Back to Journals » Journal of Inflammation Research » Volume 16
Neutrophil Extracellular Trap is Surrogate Biomarker for Prognosis and Response to Neoadjuvant Therapy in Locally Advanced Rectal Cancer
Authors Zhong W , Wang Q, Shen X, Lv Y, Sun L, An R, Zhu H, Cai H, Chen G, Liu A, Du J
Received 18 October 2023
Accepted for publication 19 December 2023
Published 28 December 2023 Volume 2023:16 Pages 6443—6455
DOI https://doi.org/10.2147/JIR.S441981
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Wentao Zhong,1– 3,* Qianyu Wang,2,* Xiaofei Shen,4,* Yuan Lv,2,3 Liang Sun,2 Ran An,5 Hongyan Zhu,5 Huiyun Cai,2 Gang Chen,2,3 Aijun Liu,5 Junfeng Du1– 3
1The Second School of Clinical Medicine, Southern Medical University, Guangdong, 510515, People’s Republic of China; 2Department of General Surgery, The 7th Medical Center, Chinese PLA General Hospital, Beijing, 100700, People’s Republic of China; 3Medical Department of General Surgery, The 1st Medical Center, Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China; 4Division of Gastric Surgery, Department of General Surgery, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, 210008, People’s Republic of China; 5Department of Pathology, the 7th Medical Center, Chinese PLA General Hospital, Beijing, 100700, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Aijun Liu, Department of Pathology, The 7th Medical Center, Chinese PLA General Hospital, NO. 5, Nanmen Cang, Beijing, 100700, People’s Republic of China, Email [email protected] Junfeng Du, Department of General Surgery, the 7th Medical Center, Chinese PLA General Hospital, NO. 5, Nanmen Cang, Beijing, 100700, People’s Republic of China, Email [email protected]
Purpose: To demonstrate the intrinsic association of Neutrophil extracellular traps (NETs) with outcome and neoadjuvant therapy response of locally advanced rectal cancer (LARC), and the mechanisms.
Patients and Methods: We enrolled 240 patients with LARC who underwent surgery without neoadjuvant therapy in two independent sets (training and validation), and 153 patients who received neoadjuvant therapy with biopsy followed by surgery. Immunohistochemistry, immunofluorescence staining and bioinformatics analysis were performed in formalin-fixed paraffin-embedded sections. NETs were identified by costaining for myeloperoxidase and citrullinated histone H3.
Results: NETs were associated with recurrence-free survival in the surgical training and validation sets. High-NET density predicted poor postoperative survival of patients with LARC. Multivariate analysis identified NETs, TNM stage, and neutrophil-to-lymphocyte ratio as independent prognostic factors for recurrence-free survival. Low-NETs LARC demonstrated increased CD8+ T cell and lower T regulatory cell infiltration, which indicated a tumor immune microenvironment with strong antitumor capacity. High-NET density was associated with epithelial–mesenchymal transition, which is considered to contribute to tumor progression. In the neoadjuvant therapy cohort, high-NET density on biopsy was significantly associated with reduced likelihood of complete/near complete response.
Conclusion: NET was an independent prognostic factor in LARC that were associated with patients’ survival, and NET density in pretreatment biopsies was an independent predictive biomarker of response to neoadjuvant therapy. This biomarker may be helpful in predicting survival in LARC with improved accuracy and selecting patients who will respond to neoadjuvant therapy.
Keywords: NETs, tumor microenvironment, locally advanced rectal cancer, outcome
Introduction
According to the latest statistics, colorectal cancer is still one of the most prevalent cancers, with the third highest incidence and mortality worldwide,1 both in men and women. Locally advanced rectal cancer (LARC) is defined as TNM stage II and III, and accounts for ~70% of rectal cancer patients. The main treatment of LARC is total mesorectal excision followed by adjuvant chemotherapy. In recent years, neoadjuvant chemoradiotherapy (nCRT) followed by surgery has demonstrated improved survival and outcomes.2,3 By downstaging the tumor, neoadjuvant therapy offers patients greater potential for R0 resection and reduced probability of recurrence. Identification of predictive biomarkers for prognosis and response to neoadjuvant therapy of LARC could offer appropriate personalized therapy. To date, no clinicopathological features have been identified as biomarkers for predicting responses to neoadjuvant therapy of LARC.
Neutrophil extracellular traps (NETs) are unique net-like structures composed mainly of neutrophil-derived DNA, histones, and various granular proteins, including myeloperoxidase (MPO) and neutrophil elastase (NE).4,5 Previously, NETs, like neutrophils, were recognized to play an important role in the immune response against infections and pathogens. There is increasing evidence of the role of neutrophils in tumor progression; therefore, the role of NETs in the biological behavior of tumors has also attracted attention.6,7 Recently, high density of NETs has been detected in various tumor types and metastases, and shown to be associated with tumor stage.8–10 However, the clinicopathological features and prognostic significance of NETs have rarely been investigated in LARC. Here, we aimed to describe the role of NETs in the tumor immune microenvironment and their value as potential biomarkers for predicting outcomes and response to neoadjuvant therapy in patients with LARC.
Materials and Methods
Patient Cohort
This multicenter retrospective study was designed and performed on rectal adenocarcinoma patients who underwent radical resection at the 7th Medical Center of Chinese PLA General Hospital and Nanjing Drum Tower Hospital. Tumors staged in pathological stage II or III [pTNM, stage II (T3–4N0) or stage III (T1–4N1–2)] according to the 8th edition of the American Joint Committee of Cancer (AJCC) TNM staging classification for rectal cancer were defined as LARC. Enhanced computed tomography of the chest and abdomen, and magnetic resonance imaging of the rectum were used to confirm the TNM stage.
The inclusion criteria were as follows: (1) diagnosed with rectal cancer by preoperative biopsy; (2) received R0 resection; (3) the surgical cohort with postoperative pathological staging of LARC, and the neoadjuvant therapy cohort diagnosed with LARC by preoperative imaging data and biopsy; (4) complete medical and follow-up records; (5) included controlled comorbidities. The exclusion criteria were as follows (1) incomplete neoadjuvant therapy; (2) comorbidities with severe infections, autoimmune diseases, and other life-threatening diseases; (3) died within 30 days after surgery; (4) rectal tumor was derived from other tumors; (5) the patient had a history of other tumors. Through the above filters, we enrolled 240 LARC patients in the surgical cohort from June 2015 to December 2017 and 153 patients in the neoadjuvant therapy cohort between June 2018 and December 2020. The period between surgery and tumor recurrence or death was referred to as recurrence-free survival (RFS) and this study was conducted under approval of the Institutional Ethics Committee.
Assessment of Tumor Regression Score
Tumor regression score (TRS) in the biopsies was assessed according to the AJCC and using a four-point system defined as follows: 0, complete response, no viable cancer cells; 1, near complete response, single cells or rare small groups of cancer cells; 2, partial response, residual cancer outgrown by fibrosis; and 3, poor or no response, extensive residual cancer with no evident tumor regression. The TRS of each patient was assessed by at least two pathologists, including the original sign-out pathologist at the time of initial pathological evaluation and one of the authors (Ran An). For some of the subsequent analyses, patients were grouped into responders (TRS 0 or 1) and nonresponders (TRS 2 or 3). Additionally, TRS 0 was considered as pathological complete response (pCR) and TRS 1–3 was incomplete pathological remission (i-pCR).
Given the certification between TRS 1 and TRS 2 may be controversial, therefore, all cases classified as TRS 1 or 2 were reviewed by two pathologists. All cases with discrepancies between any of the pathologists were arbitrated by the third pathologist (Aijun Liu).
Immunofluorescence Staining
Tumors were formalin fixed and paraffin embedded (FFPE) and sectioned (5 μm) by a pathologist. Paraffin-embedded tissue sections were deparaffinized, rehydrated, and antigen was retrieved in EDTA buffer. Slides were incubated with anti-citrullinated histone H3 (CitH3) (1:1000, Abcam, ab5103), anti-MPO (1:1000, Abcam, AF3667) overnight at 4°C. Fluorochrome-conjugated secondary antibodies (1:200, Invitrogen) were added for 1 h incubation. After rinsing counterstaining the nuclei and images were obtained by Olympus Fluoview FV3000 confocal microscope.
Identification and Quantification of NETs
Tumor regions were identified by hematoxylin and eosin staining and these regions were used to obtain the immunofluorescence images through confocal microscopy. Images of tissue sections were obtained by Olympus Fluoview FV3000 confocal microscope and analyzed by QuPath (0.4.1 version) and ImageJ (1.52c version) software. NETs were identified through co-staining for MPO and CitH3, as described previously.11,12 The average NET density was calculated by randomly selecting five images on the same slide. The density of NETs in an area was calculated using the formula: (number of NETs/number of DAPI)×100.
Immunohistochemistry of Tissues Samples
Immunohistochemistry was performed on dewaxed and hydrated 4-μm-thick FFPE sections that were antigen retrieved in EDTA, using E-cadherin (1:800, Servicebio, GB11082), N-cadherin (1:800, Servicebio, GB11135), CD8+ T cells (1:300, Abcam, ab101500), and T regulatory (Treg) cells (1:800, Abcam, ab20034). After blocking with 5% bovine serum albumin (BSA) for 30 min, the sections were incubated overnight at 4°C with primary antibody. On the next day, the sections were incubated with secondary antibody for 1 h at room temperature and diaminobenzidine (DAB) for 3 min followed by hematoxylin staining. All sections were dehydrated and scanned using Leica CS2 and analyzed using Aperio ImageScope.
Gene Expression Analysis
mRNA sequencing of specimens from 49 patients who underwent surgery without neoadjuvant therapy was performed. According to the same criteria, they were divided into low and high NETs groups. We compared the difference between them by conducting bioinformatics analysis in R software. The differentially expressed genes (DEGs) between the two groups were identified using the criteria of |log2FC|≥1 and FDR < 0.05. GO and KEGG pathway enrichment analyses of the DEGs were carried out using the “clusterProfiler” R package. The “GSEA” package was used to investigate the functions correlated with different subgroups of patients. In addition, immune cell infiltration in different groups was analyzed by “ssGSEA” package in R software.
Statistical Analysis
Categorical variables of clinical characteristics in different patient groups are demonstrated in numbers with percentages, using Fish’s exact or chi-square test, as appropriate to determine the difference. Analysis of continuous variables such as NET density and neutrophil-to-lymphocyte ratio (NLR) was performed using the Wilcoxon rank-sum test. Survival curves were calculated using the Kaplan–Meier and Log rank tests. Univariate regression analysis can exclude some irrelevant variables, while multivariate regression analysis can further eliminate the influence of other confounding factors, and the combination of univariate and multivariate regression analyses can identify stable and highly credible predictors of outcome. So, the independent risk factors for prognosis were summarized using univariate and multivariate regression Cox analyses, and logistic regression was used to analyze independent risk factors for neoadjuvant therapy response. Statistical analyses were performed using a combination of GraphPad Prism 9 (version 9.0.0) and R (version 4.2.1) and SPSS (version 26.0). A two-tailed p < 0.05 was regarded as statistically significant.
Results
Identification of NETs in LARC Patients
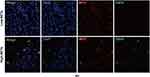
To identify the presence of NETs in LARC, double immunofluorescence analysis of CitH3 and MPO was performed in FFPE tumor tissue samples resected directly from 240 patients and biopsies from 153 nCRT patients. Representative images of NETs in surgical resection specimens are shown in Figure 1, and NETs were mainly present in the tumor stroma rather than in tumor nests.
The relationships between NET density and clinical features of all patients in the surgical cohort are listed in Supplementary Table 1. NET density in the surgical training and validation sets were positively correlated with the NLR level both (p = 0.0334 and p = 0.0063, respectively) and lymph node invasion (p = 0.0032 and p = 0.0464, respectively), but there was no obvious correlation between NETs and neurovascular invasion and tumor grade in either set.
NET Accumulation Was an Independent Predictor of Survival in the Surgical Cohort
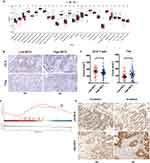
NET density was calculated in surgically resected tumor sections, and patients without neoadjuvant therapy were categorized into the low-NETs group (n = 87) and high-NETs group (n = 86), based on the median NET density (1.67). To investigate the prognostic value of NETs in LARC, we performed Kaplan–Meier analysis on patients who were subgrouped according to NET density. The Log rank test showed that patients with low tumor-infiltrating NETs had significantly improved RFS compared with those with high tumor-infiltrating NETs in the training set (p = 0.0058, Figure 2A) and validation set (p = 0.0012; Figure 2B).
In the univariate and multivariable Cox proportional hazards model of the training set, independent prognostic factors of RFS were tumor-infiltrating NETs (multivariate hazard ratio 1.679, 95% CI 1.010–2.790, p = 0.046), TNM stage (multivariate hazard ratio 1.722, 95% CI 1.005–2.952, p = 0.048) and NLR (multivariate hazard ratio 1.676, 95% CI 1.011–2.779, p = 0.045). Similar results were found in the validation set (Table 1). Based on the multivariate Cox-regression analyses, we constructed a nomogram that included NET density, TNM stage and NLR to predict the 5-year RFS in LARC patients (Figure 2C). The calibration curve showed good agreement on actual and predicted clinical outcomes in the training set and external validation (Figure 2D and E).
 |
Table 1 Cox Proportional Hazards Regression Models for the Predictors of RFS in the Surgical Cohort |
High NET Density Was Associated with Protumor Characteristics
To better understand the prognostic value of NETs, we performed bioinformatics analysis on mRNA expression data in 49 patients who received surgery directly. They were divided into low NET density (n = 25) who were infiltrated with little NETs and high NET density (n = 24) with abundant NETs. The R package “ssGSEA” was used to analyze different immune cells between the two groups. Compared with the low-NETs group, the high-NETs group had compromised immune response indicated by more Treg cell and lower CD8+ T cell infiltration (Figure 3A). This indicated that patients with high intratumoral NET density had a stronger pro-tumor immune response. We confirmed this finding using immunohistochemistry (Figure 3B and C). GO and KEGG analyses on the DEGs of both high- and low-NETs groups were performed, and we found that low-NETs group had more enrichment in IL-17 signaling pathway which is related to anti-tumor and intense immune response (Supplementary Figure 1A–D). Given previous results suggesting a robust association between NETs and epithelial–mesenchymal transition (EMT), we specifically evaluated EMT according to NET density. As expected, analysis of mRNA data and immunohistochemistry showed that high NET density was associated with increased EMT (Figure 3D and E).
NET Density in Pretreatment Biopsy Predicted Response to Neoadjuvant Therapy in LARC
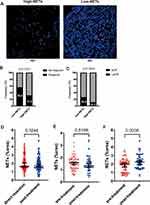
Some patients with LARC receive neoadjuvant therapy before surgery; therefore, we explored the predictive value of NET density for response to neoadjuvant therapy. We enrolled 153 patients with LARC who had pretreatment biopsies and underwent preoperative neoadjuvant therapy. Clinicopathological features are summarized in Supplementary Table 2. They were categorized into high-NETs and low-NETs tumors (Figure 4A), and the response to neoadjuvant therapy was observed in both groups.
In the low-NETs group, there were more patients sensitive to neoadjuvant therapy (55.8% vs 31.6%, p = 0.0033) (Figure 4B) with a higher proportion of pCR (26.0% vs 10.5%, p = 0.0204) (Figure 4C). Univariate and multivariate logistic regression analyses were performed to identify characteristics associated with response to neoadjuvant therapy, including complete/near complete response (TRS 0 or 1) (Table 2), and demonstrated that patients with high NET density on pretreatment biopsies were associated with a lower likelihood of complete/near complete response to neoadjuvant therapy (multivariate odds ratio 0.256, 95% CI 0.119–0.551, p < 0.001). NLR and neoadjuvant therapy type which patients received were also associated with response to neoadjuvant therapy. The effects of neoadjuvant therapy type and NLR on response rate were further explored in the high-NETs group and low-NETs group (Supplementary Table 3). The result showed that there was no significant difference between different neoadjuvant therapy type on response in both two groups (p = 0.1348 and p = 0.0614 respectively). NLR was significantly associated with response in the high-NETs group (p < 0.001), but this finding was not found in the low-NETs group (p = 0.8206). In the multivariable model of predictors of response to neoadjuvant therapy, high NLR before treatment was associated with a reduced likelihood of complete/near complete response to neoadjuvant therapy (multivariate odds ratio 0.185, 95% CI 0.081–0.425, p < 0. 001). Compared with chemotherapy, the patients who received chemoradiotherapy had a 4.177 higher likelihood of achieving complete or near complete response (95% CI 1.854–9.410, p = 0.001).
 |
Table 2 Logistic regression of clinicopathologic variables in predicting response to neoadjuvant therapy in LARC in the neoadjuvant therapy cohort |
Based on above results, we compared the NET density in patients who underwent surgery with or without neoadjuvant therapy and we did not find any significant difference in NET density between these patients in surgically resected specimens (Figure 4D). In the neoadjuvant therapy cohort, there was no significant change in NET levels after treatment in patients who were sensitive to neoadjuvant therapy, but interestingly, in the insensitive patients, we found a significant increase in NETs after treatment (Figure 4E and F).
Discussion
Our study presented unambiguous evidence for NETs in LARC. Specifically, we revealed that LARC patients with high NET density showed worse survival and response to neoadjuvant therapy. Notably, we found that NETs influenced the antitumor immune response, possibly by altering the infiltration of CD8+ T cells and Treg cells, and promoted tumor progression through facilitating EMT. We explored the association between NETs and other clinicopathological characteristics. NETs were positively related to lymph node metastasis and NLR, which is supposed to be a predictor of poor prognosis,13 it did show a predictive value in this study.
In the past few decades, the perception of neutrophils as mere bystanders in tumors has evolved and it is now well established that neutrophils play a contributory role in tumor development,14 and neutrophils have been found to correlate with poor prognosis of patients with tumors.15,16 However, some studies came to the opposite conclusion through identification of neutrophil-mediated antitumor mechanisms,17,18 which may be due to the multiple subtypes of neutrophils. Simply classifying tumor-associated neutrophils into N1 (anti-tumor) and N2 (pro-tumor) is narrow and does not indicate well the prognostic significance of neutrophils. NETs are derived from neutrophils and, like neutrophils, are an essential component of the tumor microenvironment (TME). In contrast to neutrophils, NETs have so far not been characterized by different phenotypes; therefore, they may be more easily identified in tissue, and a prognostic analysis from the perspective of NETs may be a suitable and promising strategy.
Since the progression of NET formation was first described by Brinkmann in 2004,4 research of NETs has mainly focused on inflammatory disease.19 Recently, the critical role of NETs in progression of sterile inflammation, especially in cancer, has been realized.10,20,21 The cytokines secreted by tumor cells, including interleukin-8 and granulocyte colony-stimulating factor,22,23 can promote production of reactive oxygen species in neutrophils, which is supposed to be warrant for NETs, and thus increase NET density. However, few studies have evaluated the role of NETs as a predictive biomarker for prognosis, particularly in the setting of LARC. Several recent studies have highlighted the potential for a high level of NETs or citH3 in serum to predict adverse outcomes in cancer.24–26 However, we doubt that measuring NETs or markers related to NETs in serum is reliable, because serum levels of NETs can be affected by diverse factors, while levels of NETs in tumors are more descriptive of the actual status. Jin et al23 found that NETs infiltrated in tumor were significantly associated with the prognosis of pancreatic ductal adenocarcinoma, and the model of NETs combined with TNM staging showed more accuracy, which was similar to our study.
EMT is a process in which cells lose epithelial characteristics and acquire mesenchymal features and promote the entry of tumor cells into the vascular system, which leads to metastasis.27,28 Several studies have demonstrated a strong correlation between EMT and tumor prognosis by immunohistochemistry and enrichment of hybrid EMT RNA signature.29,30 Pieterse et al showed that NETs can induce EMT.31 Similarly, in a recent study, cell motility of colorectal cancer cells was increased when cocultured with purified NETs, through promotion of EMT.32 In colon cancer, a subtype defined by upregulation of genes involved EMT and downregulation of E-cadherin showed unpromising prognosis.33,34 Consistently, our gene expression analysis and immunohistochemistry can provide clues regarding the underlying mechanism of poor prognosis in patients with high NET density through promotion of EMT. Our study provides a new insight into the relationship between EMT, NETs and prognosis. Simultaneously, in our study, N staging was notably correlated with NET density, which might also be explained by the robust association of NETs with EMT.
TME is known to play a critical role in patients’ outcome. As a part of the TME, the correlation between NETs and other components should receive attention. Treg cells are an immunosuppressive subset of CD4+ T cells that are characterized by expressing the master transcription factor forkhead box P3 and are critical for maintenance of immune tolerance and homeostasis.35 Unlike Treg cells, CD8+ T cells are deemed to be the main component of antitumor immune cells, which are usually associated with better survival. Given the protumor and antitumor characteristics of Treg and CD8+ T cells, respectively, studies have demonstrated that increased Treg cells and low CD8+ to Treg ratio are associated with poor prognosis in multiple types of tumors.36–39 However, some studies have reported contradictory results in that tumor infiltration of Treg cells was positively correlated with patients’ outcomes, particularly in colorectal cancer and Hodgkin’s lymphoma.40–42 The different conclusions may have arisen from improper interpretation of the heterogeneous subsets of Treg cells, such as functional Treg cells and non-Treg cells, as a single population of Treg cells.43 Wang et al found that hepatic NET density was positively associated with accumulation of Treg cells through the impact of mitochondrial oxidative phosphorylation.44 Similarly, we found that high NET density shifts tumor immune balance towards a protumor immune response with increased Treg and decreased CD8+ T cells.
Recently, several studies have demonstrated that increased NET density is associated with radiation and chemotherapy resistance through activating transforming growth factor-β, and compared to pretreatment, there is an increase in NETs post-treatment.45,46 Similarly, our research also showed that NET density was related to response to neoadjuvant therapy, and it might serve as a predictor of neoadjuvant treatment. We found that, compared with the high-NETs group, the low-NETs group had a significantly higher proportion of neoadjuvant therapy responders and ratio of pCR. Although there was no significant change in NET density in the sensitive patients, an increased NET density was found in the insensitive patients, which suggest that NETs were associated with drug resistance in the LARC patients.
There were some limitations to our study. First, although incorporating patients from two different centers in our study, the insufficient number of patients remains a problem, which requires multi-center studies with larger sample sizes. Second, as a retrospective analysis, unintentional selection bias was inevitably present during our data collection. Finally, we performed analysis of preoperative biopsy tissues, which were highly heterogeneous and not representative of the entire tumor, and that might have caused false NET density.
Conclusion
We demonstrated that high tumor-infiltrating NET density is associated with adverse prognosis in LARC patients and NET density in biopsy specimens was associated with sensitivity to preoperative neoadjuvant therapy of LARC patients. These observations suggest that NET might be a valuable biomarker for poor outcome in patients with LARC, which require further prospective clinical trials to validate.
Abbreviations
NETs, Neutrophil extracellular traps; LARC, Locally advanced rectal cancer; nCRT, neoadjuvant chemoradiotherapy; MPO, Myeloperoxidase; NE, Neutrophil elastase; AJCC, American Joint Committee of Cancer; RFS, recurrence-free survival; TRS, Tumor regression score; pCR, pathological complete response; i-pCR, incomplete pathological remission; FFPE, formalin fixed and paraffin embedded; CitH3, citrullinated histone H3; Treg, T regulatory; BSA, bovine serum albumin; DAB, diaminobenzidine; GSEA, Gene Set Enrichment Analysis; ssGSEA, Single sample gene set enrichment analysis; NLR, neutrophil-to-lymphocyte ratio; EMT, epithelial–mesenchymal transition; TME, tumor microenvironment; DEGs, differentially expressed genes.
Data Sharing Statement
Most of the data generated and analyzed during the current study are available from the Supplementary Data and the corresponding author upon reasonable request.
Ethics Approval and Consent to Publication
The studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards and were reviewed and approved by the Institute Research Ethics Committees of the 7th Medical Center of Chinese PLA General Hospital and Affiliated Drum Tower Hospital of Nanjing University Medical School (2023-38). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Acknowledgments
We thank Majorbio Company for offering the gene expression sequencing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China [No. 81870393, JFD, and 81970500, XFS].
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi:10.3322/caac.21763
2. Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–1740. doi:10.1056/NEJMoa040694
3. Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized Phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–1933. doi:10.1200/jco.2011.40.1836
4. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi:10.1126/science.1092385
5. Zhong W, Wang Q, Shen X, Du J. The emerging role of neutrophil extracellular traps in cancer: from lab to ward. Front Oncol. 2023;13:1163802. doi:10.3389/fonc.2023.1163802
6. Rayes RF, Mouhanna JG, Nicolau I, et al. Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight. 2019;5(16). doi:10.1172/jci.insight.128008
7. Guan X, Lu Y, Zhu H, et al. The crosstalk between cancer cells and neutrophils enhances hepatocellular carcinoma metastasis via neutrophil extracellular traps-associated cathepsin G component: a potential therapeutic target. J Hepatocell Carcinoma. 2021;8:451–465. doi:10.2147/jhc.S303588
8. Yang C, Sun W, Cui W, et al. Procoagulant role of neutrophil extracellular traps in patients with gastric cancer. Int J Clin Exp Pathol. 2015;8(11):14075–14086.
9. Yazdani HO, Roy E, Comerci AJ, et al. Neutrophil extracellular traps drive mitochondrial homeostasis in tumors to augment growth. Cancer Res. 2019;79(21):5626–5639. doi:10.1158/0008-5472.Can-19-0800
10. Tohme S, Yazdani HO, Al-Khafaji AB, et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 2016;76(6):1367–1380. doi:10.1158/0008-5472.Can-15-1591
11. Nie M, Yang L, Bi X, et al. Neutrophil extracellular traps induced by IL8 promote diffuse large B-cell lymphoma progression via the TLR9 signaling. Clin Cancer Res. 2019;25(6):1867–1879. doi:10.1158/1078-0432.Ccr-18-1226
12. Yang L, Liu Q, Zhang X, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583:7814):133–138. doi:10.1038/s41586-020-2394-6
13. Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi:10.1093/jnci/dju124
14. Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol. 2019;16(10):601–620. doi:10.1038/s41571-019-0222-4
15. Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–945. doi:10.1038/nm.3909
16. Li YW, Qiu SJ, Fan J, et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54(3):497–505. doi:10.1016/j.jhep.2010.07.044
17. Cui C, Chakraborty K, Tang XA, et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell. 2021;184(12):3163–3177.e21. doi:10.1016/j.cell.2021.04.016
18. Gershkovitz M, Caspi Y, Fainsod-Levi T, et al. TRPM2 mediates neutrophil killing of disseminated tumor cells. Cancer Res. 2018;78(10):2680–2690. doi:10.1158/0008-5472.Can-17-3614
19. Silva CMS, Wanderley CWS, Veras FP, et al. Gasdermin D inhibition prevents multiple organ dysfunction during sepsis by blocking NET formation. Blood. 2021;138(25):2702–2713. doi:10.1182/blood.2021011525
20. Honda M, Kubes P. Neutrophils and neutrophil extracellular traps in the liver and gastrointestinal system. Nat Rev Gastroenterol Hepatol. 2018;15(4):206–221. doi:10.1038/nrgastro.2017.183
21. Arelaki S, Arampatzioglou A, Kambas K, et al. Gradient infiltration of neutrophil extracellular traps in colon cancer and evidence for their involvement in tumour growth. PLoS One. 2016;11(5):e0154484. doi:10.1371/journal.pone.0154484
22. de Andrea CE, Ochoa MC, Villalba-Esparza M, et al. Heterogenous presence of neutrophil extracellular traps in human solid tumours is partially dependent on IL-8. J Pathol. 2021;255(2):190–201. doi:10.1002/path.5753
23. Li Y, Yuan R, Ren T, et al. Role of Sciellin in gallbladder cancer proliferation and formation of neutrophil extracellular traps. Cell Death Dis. 2021;12(1):30. doi:10.1038/s41419-020-03286-z
24. Thålin C, Lundström S, Seignez C, et al. Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer. PLoS One. 2018;13(1):e0191231. doi:10.1371/journal.pone.0191231
25. Decker AS, Pylaeva E, Brenzel A, et al. Prognostic role of blood NETosis in the progression of head and neck cancer. Cells. 2019;8(9):946. doi:10.3390/cells8090946
26. Kaltenmeier CT, Yazdani H, van der Windt D, et al. Neutrophil extracellular traps as a novel biomarker to predict recurrence-free and overall survival in patients with primary hepatic malignancies. HPB (Oxford). 2021;23(2):309–320. doi:10.1016/j.hpb.2020.06.012
27. Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–695. doi:10.1016/j.cell.2006.11.001
28. Pastushenko I, Blanpain C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019;29(3):212–226. doi:10.1016/j.tcb.2018.12.001
29. George JT, Jolly MK, Xu S, Somarelli JA, Levine H. Survival outcomes in cancer patients predicted by a partial EMT gene expression scoring metric. Cancer Res. 2017;77(22):6415–6428. doi:10.1158/0008-5472.Can-16-3521
30. Grosse-Wilde A, Fouquier D, Hérouël A, et al. Stemness of the hybrid epithelial/mesenchymal state in breast cancer and its association with poor survival. PLoS One. 2015;10(5):e0126522. doi:10.1371/journal.pone.0126522
31. Pieterse E, Rother N, Garsen M, et al. Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arterioscler Thromb Vasc Biol. 2017;37(7):1371–1379. doi:10.1161/atvbaha.117.309002
32. Stehr AM, Wang G, Demmler R, et al. Neutrophil extracellular traps drive epithelial-mesenchymal transition of human colon cancer. J Pathol Apr. 2022;256(4):455–467. doi:10.1002/path.5860
33. Jie D, Zhongmin Z, Guoqing L, et al. Positive expression of LSD1 and negative expression of E-cadherin correlate with metastasis and poor prognosis of colon cancer. Dig Dis Sci. 2013;58(6):1581–1589. doi:10.1007/s10620-012-2552-2
34. De Sousa EMF, Wang X, Jansen M, et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med. 2013;19(5):614–618. doi:10.1038/nm.3174
35. Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16(6):356–371. doi:10.1038/s41571-019-0175-7
36. Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi:10.1038/nm1093
37. Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98(5):1089–1099. doi:10.1002/cncr.11618
38. Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5:15179. doi:10.1038/srep15179
39. Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102(51):18538–18543. doi:10.1073/pnas.0509182102
40. Alvaro T, Lejeune M, Salvadó MT, et al. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11(4):1467–1473. doi:10.1158/1078-0432.Ccr-04-1869
41. Frey DM, Droeser RA, Viehl CT, et al. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010;126(11):2635–2643. doi:10.1002/ijc.24989
42. Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137(4):1270–1279. doi:10.1053/j.gastro.2009.06.053
43. Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27(1):109–118. doi:10.1038/cr.2016.151
44. Wang H, Zhang H, Wang Y, et al. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J Hepatol. 2021;75(6):1271–1283. doi:10.1016/j.jhep.2021.07.032
45. Mousset A, Lecorgne E, Bourget I, et al. Neutrophil extracellular traps formed during chemotherapy confer treatment resistance via TGF-β activation. Cancer Cell. 2023;41(4):757–775.e10. doi:10.1016/j.ccell.2023.03.008
46. Shinde-Jadhav S, Mansure JJ, Rayes RF, et al. Role of neutrophil extracellular traps in radiation resistance of invasive bladder cancer. Nat Commun. 2021;12(1):2776. doi:10.1038/s41467-021-23086-z
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.