicate.
Antibacterial Combination Assay (Checkerboard)
After determining MIC of isolated compounds, we tested two compounds (AgNPbio and simvastatin) together to evaluate the antibacterial interaction between them. The checkerboard assay was performed in 96-well microplates,38 where both compounds were diluted in MHB in combination, with concentrations ranging from 0.015.6 to 0.125 mg mL−1 and from 0.013 to 0.212 mg mL−1 for simvastatin and AgNPbio, respectively. Previously grown bacteria were suspended in saline according to 0.5 McFarland. This bacterial suspension was diluted in MHB to a ratio of 1:100 and inoculated in 96-well microplates at a density of 5.0 × 105 CFU mL−1 per well. After 24 hrs of incubation at 37°C, the checkerboard was read visually and defined as minimal concentration that inhibits bacterial growth visually according to turbidity. The assay was performed in triplicate.
To qualify the interaction between both compounds, we calculated the fractional inhibitory concentration index (FICI) as previously described,39 using MIC combined of both compounds (MICc) and MIC alone of each compounds (MICa) the following equation:
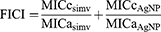
We interpreted FICI according to the following index: ≤0.5, synergistic interaction effect; >0.5 and ≤1.0, additive interaction effect; >1 and <4, indifferent; and ≥4, antagonistic interaction effect.
Time-Kill Curve Assay
Time-kill curves were determined according to the National Committee for Clinical Laboratory Standards40 to evaluate the effect of simvastatin and AgNPbio on growth kinetics of MRSA N315 and E. coli ESBL 176 producing. Bacterial strains were grown previously, and we prepared an inoculum corresponding to 0.5 on the McFarland scale and diluted in MHB to a ratio of 1:100. The compounds were tested alone and in combination, according to MIC and checkerboard assay, respectively, and compared with the bacterial positive control. At different time points of treatment and incubation (0, 2, 4, 7, 10, and 24 hrs) aliquots of bacterial culture were diluted and transferred to a plate with MHA to quantify the number of viable cells. After incubation of the MHA plate at 37ºC for 24 hrs, CFUs were counted and a time-kill curve was constructed. The assay was performed in triplicate.
Cytotoxicity Assay In Human Red Blood Cells
Hemolytic assay of AgNPbio and simvastatin was performed as Izumi et al 201241 with modifications. Human red blood cells (HRBC) were taken from a healthy donor and approved by the human ethics committee (CAAE 47661115.0.0000.5231, No. 1.268.019 – UEL). HRBC were collected in heparinized tubes (vacutainer), separated by centrifugation (5000 rpm, 4ºC, 5 mins) and diluted in 6% v v −1 of phosphate-buffered saline (0.1 M PBS, pH 7,2). In a 96-well plate, 100 µL of HRBC 6% was added in 100 µL of AgNPbio and simvastatin alone.
After 3 hrs of incubation at 37ºC, the supernatant was removed and read 550 nm. Triton-X 100 1% (Sigma-Aldrich) was used as a positive control for hemolysis. Concentration range tested was 0.015.6 to 0.125 mg mL−1 simvastatin and to AgNPbio was 0.013 to 0.212 mg mL−1. Cytotoxic concentration in 50% (CC50) of HRBC was calculated for each compound through linear regression. Selectivity index (SI) was determined using following equation: SI=CC50/IC50.
Scanning Electron Microscopy (SEM)
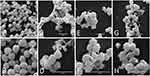
The MRSA N315 strain exposed to 4 situations was analyzed by SEM, as follows: (1) bacteria without antimicrobial treatment (control), (2) bacteria treated with 0.500 mg mL−1 of simvastatin, (3) bacteria treated with AgNPbio at 0.212 mg mL−1, and (4) bacteria exposed to a combination of 0.125 mg mL−1 of simvastatin and AgNPbio at 0.106 mg mL−1 respectively. Previously grown MRSA N315 strains were suspended according to 0.5 McFarland turbidity. A suspension containing approximately 108 CFU mL−1 was prepared in MHB and all 4 samples were incubated at 37°C for 3 hrs. Next, the bacterial cells were obtained by centrifugation (5310 × g, for 5 mins at 10ºC) and washed and suspended with 0.1 M phosphate-buffered saline (PBS) at pH 7.4. 20 µL cell suspensions of MRSA N315 were spotted on glass slides previously coated with a thin layer of 10% poly-L-lysine. Afterwards, we fixed each slide containing MRSA N315 through immersion in 1 mL of 2% glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) solution for 12 hrs, followed by post-fixation in 1% OsO4 for 2 hrs.
Subsequently, the samples were dehydrated in graded ethanol series (70, 80, 90, and 100 GL) and critical point dried using CO2 (BALTEC CPD 030 Critical Point Dryer). The slides were taped onto stubs, coated with gold (BALTEC SDC 050 Sputter Coater), and finally examined using a FEI Quanta 200 scanning electron microscope.42
Statistical Methods
We evaluated the results by two-way ANOVA and standard deviation using R cran and considering p < 0.05 significant. All samples were made in triplicate. Linear regression was performed to determine CC50 of cytotoxic assay.
Results
Characterization Of The AgNPbio And Simvastatin
The average AgNPbio size was 77.68±33.95 nm and average zeta potential was −34.6±12.7 mV (Supplementary data). PDI for AgNPbio was 0.182. TEM images show AgNPbio average size of 50nm (Figure 1). Particle size of simvastatin was 110.8±46.8 nm. UV-Vis wavelength corresponding to AgNPbio was 420 nm22 (Figure 2).
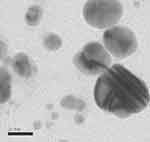 |
Figure 1 Characterization by transmission electron microscopy (TEM) of AgNPbio synthesized by Fusarium oxysporum (300,000×). |
 |
Figure 2 UV-Vis spectrophotometry of nanoparticles synthetized by Fusarium oxysporum. |
Antimicrobial Disk Susceptibility Test – Disk Diffusion
The antimicrobial disk susceptibility assay showed that AgNPbio formed inhibition zones against MSSA ATCC 25923, E. coli ATCC 25922, and multidrug-resistant strains. The disk containing simvastatin showed no inhibition of either Gram-positive or Gram-negative bacteria, not forming inhibition zones (Table 1).
 |
Table 1 Results Of Antimicrobial Disk Susceptibility And Minimal Inhibitory Concentration (MIC), Checkerboard, And Fractional Inhibitory Concentration (FICI) For Bacterial Strains |
Minimal Inhibitory Concentration Of Simvastatin And AgNPbio
Simvastatin only demonstrated antibacterial activity against the Gram-positive bacterial strains MSSA ATCC 25923, MSSA ATCC 29213, MRSA BEC 9393, and MRSA N315, with MIC values ranging from 0.062 mg mL−1 to 0.25 mg mL−1. AgNPbio showed a broad spectrum of action, acting against Gram-positive and Gram-negative bacterial strains, with an MIC value of 0.212 mg mL−1 against MSSA ATCC 25923, MSSA ATCC 29213, MRSA N315, and MRSA BEC 9393, and 0.106 mg mL−1 against E. coli ATCC 25922 and E. coli ESBL 176 producing (Table 1). Simvastatin and AgNPbio presented MICs of 0.062 mg mL−1 and 0.212 mg mL−1, respectively, against MRSA N315. E. coli ESBL 176 demonstrated no susceptibility to simvastatin, whereas AgNPbio was active against this strain at an MIC value of 0.106 mg mL−1. DMSO control not inhibited bacterial growth.
Antibacterial Combination Assay (Checkerboard)
The results of the checkerboard assay (Table 1) showed that there were synergistic and additive antibacterial effects between simvastatin and AgNPbio. The combination demonstrated potentiated antibacterial activity against MRSA N315 and MSSA ATCC 25923, decreasing the concentration used for antibacterial effect. The concentration was reduced to 75% when simvastatin and AgNPbio were in combination.
Time-Kill Curve Assay
The results showed that simvastatin, when used alone, had a bacteriostatic effect against MRSA N315 (Figure 3), while AgNPbio, at 24 hrs, had a bactericidal effect. Combination of AgNPbio with simvastatin shows difference between treatments alone of AgNPbio (p < 0.05) e simvastatin (p < 0.05) eliminate entire bacterial population.
Afer 10 hrs of incubation, all cells of the bacterial population were eradicated by simvastatin and AgNPbio combined (p < 0.05), against N315 MRSA. In comparison, simvastatin when used alone against E. coli ESBL 176 not showed antibacterial activity (Figure 4). AgNPbio was bactericidal in 24 hrs against MRSA N315 and decreased the concentration cells in 4 hrs. Simvastatin was bacteriostatic, and the combination was more effective than simvastatin used alone, showing bactericidal activity in 4 hrs.
Cytotoxicity Assay In HRBC
Simvastatin showed a CC50 in HRBC in range of 0.260 mg mL−1. CC50 AgNPbio was 9283.4 mg mL−1. CC50 of simvastatin and AgNPbio was the concentration above MIC 0.260 mg mL−1 and 9.283.4 mg mL−1, respectively. SI was 4.193 for simvastatin and 43.789 for AgNPbio.
Scanning Electron Microscopy (SEM)
SEM analysis showed that the untreated MRSA N315 sample presented a large number of smooth cells, an intact surface, and unaltered average size, with typical features unchanged in format, arrangement, and appearance as found in treated cells (Figure 5A and B). The MRSA N315 sample exposed to simvastatin treatment presented some cells with normal appearance, arrangement, and format, while others presented deformations; the micrographs demonstrated the formation of a large amorphous mass caused by destruction of the bacterial cells in the treatment with simvastatin (Figure 5C and D). The AgNPbio treatment caused alterations in cell morphology, such as protrusions of numerous small bubbles a few nanometers in size; the metal nanoparticles also resulted in numerous lysed cells and cell debris (Figure 5E and F). Cells treated with a combination of simvastatin and AgNPbio showed both types of alterations: formation of a large amorphous mass caused by simvastatin and a protrusion of numerous small bubbles caused by AgNPbio. It was possible to identify cell surface protrusions, amorphous mass, small bubbles, lysed cells, and cell debris, showing the interaction between the two compounds (Figure 5G and H).
Discussion
Statins are used for treatment antihyperlipidemic effects in patients with high cholesterol by competitively inhibiting the enzyme HMG-CoA reductase, decreasing cholesterol biosynthesis.5 Simvastatin is also known to present pleiotropic effects, such as anti-inflammatory, antithrombotic, and antimicrobial effect.6–8 and shows hepatocyte cytotoxicity.43 Therefore, the combination and consequent decrease in the concentrations of simvastatin have advantages in reducing cytotoxicity.
Results obtained by disk diffusion assay showed no inhibition by simvastatin in any bacteria tested. Antibacterial activity was demonstrated by broth microdilution assay, showing an inhibitory effect of simvastatin against MSSA and MRSA strains. Several studies have performed only disk or well diffusion into agar for evaluation of antimicrobial activity, mainly assay with nanoparticles, but some compounds do not diffuse into agar very well, other methods being recommended such as microdilution in broth and time-kill curves.22 Our results showed small halos (inhibition zones) for AgNPbio and none for simvastatin, but high antibacterial activity in the broth microdilution and time-kill curves. This method can be performed as screening assay and other techniques are recommended for complete evaluation for antibacterial activity.
Several studies have shown that simvastatin has an antibacterial effect against S. aureus strains, with MIC ranging from 0.050 mg mL−1 to 0.300 mg mL−1.8,44–46 Results obtained in the literature show that MIC values are similar to those obtained in our study. In general, MIC values are higher in MRSA than MSSA, although this was not observed in our study. The results obtained for simvastatin against E. coli (ESBL 176 and ATCC 25922) demonstrated no antibacterial effect. A recent study obtained the same results: simvastatin showed no antibacterial effect against Gram-negative bacteria.47 Structural differences between Gram-positive and Gram-negative bacteria could lead to the difference in the antibacterial activity of simvastatin. According to a previous study, statins have a bacteriostatic effect against S. aureus strains,46 and similar results were obtained in the present study, where simvastatin showed growth inhibition of the MRSA tested.
Our results demonstrated an inhibitory effect of AgNPbio against S. aureus and E. coli. The broad spectrum of antibacterial activity of AgNPbio could be due to different targets in bacteria cells such as DNA, vital enzymes, and cell membrane, structures present in both Gram-positive and Gram-negative bacteria.16 The same inhibitory effects against S. aureus and E. coli were reported in earlier studies.48,49 Our silver nanoparticles are biogenic (by F. oxysporum) with an average diameter of 77.68 ± 33.95 nm. AgNPbio MIC values were 212.33 mg mL−1 for S. aureus and 106.16 mg mL−1 for E. coli strains, keeping the same MIC for more than one year (data not shown). MIC value was lower for E. coli than S. aureus strains, showing that the antibacterial effect of AgNPbio was higher for E. coli strains (Gram-negative). Gram-positive and Gram-negative bacteria present differences in structure such as peptidoglycan (cell wall) and this difference may interfere in antibacterial activity in Gram-positive bacteria (peptidoglycan thicker). Other factors that influence the antibacterial activity of AgNPbio are size, morphology, and coating of nanoparticles. AgNPbio with smaller sizes are more efficient than nanoparticles of larger size.16,50,51
Resistance to antimicrobials reduces the range of treatment options by increasing the cost and making it more difficult to eliminate microorganisms through enhanced severity of infections.1 Therefore, there is a necessity to discover new treatment options. Studies have recommended the combination of drugs as a strategy to control antimicrobial resistance.52,53 Studies involving synergism have been especially important for multidrug-resistant bacteria therapy.17,31 Therefore, there is a need to search for new antibiotics. Regarding the search for new treatments, in addition to the investigation of new drugs, combinations with AgNP and another compound such as amoxicillin,18 cinnamaldehyde,32 eugenol,31 oregano essential oil,14 and phenazine-1-carboxamide12 have demonstrated better antibacterial action when combined. Simvastatin when combined with AgNPbio presents a new treatment option against infections caused mainly by S. aureus and E. coli. Our results showed that simvastatin when used alone has a bacteriostatic effect, not being as effective against bacteria. Although AgNPbio has a bactericidal effect when used alone, bacterial resistance has been described.28 The use of the combination, in addition to improving activity, decreases the time of action and concentration, and minimizes the occurrence of resistance (lower dose). This combination has a greater effect than isolated drugs, establishing a new perspective for treating infections.
A recent study shows antifungal activity of the simvastatin combined with AgNPbio synthetized by F. oxysporum against species of Aspergillus.33 This study reported, for the first time, combination of statins and nanoparticles against bacteria including multidrug-resistant bacteria strains. In the checkerboard test, the combination of simvastatin and AgNPbio showed a synergistic effect against MSSA ATCC 25923 and MRSA N315, demonstrating that the combination decreased to 75% the concentration used to eliminate these bacteria. In addition, the combination of both compounds against MRSA BEC 9393 and MSSA ATCC 29213 presented an additive antibacterial effect, decreasing by 50% the concentration to eliminate these bacteria. Our results showed that simvastatin used in combination with AgNPbio caused a 2-log decrease in the bacterial population at 4 hrs when used against MRSA. The MIC values decreased 4-fold for both, indicating a synergistic effect between the two compounds. When the combination was used, all bacteria were eliminated within 10 hrs, which was less than half the time observed for separate applications.
Studies have used AgNPbio with other compounds, showing a synergistic interaction effect. The combination of AgNPbio with phenazine-1-carboxylic caused a decrease in MIC.12 AgNPbio in combination with eugenol showed a synergistic interaction effect against S. agalactiae.31 AgNPbio combined with oregano essential oil demonstrated a synergistic and additive effect against multidrug-resistant bacterial strains.14 Simvastatin, when combined with AgNPbio, showed a synergistic effect against some strains of S. aureus, including MRSA. The synergic effect of the simvastatin with AgNPbio decreased the inhibitory concentration to 4-fold for both compounds, also therapeutic concentration.
Another important aspect of this synergism is the toxicity of simvastatin and AgNPbio. Simvastatin has presented hepatotoxicity in humans.54 In relation to AgNPbio, there is also concern with the environment. Thus, our results showed that low concentrations of both compounds had a higher effect than alone (synergism).22 In HRBC cells, simvastatin shows a CC50 in concentration of 0.260 mg mL−1 and AgNPbio CC50 of 9283.4 mg mL−1. Results of SI showed viable application in medical area.
There are no reports of SEM in the literature of bacteria treated with simvastatin. Our results showed morphological alteration in the bacterial cells similar to an amorphous mass. Through SEM, we observed cellular morphological alterations within a few hours of incubation with treatments of simvastatin, AgNPbio, and a combination of both compounds. A previous study obtained images of samples treated with AgNPbio showing a formation of protrusions on the surface of most cells. Studies have demonstrated that the cytoplasmic material is lost, suggesting that AgNPbio interferes with the permeability of the bacterial cell membrane.14,31,55 In our study, treatment with AgNPbio caused the formation of protrusions of numerous small bubbles a few nanometers in size, numerous lysed cells, and cell debris, suggesting the similar mechanism of action. In SEM, the combination of AgNPbio and simvastatin showed cellular morphological alterations characteristic of both compounds. Studies using the combination of AgNPbio and other compounds obtained similar results.14,31
Conclusion
In conclusion, our study showed synergism interactions effect between simvastatin and AgNPbio synthetized by F. oxysporum, on antibacterial activity against a MRSA N315. These data suggest that the combination of these compounds is a possible treatment option for fighting resistant bacterial infections. In addition, it was possible to observe different cell changes under simvastatin and AgNPbio by SEM.
The combination of simvastatin and AgNPbio has potential to be applied in industry (pharmaceutical) and hospitals (impregnated in materials and treatment of wounds and burns infections).
Mechanism of action and physical-chemical compatibility of the combination of compounds are the next steps of our group.
Acknowledgments
This study was supported by CNPq BIOTEC 402728/2013-0 and CAPES, which made this study possible. Support from INOMAT (MCTI/CNPq), NanoBioss (MCTI), and the Brazilian Network of Nanotoxicology (MCTI/CNPq) is also acknowledged. The authors would also like to thank the Laboratory for Electron Microscopy and Microanalysis – LMEM/Universidade Estadual de Londrina and Central de Microscopia – COMCAP/Universidade Estadual de Maringá for help with the electron microscopy experiments. We thank Dr. Elsa Masae and Dr. Agnes Marie Sá Figueiredo who donated the MRSA N315 and MRSA BEC9393 strains. Dr. A. Leyva helped with English editing of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. Rev Antimicrob Resist. 2016;1–80.
2. Cantas L, Shah SQA, Cavaco LM, et al. A brief multi-disciplinary review on antimicrobial resistance in medicine and its linkage to the global environmental microbiota. Front Microbiol. 2013;4:1–14. doi:10.3389/fmicb.2013.00077
3. Silva KC, Lincopan N. Epidemiologia das betalactamases de espectro estendido no Brasil: impacto clínico e implicações para o agronegócio. J Bras Patol E Med Lab. 2012;48(2):91–99. doi:10.1590/S1676-24442012000200004
4. Bodnar GC, Martins HM, De Oliveira CF, et al. Comparison of HRM analysis and three REP-PCR genomic fingerprint methods for rapid typing of MRSA at a Brazilian hospital. J Infect Dev Ctries. 2016;10(12):1306–1317. doi:10.3855/jidc.7887
5. Graziano TS, Cuzzullin MC, Franco GC, et al. Statins and antimicrobial effects: simvastatin as a potential drug against Staphylococcus aureus biofilm. PLoS One. 2015;10(5):1–17. doi:10.1371/journal.pone.0128098
6. Friesen JA, Rodwell VW. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol. 2004;5(11):248. doi:10.1186/gb-2004-5-11-248
7. Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4(12):977–987. doi:10.1038/nrd1876
8. Jerwood S, Cohen J. Unexpected antimicrobial effect of statins. J Antimicrob Chemother. 2008;61(2):362–364. doi:10.1093/jac/dkm496
9. Chen X, Schluesener HJ. Nanosilver: a nanoproduct in medical application. Toxicol Lett. 2008;176(1):1–12. doi:10.1016/j.toxlet.2007.10.004
10. Ansari MA, Khan HM, Khan AA, Cameotra SS, Saquib Q, Musarrat J. Gum arabic capped-silver nanoparticles inhibit biofilm formation by multi-drug resistant strains of Pseudomonas aeruginosa. J Basic Microbiol. 2014;54(7):688–699. doi:10.1002/jobm.v54.7
11. Bibbs RK, Harris RD, Peoples VA, et al. Silver polyvinyl pyrrolidone nanoparticles exhibit a capsular polysaccharide influenced bactericidal effect against Streptococcus pneumoniae. Front Microbiol. 2014;5:1–8. doi:10.3389/fmicb.2014.00547
12. Cardozo VF, Oliveira AG, Nishio EK, et al. Antibacterial activity of extracellular compounds produced by a Pseudomonas strain against methicillin-resistant Staphylococcus aureus (MRSA) strains. Ann Clin Microbiol Antimicrob. 2013;12:12. doi:10.1186/1476-0711-12-12
13. Palanisamy NK, Ferina N, Amirulhusni AN, et al. Antibiofilm properties of chemically synthesized silver nanoparticles found against Pseudomonas aeruginosa. J Nanobiotechnology. 2014;12:2. doi:10.1186/1477-3155-12-2
14. Scandorieiro S, De Camargo LC, Lancheros CAC, et al. Synergistic and additive effect of oregano essential oil and biological silver nanoparticles against multidrug-resistant bacterial strains. Front Microbiol. 2016;7. doi:10.3389/fmicb.2016.00760
15. Singh K, Panghal M, Kadyan S, Chaudhary U, Yadav J. Green silver nanoparticles of Phyllanthus amarus: as an antibacterial agent against multi drug resistant clinical isolates of Pseudomonas aeruginosa. J Nanobiotechnology. 2014;12(1):40. doi:10.1186/s12951-014-0040-x.
16. Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346–2353. doi:10.1088/0957-4484/16/10/059
17. Lok C, Ho C, Chen R, et al. Proteomic analysis of the mode of antibacterial action of silver. J Proteome Res. 2006;5:916–924. doi:10.1021/pr0504079
18. Li P, Li J, Wu C, Wu Q, Li J. Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology. 2005;16(9):1912–1917. doi:10.1088/0957-4484/16/9/082
19. Durán N, Durán M, de Jesus MB, Seabra AB, Fávaro WJ, Nakazato G. Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomedicine. 2015;12(3):789–799. doi:10.1016/j.nano.2015.11.016.
20. Herman A, Herman AP. Nanoparticles as antimicrobial agents: their toxicity and mechanisms of action. J Nanosci Nanotechnol. 2014;14(1):946–957. doi:10.1166/jnn.2014.8735.
21. Seabra A, Durán N. Nanotoxicology of metal oxide nanoparticles. Metals (Basel). 2015;5(2):934–975. doi:10.3390/met5020934
22. Durán N, Nakazato G, Seabra AB. Antimicrobial activity of biogenic silver nanoparticles, and silver chloride nanoparticles: an overview and comments. Appl Microbiol Biotechnol. 2016;100(15):6555–6570. doi:10.1007/s00253-016-7657-7
23. Durán N, Seabra AB. Metallic oxide nanoparticles: state of the art in biogenic syntheses and their mechanisms. Appl Microbiol Biotechnol. 2012;95(2):275–288. doi:10.1007/s00253-012-4118-9
24. Ingale AG, Chaudhari AN. Biogenic synthesis of nanoparticles and potential applications: an eco- friendly approach. J Nanomed Nanotechnol. 2013;04(02). doi:10.4172/2157-7439.1000165
25. Jelenko C. Silver nitrate resistant E. coli: report of case. Ann Surg. 1969;170(2):296–299. doi:10.1097/00000658-196908000-00021
26. Haefeli C, Franklint C, Hardy K, Biogen SA, Acacias R. Plasmid-determined silver resistance in Pseudomonas stutzeri isolated from a silver mine. J Bacteriol. 1984;158(1):389–392.
27. McHugh GL, Hopkins CC, Moellering RC, Swartz MN. Salmonella typhymurium resistant to silver nitrate, chloramphenicol, and ampicillin. Lancet. 1975;305:235–240.
28. Graves JL, Tajkarimi M, Cunningham Q, et al. Rapid evolution of silver nanoparticle resistance in Escherichia coli. Front Genet. 2015;5:1–13.
29. Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, Venketesan R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomedicine. 2010;6(1):103–109. doi:10.1016/j.nano.2009.04.006
30. Jain J, Arora S, Rajwade JM, Omray P, Khandelwal S, Paknikar KM. Silver nanoparticles in therapeutics: development of an antimicrobial gel formulation for topical use. Mol Pharm. 2009;6(5):1388–1401. doi:10.1021/mp800174g
31. Biasi-garbin RP, Otaguiri ES, Morey AT, et al. Effect of eugenol against Streptococcus agalactiae silver nanoparticles. Evid Based Complement Alternat Med. 2015;2015:1–8. doi:10.1155/2015/861497
32. Ghosh IN, Patil SD, Sharma TK, Srivastava SK, Pathania R, Navani NK. Synergistic action of cinnamaldehyde with silver nanoparticles against spore-forming bacteria: a case for judicious use of silver nanoparticles for antibacterial applications. Int J Nanomedicine. 2013;8:4721–4731. doi:10.2147/IJN.S37465
33. Bocate KP, Reis GF, de Souza PC, et al. Antifungal activity of silver nanoparticles and simvastatin against toxigenic species of Aspergillus. Int J Food Microbiol. 2019;291:79–86. doi:10.1016/j.ijfoodmicro.2018.11.012
34. Durán N, Marcato PD, Alves OL, De GIH, Esposito E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Nanobiotechnology. 2005;7:1–7.
35. Bauer AW, Kirby MWM, Jherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–496. doi:10.1093/ajcp/45.6_ts.764
36. CLSI. M100-S23 Performance Standards for Antimicrobial Susceptibility Testing; Vol 23; 2013.
37. CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Vol. 32; 2012:69
38. Kelly MT, Matsen JM. Testing parameters of amikacin, with comparisons to other aminoglycoside antibiotics in vitro activity, synergism, and testing parameters of amikacin, with comparisons to other aminoglycoside antibiotics. Antimicrob Agents Chemother. 1976;9(3):440–447. doi:10.1128/AAC.9.3.440
39. Chin NX, Weitzman I. In vitro activity of fluvastatin, a cholesterol-lowering agent, and synergy with flucanazole and itraconazole against Candida species and Cryptococcus neoformans. Antimicrob Agents Chemother. 1997;41(4):850–852. doi:10.1128/AAC.41.4.850
40. NCCLS. Methods for Determinating Bactericidal Activity of Antimicrobial Agents: Aproved Guideline. NCCLS, Vol. 19; 1999:50
41. Izumi E, Veiga VF, Pinto AC, Nakamura CV. Terpenes from copaifera demonstrated in vitro antiparasitic and synergic activity. J Med Chem. 2012;55(7):2994–3001.
42. Gonçalves A, Oliveira D, Sayuri L, et al. Evaluation of the antibiotic activity of extracellular compounds produced by the Pseudomonas strain against the Xanthomonas citri pv. Citri 306 Strain. Biol Control. 2011;56:125–131. doi:10.1016/j.biocontrol.2010.10.008
43. Abdoli N, Heidari R, Azarmi Y, Eghbal MA. Mechanisms of the statins cytotoxicity in freshly isolated rat hepatocytes. J Biochem Mol Toxicol. 2013;29(4):165–172.
44. Çoban AY, Tekeli HO, Guney AK, Durupinar B. Investigation of the in vitro antibacterial effects of statins. Mikrobiyol Bul. 2010;161–163.
45. Masadeh M, Mhaidat N, Alzoubi K, Al-azzam S, Alnasser Z. Antibacterial activity of statins: a comparative study of Atorvastatin, Simvastatin, and Rosuvastatin. Ann Clin Microbiol Antimicrob. 2012;11:1–5.
46. Wang -C-C, Yang P-W, Yang S-F, Hsieh K-P, Tseng S-P, Lin Y-C. Topical simvastatin promotes healing of Staphylococcus aureus -contaminated cutaneous wounds. Int Wound J. 2015;1–10. doi:10.1111/iwj.12431
47. Thangamani S, Mohammad H, Abushahba MFN, et al. Exploring simvastatin, an antihyperlipidemic drug, as a potential topical antibacterial agent. Sci Rep. 2015;5:1–13. doi:10.1038/srep16407
48. Kim JS, Kuk E, Yu N, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi:10.1016/j.nano.2006.12.001
49. Mirzajani F, Ghassempour A, Aliahmadi A, Esmaeili MA. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Res Microbiol. 2011;162(5):542–549. doi:10.1016/j.resmic.2011.04.009
50. Panacek A, Kvítek L, Prucek R, et al. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem. 2006;33:16248–16253. doi:10.1021/jp063826h
51. Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. J Biol Chem. 2015;290(42):1712–1720.
52. Bollenbach T. Antimicrobial interactions: mechanisms and implications for drug discovery and resistance evolution. Curr Opin Microbiol. 2015;27:1–9. doi:10.1016/j.mib.2015.05.008
53. Fischbach MA. Combination therapies for combating antimicrobial resistance. Curr Opin Microbiol. 2011;14(5):519–523. doi:10.1016/j.mib.2011.08.003
54. Stossel TP. The Discovery of Statins. BenchMarks. 2008;903–905.
55. Kim SH, Lee HS, Ryu DS, Choi SJ, Lee DS. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J Microbiol Biotechnol. 2011;39(1):77–85.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.