Back to Journals » Journal of Pain Research » Volume 15
Neutrophil-to-Lymphocyte Ratio as an Indicator of Opioid-Induced Immunosuppression After Thoracoscopic Surgery: A Randomized Controlled Trial
Authors Chen Q , Liang J, Liang L, Liao Z, Yang B, Qi J
Received 16 April 2022
Accepted for publication 27 June 2022
Published 30 June 2022 Volume 2022:15 Pages 1855—1862
DOI https://doi.org/10.2147/JPR.S371022
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ellen M Soffin
Qi Chen,1,* Jingqiu Liang,2,* Ling Liang,2 Zhongli Liao,2 Bin Yang,3 Jun Qi2
1Department of Anesthesiology, Chongqing University Cancer Hospital, Chongqing, People’s Republic of China; 2Chongqing Cancer Multi-Omics Big Data Application Engineering Research Center, Chongqing University Cancer Hospital, Chongqing, People’s Republic of China; 3Department of Anesthesiology, the First Affiliated Hospital of Xiamen University, Xiamen, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bin Yang, Department of Anesthesiology, the First Affiliated Hospital of Xiamen University, Xiamen, People’s Republic of China, Email [email protected] Jun Qi, Chongqing Cancer Multi-Omics Big Data Application Engineering Research Center, Chongqing University Cancer Hospital, Chongqing, People’s Republic of China, Email [email protected]
Purpose: The neutrophil-to-lymphocyte ratio (NLR) is a useful prognostic marker for various diseases and surgery-induced immunosuppression. While opioids are important in general anesthesia, the association between immediate perioperative immune monitoring and opioid consumption for postoperative analgesia after video-assisted thoracoscopic surgery (VATS) is unknown. We aimed to investigate the effect of analgesic techniques on opioid-induced immune perturbation, and the feasibility of NLR as an indicator of opioid-induced immune changes.
Patients and Methods: Patients were randomly assigned to two groups: Group P (n=40) or Group C (n=40). Patients in group P received ultrasound-guided paravertebral block (PVB) before surgery, and followed by sufentanil patient-controlled intravenous analgesia (PCIA) after surgery, and group C received sufentanil PCIA only. The total and differential white blood cell counts, including CD4+ T lymphocyte counts, CD8+ T lymphocyte were recorded before surgery and at 24 and 72 hours after surgery. NLR was determined using the frequencies of lymphocyte subpopulations. The cumulative dose of sufentanil were recorded at 24 and 24h after surgery while the 40-item quality of recovery questionnaire (QoR-40) score were assessed at 48h after the surgery.
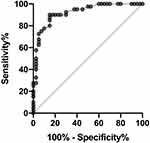
Results: At 24 and 48 hours after surgery, a lower sufentanil consumption, and higher QoR-40 recovery scores were found in group P than in group C (P< 0.05). In biochemical analyses, the values of NLR were lower in group P compared to group C (p< 0.0001) and ratio of CD4/CD8 were higher in group P compared to group C (p< 0.05) on day three after surgery. NLR showed excellent predictive capability for immunosuppression, with an area under the curve (AUC) of 0.92 [95% confidence interval (CI), 0.86– 0.98, P < 0.0001].
Conclusion: Opioid-sparing pain management strategies may affect postoperative immunosuppression and NLR could be a reliable indicator of opioid-related immunosuppression. Moreover, opioid-sparing pain management strategies could improve patient’s satisfaction in VATS.
Keywords: neutrophil-to-lymphocyte ratio, immunosuppression, paravertebral block, opioid, analgesia
Introduction
Inflammation seems to be one of the most important perioperative factors for cancer recurrence, especially in kidney, lung, and breast tissues.1,2 Animal models and retrospective clinical data suggest that regional anesthesia, particularly central blocks, can attenuate immunosuppression and minimize inflammation after cancer surgery.3,4 Various inflammatory markers have been widely used in cancer patients, such as prognostic index (PI), prognostic nutritional index (PNI), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), CD4+ T lymphocyte counts, modified Glasgow prognostic score (mGPS).5,6 Meanwhile, mGPS and NLR have the moderate predictive ability in overall survival and disease-free survival of oesophageal cancer.7 In the absence of pain, opioids induce a decrease in natural killer (NK) cell activity and may be responsible for opioid-induced immunosuppression.8 Data on synthetic opioids have revealed a phenomenon similar to that of fentanyl and sufentanil.9 Neutrophils are critical in surveilling circulating tumor cells to enable cell cycle progression and anti-tumor immune response.10 NLR is a useful marker to predict inflammation and surgery-induced immunosuppression which reflects trends in both neutrophils and lymphocytes, combining the strengths of both systems rather than a single one.11,12 Whether it is associated with opioid-related immunosuppression after thoracoscopic surgery is unknown. We investigated the effect of analgesic techniques on opioid-induced immune perturbation in patients undergoing video-assisted thoracoscopic surgery (VATS), and the feasibility of NLR as an indicator of opioid-induced immune changes.
Materials and Methods
Study Design
Eighty patients in the age range of 18–65 years, with American Society of Anesthesiologists (ASA) class I–III status and body mass index (BMI) of 18.5–28.0, who received thoracoscopic lobectomy were enrolled finally. All surgeries were performed using a lateral chest wall incision with unilaterally inserted drainage tube. Patients with infection at the injection site, bleeding diathesis, opioid dependence, neuropathies, or psychiatric illnesses which could distracted perception and pain assessment were excluded from the study (Figure 1). Preoperative guidance on QoR-40 questionnaire evaluation was provided by the same anesthesiologist. The patients were randomly allocated to one of the two study groups using random numbers in a one-to-one ratio. The numbers for group allocation were concealed in sealed opaque envelopes and investigator opened the envelopes after anesthesia induction.
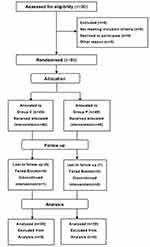 |
Figure 1 CONSORT flow diagram showing the number of patients at each phase of the study. |
Anesthesia Procedure
General anesthesia with tracheal intubation were taken after 1–2 mg midazolam and 0.3–0.4 μg/kg sufentanil along with target-controlled infusion of 3–4 ng/min propofol and 0.8 mg/kg rocuronium.
After the onset of muscle relaxation, the left-side double-lumen endotracheal tube (32–35 Fr for women and 35–37 Fr for men) was inserted under video laryngoscopy. The appropriate depth of double-lumen endotracheal tube was checked immediately after intubation using an Olympus BF 3C30-type fibreoptic bronchoscope (FOB). After anesthesia induction, the patients were placed carefully in the lateral decubitus position and thoracic paravertebral nerve block (TPVB) with ultrasound guidance was performed in all patients in group P by the same investigator. Under sterile conditions, the costal space corresponding to the surgical incision was located using ultrasound probe (Mindray M9 super, Shenzhen, China; linear high-frequency probe, 6–13 MHz). The ultrasound probe was placed on the midline in the craniocaudal direction, showing an image of the spinous process. The probe could be moved laterally to identify the hyperechoic transverse process, slidable parietal pleura and superior costotransverse ligament. The space between the pleura and the superior costotransverse ligament was injected with 20 mL of 0.3% ropivacaine (AstraZeneca AB, Sodertalje, Sweden). General anesthesia was maintained with sevoflurane and remifentanil in conjunction with intermittent cisatracurium, which maintained a minimum alveolar concentration of 1–1.5. According to the grouping results, anesthesiologists used different analgesic strategies during the operation. In group C, sufentanil was administered intermittently according to the condition of the patients during the operation. The total dose of sufentanil was maintained at 0.8–0.9 µg/kg. In group P, sufentanil was administered only before the end of the operation. Its total dose was maintained below 0.5 µg/kg. The neuromuscular block was antagonized with 0.04 mg/kg neostigmine and 0.01 mg/kg atropine if needed. The trachea was extubated when the patients were fully awake and breathing adequately in the postanesthesia care unit (PACU). Once the VAS scores expressed by the patients were ≥3, patient-controlled intravenous anesthesia (PCIA) was programmed to deliver 2 μg of sufentanil boluses with a lockout interval of 10 minutes. No background infusion was allowed in both groups. If the patient’s VAS score> 4, additional flurbiprofen axetil 50mg will be given.
Outcome Measures
The primary outcome measures of the study were NLR three days before surgery, one day and three days after surgery, and the dose of remedial analgesics at 24 and 48h postoperatively. The secondary outcome measures were ratio of CD4/CD8 one day and three days after surgery and the QoR-40 scores at 48h postoperatively.
Blood Sample Analysis
All complete blood cell (CBC) counts, CD4+T lymphocyte counts and CD8+T lymphocyte counts were recorded three days prior to surgery and one day and three days after surgery. Automated hematology analyzer XE- 5000 (SYSMEX K1000 hematology analyzer; Medical Electronics, Kobe, Japan) were used for measuring the CBC in ethylenediaminetetraacetic acid-treated blood. Peripheral blood lymphocytes were counted using Sysmex. Flow cytometry (FACSCanto, BD) was used to determine the proportion of CD4+ T lymphocytes and CD8+ T lymphocytes.
Statistical Analysis
SPSS (version 21.0; IBM Corporation, Armonk, NY, USA) was used statistical analysis. Normally, distributed interval data (age, weight, height, BMI, time of surgery and QoR-40 scores) were reported as means ± standard deviations (SDs) and analyzed by the Student’s unpaired t-tests. Non-normally distributed interval and ordinal data (cumulative of remedial sufentanil) were reported as median values with interquartile and analyzed by the Mann–Whitney U-tests. Biochemical indicators at different times were compared with baseline using repeated-measures ANOVA, followed by the Student’s paired t-tests. Post-hoc analysis with Bonferroni correction was applied for multiple comparisons. Statistical significance threshold was set at p < 0.05.
The sample size was calculated from a pilot study.13 Given the mean sufentanil consumption of 20.4 µg with SD of 5.9 µg, for a 50% difference in the 24-hour postoperative sufentanil consumption at a significance level of 0.05 and power of 0.8, a minimum of 35 patients were required in each group. To account for possible dropouts, 80 patients were recruited for this randomized study.
Results
Two study groups were comparable in terms of age, height, weight, ASA physical status, smoking history, and duration of surgery (Table 1). One patients were excluded from the study due to refused to continue participating in the trial after surgery in group C while one patients were excluded from the study due to acute coronary syndrome occurred after operation and entered ICU for treatment in group P. Total sufentanil consumption in group P was significantly reduced in comparison to that of group C at 24 hours (14 µg vs 26 µg) and 48 hours (52 µg vs 68 µg) after surgery (Table 2). The ratio of CD4/CD8 were decreased significantly in both groups one day after operation when compared with preoperation, but the postoperative ratio of CD4/CD8 in group P were higher than those in group C three days after operation (p<0.05). Meanwhile, NLR were increased significantly in both groups one day after operation but were significantly decreased in group P when compared to those in group C on day three after surgery (p<0.0001) (Table 3). Using ratio of CD4/CD8 as the gold standard for immunosuppression, the ROC curve of NLR and immunosuppression is shown in Figure 2). NLR showed excellent predictive capability for immunosuppression, with an area under the curve (AUC) of 0.92 [95% confidence interval (CI), 0.86–0.98, P < 0.0001].
 |
Table 1 Characteristic of Participants |
 |
Table 2 Total Analgesic Requirement After Surgery |
 |
Table 3 Perioperative Changes of Immunocyte in Patients |
The assessment of recovery after surgery shows that patients in group P had better QoR-40 scores than those in group C on day two after surgery (Table 4, P<0.05). The incidence of postoperative nausea and vomiting (PONV) was recorded and assessed using a QoR-40 questionnaire. As noted, we did not observe any obvious delay in the discharge of the patients from the PACU and no patients used flurbiprofen axetil as rescue analgesia postoperatively in either group.
 |
Table 4 QoR-40 Questionnaire Scores |
Discussion
In this study, we evaluated the effects of perioperative opioid use on immunological functions by using the NLR value. NLR is regarded as a reliable indicator of opioid-induced immune changes. We confirmed that the reduction in perioperative opioids by regional block may have a positive effect on the reduction of immunosuppression and improve the quality of postoperative recovery.
Opioids have been a major focus of medical research because of their critical role in pain management, especially in anesthesia during perioperation.14,15 Opioids can produce powerful analgesia, which is effective in treating severe pain. However, it has some common adverse effects, one of which is related to immune function.16,17 While it is believed that most opioids suppress the immune system, recent research indicates that they may have various effects on immune function.18,19 However, the mechanisms by which opioids and opioid receptors regulate immune responses are still not clearly. Recent literature suggests that morphine-induced inhibition of NK cell activity may be a consequence of opioid receptor activation in the central nervous system. This impact on NK cell activity seems to be related to the dose of opioids.20,21 In our study, regional anesthesia was used to ensure adequate analgesia while reducing the use of opioids to observe the effect of different doses of opioids on postoperative immunity.
CD4+ regulatory T cells and NK cells play important roles in the immune system.22 In our study, although the ratio of CD4/CD8 were decreased significantly in both groups one day after operation when compared with preoperation, the postoperative ratio of CD4/CD8 in group P were higher than those in group C three days after operation. These findings suggest that reducing the use of opioids may alleviate immunosuppression in the body. At the same time, NLR in group P also decreased significantly three days after the operation, suggesting that NLR can also be used as an indicator of opioid-induced immune changes. Frequencies of leukocytes and their subtypes are well-known inflammatory markers.23 In recent years, some studies have investigated the potential diagnostic role of NLR in the inflammatory processes of different chronic diseases.24,25 NLR is a readily available and inexpensive marker of systemic inflammation. This indicator is driven by elevated concentrations of circulating cytokines. It has been shown to modulate myocardial injury and used for risk stratification in different diseases. This is the first study to investigate the relationship between NLR and opioid immunosuppression. It also demonstrated the effect of multimodal analgesia for opioid-sparing in reducing perioperative immunosuppression.
Our study has several limitations. First, we observed only the acute immunosuppressive period. Therefore, the long-term impact of opioids on immune function remains to be determined. Second, the relationship between postoperative opioid-induced immunosuppression and the incidence of related complications has not been recorded and requires further investigation.
Conclusion
NLR might be a reliable indicator of opioid-related immunosuppression after surgery with its advantage of rapid, easy and cost-effective. Meanwhile, opioid-sparing pain management strategies may affect postoperative immunosuppression and improve patient’s satisfaction in thoracoscopic surgery.
Abbreviations
ASA, American Society of Anesthesiologists; BMI, body mass index; CBC, complete blood count; mGPS, modified Glasgow prognostic score (mGPS); NK, natural killer; NLR, neutrophil to lymphocyte ratio; PACU, postanesthesia care unit; PCIA, patient-controlled intravenous analgesia; PI, prognostic index (PI); PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; PONV, postoperative nausea and vomiting; PVB, paravertebral block; QoR-40, 40-item quality of recovery questionnaire; SD, standard deviation; TPVB, thoracic paravertebral block; VATS, video-assisted thoracoscopy.
Data Sharing Statement
When needed, we can provide our original data, such as dose of sufentanil, values of NLR, CD4 and CD8. The data will be available for anyone who wishes to access them for academic purpose. The data will be accessible from immediately following publication to 6 months after publication by sending email to corresponding author via [email protected].
Ethics Approval and Informed Consent
This prospective, randomized, double-blind clinical trial was approved by the Ethics Committee of Chongqing University Cancer Hospital China (2019-177). The study was conducted in accordance with the Declaration of Helsinki and implemented from January 2020 to August 2020. It was also registered at www.clinicaltrials.gov (ChiCTR1900027208). Participants who underwent elective thoracoscopic surgery were provided with written informed consent prior to surgery.
Consent for Publication
Written consent was obtained from each of three patients for all procedures and publication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Laboratory Open Fund of Chongqing University Cancer Hospital (2021); High-Level Medical Personnel Training Project of Chongqing (2019GDRC017); Basic science and advanced technology foundation of Chongqing Science and Technology Commission (cstc2018jcyjAX0775).
Disclosure
The authors declare no conflicts of interest.
References
1. Kinoshita T, Goto T. Links between inflammation and postoperative cancer recurrence. J Clin Med. 2021;10(2):228. doi:10.3390/jcm10020228
2. García-López L, Adrados I, Ferres-Marco D, et al. A blueprint for cancer-related inflammation and host innate immunity. Cells. 2021;10(11):3211. doi:10.3390/cells10113211
3. Muncey AR, Patel SY, Whelan CJ, et al. The intersection of regional anesthesia and cancer progression: a theoretical framework. Cancer Control. 2020;27(1):1073274820965575. doi:10.1177/1073274820965575
4. Byrne K, Levins KJ, Buggy DJ. Can anesthetic-analgesic technique during primary cancer surgery affect recurrence or metastasis? Can J Anaesth. 2016;63(2):184–192. doi:10.1177/1073274820965575
5. Hayama T, Ozawa T, Asako K, et al. Impact of colon cancer location on the prognostic significance of nutritional indexes and inflammatory markers. Vivo. 2021;35(2):1261–1269. doi:10.21873/invivo.12377
6. Dolan RD, McSorley ST, Park JH, et al. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: comparison of composite ratios and cumulative scores. Br J Cancer. 2018;119(1):40–51.
7. Jiang Y, Xu D, Song H, et al. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: a systematic review and meta-analysis. BMJ Open. 2021;11(9):e048324. doi:10.1136/bmjopen-2020-048324
8. Kim R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med. 2018;16(1):8. doi:10.1186/s12967-018-1389-7
9. Wen S, Jiang Y, Liang S, et al. Opioids regulate the immune system: focusing on macrophages and their organelles. Front Pharmacol. 2022;12:814241. doi:10.3389/fphar.2021.814241
10. Cho U, Park HS, Im SY, et al. Prognostic value of systemic inflammatory markers and development of a nomogram in breast cancer. PLoS One. 2018;13(7):e0200936. doi:10.1371/journal.pone.0200936
11. Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23(34):6261–6272. doi:10.3748/wjg.v23.i34.6261
12. Eisenstein TK. The role of opioid receptors in immune system function. Front Immunol. 2019;12:2904. doi:10.3389/fimmu.2019.02904
13. Gao Z, Xiao Y, Wang Q, et al. Comparison of dexmedetomidine and dexamethasone as adjuvant for ropivacaine in ultrasound-guided erector spinae plane block for video-assisted thoracoscopic lobectomy surgery: a randomized, double-blind, placebo-controlled trial. Ann Transl Med. 2019;7(22):668. doi:10.21037/atm.2019.10.74
14. Kwanten LE, O’Brien B, Anwar S. Opioid-based anesthesia and analgesia for adult cardiac surgery: history and narrative review of the literature. J Cardiothorac Vasc Anesth. 2019;33(3):808–816. doi:10.1053/j.jvca.2018.05.053
15. Malafoglia V, Ilari S, Vitiello L, et al. The interplay between chronic pain, opioids, and the immune system. Neuroscientist. 2021;16:10738584211030493. doi:10.1177/10738584211030493
16. Soffin EM, Lee BH, Kumar KK, et al. The prescription opioid crisis: role of the anaesthesiologist in reducing opioid use and misuse. Br J Anaesth. 2019;122(6):e198–e208. doi:10.1016/j.bja.2018.11.019
17. Machelska H, Celik MÖ. Opioid receptors in immune and glial cells-implications for pain control. Front Immunol. 2020;4; 11:300. doi:10.3389/fimmu.2020.00300
18. Zhang P, Yang M, Chen C, et al. Toll-Like Receptor 4 (TLR4)/Opioid Receptor Pathway Crosstalk and Impact on Opioid Analgesia, Immune Function, and Gastrointestinal Motility. Front Immunol. 2020;11(8):1455. doi:10.3389/fimmu.2020.01455
19. Cascella M, Cuomo A, Bifulco F, et al. Could the perioperative use of opioids influence cancer outcomes after surgery? A scoping review protocol. BMJ Open. 2022;12(3):e054520. doi:10.1136/bmjopen-2021-054520
20. Wodehouse T, Demopoulos M, Petty R, et al. A randomized pilot study to investigate the effect of opioids on immunomarkers using gene expression profiling during surgery. Pain. 2019;160(12):2691–2698. doi:10.1097/j.pain.0000000000001677
21. Maher DP, Walia D, Heller NM. Suppression of human natural killer cells by different classes of opioids. Anesth Analg. 2019;128(5):1013–1021. doi:10.1213/ANE.0000000000004058
22. Zhu X, Zhu J. CD4 T helper cell subsets and related human immunological disorders. Int J Mol Sci. 2020;21(21):8011. doi:10.3390/ijms21218011
23. Mahapatro M, Erkert L, Becker C. Cytokine-mediated crosstalk between immune cells and epithelial cells in the gut. Cells. 2021;10(1):111. doi:10.3390/cells10010111
24. Elias-Oliveira J, Leite JA, Pereira ÍS, et al. NLR and intestinal dysbiosis-associated inflammatory illness: drivers or dampers? Front Immunol. 2020;11(11):1810. doi:10.3389/fimmu.2020.01810
25. Yao C, Liu X, Tang Z. Prognostic role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio for hospital mortality in patients with AECOPD. Int J Chron Obstruct Pulmon Dis. 2017;3(12):2285–2290. doi:10.2147/COPD.S141760
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.