Back to Journals » Breast Cancer: Targets and Therapy » Volume 12
Network Pharmacology-Oriented Identification of Key Proteins and Signaling Pathways Targeted by Xihuang Pill in the Treatment of Breast Cancer
Received 28 September 2020
Accepted for publication 18 November 2020
Published 8 December 2020 Volume 2020:12 Pages 267—277
DOI https://doi.org/10.2147/BCTT.S284076
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Jiafa Wu,1,2 Dongping Luo,3 Shengnan Li4
1School of Food and Bioengineering, Henan University of Science and Technology, Luoyang, Henan, People’s Republic of China; 2Henan Engineering Research Center of Food Microbiology, Henan University of Science and Technology, Luoyang, Henan, People’s Republic of China; 3The First Affiliated Hospital, Henan University of Science and Technology, Luoyang, Henan, People’s Republic of China; 4School of Medicine, Henan Polytechnic University, Jiaozuo, Henan, People’s Republic of China
Correspondence: Jiafa Wu
School of Food and Bioengineering, Henan University of Science and Technology, Luoyang 471023, Henan, People’s Republic of China
Email [email protected]
Purpose: The compound traditional Chinese medicine Xihuang pill (XHP) has been adopted to treat breast cancer (BC) for centuries, but its specific mechanism of action is unclear.
Materials and Methods: The active ingredients and related targets of XHP were screened using the TCMSP and TCMID databases. GSE139038 was downloaded from the GEO database, and differentially expressed genes (DEGs) were analyzed. The intersection of targets and DEGs were chosen to build an ingredients–target genes network. Protein–protein interaction network construction and functional enrichment analysis of target genes were conducted.
Results: A PPI network of 37 targets was constructed, and seven core nodes (FOS, MYC, JUN, PPARG, MMP9, PTGS2, SERPINE1) were identified. Functional enrichment analysis revealed that the aforementioned targets were mainly enriched in the IL-17, toll-like receptor, and tumor necrosis factor signaling pathways, which are deeply involved in the inflammatory microenvironment of tumors.
Conclusion: This network pharmacology study indicated that XHP can inhibit the development of BC by targeting a variety of proteins and signaling pathways involved in the inflammatory microenvironment.
Keywords: traditional Chinese medicine, Xihuang pill, breast cancer, network pharmacology
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer among women worldwide.1 Thanks to early detection, the mortality rate of BC has decreased even though its incidence has actually increased.2 It is estimated that more than 90% of cancer-related deaths are caused by metastasis.3–5 Metastasis involves both cancer cells and other cells (eg, stromal cells, immune cells) in the tumor microenvironment. These cells secrete various cytokines and/or growth factors, which in turn affect the progression of BC through various mechanisms.6 Inflammation can influence the progression, metastasis, and outcome of BC by establishing favorable immune microenvironments.7,8 Therefore, targeting inflammatory pathways could be helpful in the development of novel prevention and therapeutic strategies.
Xihuang pill (XHP) is a well-known compound traditional Chinese medicine (TCM) with anti-cancer activity. It has been used to treat BC, furunculosis, and scrofula and to relieve swelling and pain.9 A meta-analysis revealed that XHP combined with chemotherapy could significantly enhance the tumor response and alleviate toxicity induced by chemotherapy in patients with BC.10 Studies illustrated that XHP can inhibit growth and induce apoptosis in BC cells (MCF-7, MDA-MB-231).9 XHP induced Bcl-2–independent apoptosis and S phase arrest in Hs578T cells.11 XHP induced apoptosis in estrogen receptor-negative BC cells, possibly by upregulating the mRNA expression of TP53, which induced the expression of the apoptotic protein Bax.12 XHP contains Niuhuang (Calculus Bovis), Shexiang (Moschus), Ruxiang (Olibanum), and Moyao (Myrrh). Modern pharmacological studies illustrated that Ruxiang and Moyao have strong anti-inflammatory effects.13 Network pharmacology analysis indicated that XHP might target the IL-17, Toll-like receptors (TLRs), and tumor necrosis factor (TNF) signaling pathways. These signaling pathways are closely related to immunity and inflammation. Exploration of the molecular mechanism of XHP is of great significance for promoting its clinical application.
Materials and Methods
Identification of the Differentially Expressed Genes (DEGs) of BC
The gene expression dataset GSE139038 was downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). GSE139038 was based on the GPL27630 Print_1437 platform. GSE139038 contains 65 samples including 41 breast tumors (24 early stage, 17 locally advanced), 18 adjacent normal tissues (paired normal), and 6 apparently normal tissues from breasts in patients who underwent surgery for non-malignant conditions. R packages (“limma” and “pheatmap”) were used to analyze GSE139038. DEGs between normal tissue and breast tumors were detected on the basis of the criteria adjusted P-value < 0.05 and |logFC| > 1.
Screening the Chemical Ingredients and Related Targets of XHP
The ingredients of XHP and related targets were screened using the TCMSP (http://tcmspw.com/index.php) and TCMID databases (http://119.3.41.228:8000/tcmid/). Ingredients meeting the criteria oral bioactivity (OB) ≥30% and drug-likeness (DL) ≥0.18 were selected to identify related targets.
Ingredients–Targets Network Construction
The common genes between ingredients-related targets and the DEGs of BC were collected and sorted. The ingredients–targets network was visualized using Cytoscape software (www.cytoscape.org/).14
Protein–Protein Interaction (PPI) Network Construction and Analysis
The STRING database (http://string-db.org/) is designed to analyze PPI information. To evaluate potential PPIs, DEGs were uploaded to the STRING database. The PPI pairs were extracted with a combined score >0.4. Subsequently, the PPI network was visualized using Cytoscape software. Nodes with a higher degree of connectivity tend to be more essential in maintaining the stability of the entire network. CytoHubba, an app plugin in Cytoscape, was used to calculate the degree of each protein node.15 In our study, degree, betweenness, and closeness were used to determine the degree of connectivity. The intersections of the top 10 genes in each method were regarded as core proteins.
Functional Enrichment Analysis
R packages (“clusterProfiler,” “org.Hs.eg.db,” “enrichplot,” and “ggplot2”) were used to conduct biological process (BP), cellular component (CC), molecular function (MF), and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses (p-value cutoff = 0.05, q-value cutoff = 0.05).
Results
Identification of the DEGs of BC
The GSE139038 samples were separated into normal and tumor group (24 and 41 samples, respectively). In total, 1464 DEGs were identified, including 990 downregulated and 474 upregulated genes, as presented in the volcano map (Figure 1) and heatmap (Figure 2).
 |
Figure 2 Heatmap of differentially expressed genes. Red indicates that the gene is highly expressed in the sample, and green indicates that the gene has lower expression in the sample. |
Screening of the Active Ingredients and Targets of XHP
In total, 46 ingredients and 209 targets (Table S1) of XHP were identified using the TCMSP and TCMID databases. Targets and DEGs were integrated to form an ingredients–targets network using Cytoscape software. The integrated network contained 37 molecules and 37 target proteins (Figure 3).
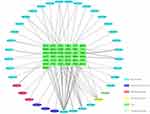 |
Figure 3 Ingredients–target genes network of Xihuang pill. Ellipse, chemical compounds from different herbs; Green rectangle, target genes. |
PPI Network Construction
The aforementioned 37 targets were mapped to the STRING database to construct the PPI network (Figure 4). The PPI pairs were extracted with a combined score >0.4, and disconnected nodes in the network were deleted. The network contained 36 nodes and 151 edges, and the PPI enrichment p-value was <1.0e−16. The CytoHubba plugin was used to located hub nodes according to degree, betweenness, and closeness (Tables 1–3). The intersections of the top 10 nodes in each method were identified as core targets (FOS, MYC, JUN, PPARG, MMP9, PTGS2, SERPINE1). The PPI network (Figure 5) of core targets has significantly more interactions than expected (enrichment p-value = 1.13e−07).
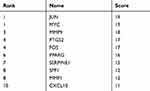 |
Table 1 Top 10 Genes in the Network Ranked by Degree |
 |
Table 2 Top 10 Genes in the Network Ranked by Betweenness |
 |
Table 3 Top 10 Genes in the Network Ranked by Closeness |
 |
Figure 4 Protein–protein interaction (PPI) network of target genes. The network featured 36 nodes and 151 edges with a combined score >0.4 and PPI enrichment p-value < 1.0e−16. |
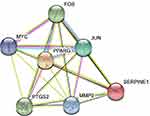 |
Figure 5 Protein–protein interaction network of core proteins. Seven genes were found in the intersections of the top 10 genes according to degree, betweenness, and closeness. |
Functional Enrichment Analysis
R packages were adopted to conduct functional enrichment analysis of the 37 targets. BP analysis indicated that targets were significantly enriched in response to lipopolysaccharide (GO: 0032496), response to molecule of bacterial origin (GO: 0002237), and response to purine-containing compound pathways (GO: 0014074; Figure 6). CC analysis revealed that the targets were mainly enriched in RNA polymerase II transcription factor complex (GO: 0090575), nuclear transcription factor complex (GO: 0044798), and apical plasma membrane pathways (GO: 0016324; Figure 7). MF analysis indicated that targets were significantly enriched in transcription factor activity, RNA polymerase II proximal promoter sequence-specific DNA binding (GO: 0000982), chemokine receptor binding (GO: 0042379), and activating transcription factor binding pathways (GO: 0033613; Figure 8). To further reveal the potential mechanism of action of XHP in the treatment of BC, KEGG pathway enrichment analysis was conducted. The IL-17 signaling pathway (hsa04657), TLR signaling pathway (hsa04620), TNF signaling pathway (hsa04668), and BC (hsa05224) were screened using q-value <0.05 (Figure 9). The interaction network between the KEGG pathways and targets is presented in Figure 10.
 |
Figure 9 Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of the target genes. The top 20 enriched KEGG pathways are presented. |
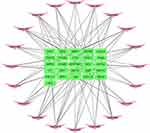 |
Figure 10 Network of the target genes–Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. The pink darts indicate KEGG pathways, and the green rectangles indicate target genes. |
Discussion
Compound TCMs have the characteristics of “multi-component, multi-pathway and multi-target” synergistic effects, and they have unique advantages in the treatment of a series of complex diseases such as cancer, diabetes, and stroke.16–18 Network pharmacology has integral and systematic characteristics, and thus, it can effectively explore the basis of medicinal substance and mechanism of action, which has great significance for improving the therapeutic application of compound Chinese medicines.
In recent years, the inhibitory effects of TCM on tumor recurrence and metastasis have attracted increasing attention.19,20 Relevant studies discovered that TCMs can improve the quality of life of patients with cancer, reduce the risks of tumor recurrence and metastasis, and prolong survival.21–23 Although a few reports revealed that XHP can inhibit proliferation and induce apoptosis in BC cells,9,11,12 the mechanism of action is unclear.
We screened 37 chemical molecules in XHP and 37 target proteins. Some molecules targeted multiple proteins, among which seven proteins (FOS, MYC, JUN, PPARG, MMP9, PTGS2, SERPINE1) were identified as core nodes. FOS forms a tight complex (non-covalently) with the transcription factor JUN/AP-1, which plays important roles in the development of BC.24,25 A recent study also found XHP could regulate the MEKK1/SEK1/JNK1/AP-1 pathway, which confirmed the possible interaction between XHP and the FOS/JUN/AP-1 complex.26 Chen et al discovered that the overexpression of MMP9 stimulated the invasiveness of BC cell-formed spheroids in vitro and DCIS in vivo, whereas depletion of MMP9 inhibited their invasiveness.27 Upregulation of PTGS2 is also associated with increased cell adhesion, phenotypic changes, anti-apoptosis, and tumor angiogenesis.28 In cancer cells, PTGS2 plays key roles in the production of prostaglandin E2 (PGE2) and development of tumors.29 A study by Bos et al indicated that COX2 (also known as PTGS2) and other genes mediate BC metastasis to the brain.30 Nandi et al found that PGE2 promotes lymphangiogenesis associated with BC by activating EP4 receptors on lymphatic endothelial cells.31 Kochel et al discovered that multidrug resistance-related protein can export PGE2 and promote metastasis in basal/triple-negative BC.32 SERPINE1 (also known as plasmin activator inhibitor-1) has been demonstrated to be highly expressed in various types of tumor biopsies.33 More importantly, SERPINE1 and urokinase-type plasminogen activator have been identified as prognostic factors for disease progression and relapse in BC.33,34
Tumor cells have the ability to produce cytokines and chemokines by activating transcription factors such as nuclear factor-κB (NF-κB) and interferon regulatory factors (IRFs).35 These cytokines and chemokines induce the mobilization and reprogramming of immune cells and activate stromal cells in the extracellular matrix adjacent to the tumor. These cells in turn produce more cytokines and chemokines, thereby amplifying the entire process. Cytokines and/or growth factors secreted by these immune or stromal cells further regulate the activity of recruited immune cells, leading to the sustenance of a tumor-friendly inflammatory microenvironment.7,36 Therefore, cytokine and chemokine pathways and the inflammatory tumor microenvironment could be therapeutic targets with great potential. Su et al found that XHP might promote Treg cell apoptosis in the tumor microenvironment and further inhibit the tumor growth of 4T1 mouse breast cancer cells.26 Functional enrichment analyses indicated that target proteins were significantly enriched in the IL-17, TLR, and TNF signaling pathways. These pathways are well known to play significant roles in inflammation and tumor development.35,37–43 It has been reported that IL-17 activates the Src/PI3K/Akt/NF-κB, MAPK, Stat3, and COX-2 pathways, which play significant roles in tumorigenesis, angiogenesis, and metastasis.44,45 IL-17 is overexpressed in the intratumoral stromal cells of triple-negative BC. Upon overexpression, IL-17 can activate tumor microangiogenesis through its signal transduction pathways, resulting in increased tumor secretion of VEGFA, thereby promoting tumor progression.46 TLRs trigger multiple signaling pathways involving nuclear factor B, IRFs, and MAPKs to produce various cytokines with important roles in diseases such as cancer.47 Inhibition of TLR signaling can suppress human BC cell viability, invasion, and migration.48,49 TNF-α is involved in all stages of BC progression, participating in cell proliferation, survival, and motility; inflammation maintenance; acquisition of stemness; and resistance to chemotherapy.43,50 TNF-α levels at the tumor site or in plasma/serum in patients with BC are correlated with the clinical status and outcome.51
Conclusion
This network pharmacology study indicated that XHP can inhibit the development of BC by targeting a variety of proteins and signaling pathways involved in the inflammatory microenvironment. Additional research is needed to clarify the effects of XHP on different molecular subtypes of BC.
Acknowledgment
We thank Joe Barber Jr. PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
This research was funded by the Natural Science Foundation of Henan Province (No. 162300410099), Doctor Scientific Research start-up Fund from Henan University of Science and Technology (No. 13480064), and National Natural Science Foundation of China (No. 81700775).
Disclosure
All authors declare no conflicts of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Narod SA, Iqbal J, Miller AB. Why have breast cancer mortality rates declined? J Cancer Policy. 2015;5:8–17. doi:10.1016/j.jcpo.2015.03.002
3. Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6(6):449–458.
4. Monteiro J, Fodde R. Cancer stemness and metastasis: therapeutic consequences and perspectives. Eur J Cancer. 2010;46(7):1198–1203. doi:10.1016/j.ejca.2010.02.030
5. Guan X. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5(5):402–418.
6. Deshmukh SK, Srivastava SK, Tyagi N, et al. Emerging evidence for the role of differential tumor microenvironment in breast cancer racial disparity: a closer look at the surroundings. Carcinogenesis. 2017;38(8):757–765. doi:10.1093/carcin/bgx037
7. Deshmukh SK, Srivastava SK, Poosarla T, et al. Inflammation, immunosuppressive microenvironment and breast cancer: opportunities for cancer prevention and therapy. Ann Transl Med. 2019;7(20):593. doi:10.21037/atm.2019.09.68
8. Zahid H, Simpson ER, Brown KA. Inflammation, dysregulated metabolism and aromatase in obesity and breast cancer. Curr Opin Pharmacol. 2016;31:90–96. doi:10.1016/j.coph.2016.11.003
9. Pan G, Wang W, Wang L, et al. Anti-breast cancer effects and mechanisms of Xihuang pill on human breast cancer cell lines. J Tradit Chin Med. 2013;33(6):770–778. doi:10.1016/S0254-6272(14)60011-X
10. Mao D, Feng L, Huang S, Zhang S, Peng W, Zhang S. Meta-analysis of Xihuang pill efficacy when combined with chemotherapy for treatment of breast cancer. Evid Based Complement Alternat Med. 2019;2019:3502460. doi:10.1155/2019/3502460
11. Zheng W, Han S, Jiang S, et al. Multiple effects of Xihuang pill aqueous extract on the Hs578T triple-negative breast cancer cell line. Biomed Rep. 2016;5(5):559–566. doi:10.3892/br.2016.769
12. He LJ, Li JS, Chen X, et al. [Effect of serum containing Xihuang pill on proliferation of human breast cancer cell line MDA-MB-435 and MCF-7 cells]. Zhongguo Zhong Yao Za Zhi. 2018;43(13):2784–2788. Chinese.
13. Guo Q, Lin J, Liu R, et al. Review on the applications and molecular mechanisms of Xihuang pill in tumor treatment. Evid Based Complement Alternat Med. 2015;2015:854307. doi:10.1155/2015/854307
14. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi:10.1101/gr.1239303
15. Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. CytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8(Suppl 4):S11. doi:10.1186/1752-0509-8-S4-S11
16. Luo H, Vong CT, Chen H, et al. Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine. Chin Med. 2019;14:48.
17. Xiao E, Luo L. Alternative therapies for diabetes: a comparison of western and Traditional Chinese Medicine (TCM) approaches. Curr Diabetes Rev. 2018;14(6):487–496. doi:10.2174/1573399813666170519103230
18. Liu T, Ding Y, Wen A. Traditional Chinese medicine for ischaemic stroke. Lancet Neurol. 2018;17(9):745. doi:10.1016/S1474-4422(18)30290-4
19. Tang K-Y, Du S-L, Wang Q-L, Zhang Y-F, Song H-Y. Traditional Chinese medicine targeting cancer stem cells as an alternative treatment for hepatocellular carcinoma. J Integr Med. 2020;18:196–202. doi:10.1016/j.joim.2020.02.002
20. Yan Z, Lai Z, Lin J. Anticancer properties of traditional Chinese medicine. Comb Chem High Throughput Screen. 2017;20(5):423–429. doi:10.2174/1386207320666170116141818
21. Qi FH, Zhao L, Zhou AY, et al. The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. Biosci Trends. 2015;9(1):16–34. doi:10.5582/bst.2015.01019
22. Tao WW, Jiang H, Tao XM, Jiang P, Sha LY, Sun XC. Effects of Acupuncture, Tuina, Tai Chi, Qigong, and traditional Chinese medicine five-element music therapy on symptom management and quality of life for cancer patients: a meta-analysis. J Pain Symptom Manage. 2016;51(4):728–747. doi:10.1016/j.jpainsymman.2015.11.027
23. Liao YH, Li CI, Lin CC, Lin JG, Chiang JH, Li TC. Traditional Chinese medicine as adjunctive therapy improves the long-term survival of lung cancer patients. J Cancer Res Clin Oncol. 2017;143(12):2425–2435. doi:10.1007/s00432-017-2491-6
24. Min DY, Jung E, Kim J, Lee YH, Shin SY. Leptin stimulates IGF-1 transcription by activating AP-1 in human breast cancer cells. BMB Rep. 2019;52(6):385–390. doi:10.5483/BMBRep.2019.52.6.189
25. Xu J, Chen Y, Olopade OI. MYC and breast cancer. Genes Cancer. 2010;1(6):629–640. doi:10.1177/1947601910378691
26. Su L, Jiang Y, Xu Y, et al. Xihuang pill promotes apoptosis of Treg cells in the tumor microenvironment in 4T1 mouse breast cancer by upregulating MEKK1/SEK1/JNK1/AP-1 pathway. Biomed Pharmacother. 2018;102:1111–1119. doi:10.1016/j.biopha.2018.03.063
27. Chen G, Ding XF, Pressley K, et al. Everolimus inhibits the progression of ductal carcinoma in situ to invasive breast cancer via downregulation of MMP9 expression. Clin Cancer Res. 2019.
28. Rodrigues S, Bruyneel E, Rodrigue CM, Shahin E, Gespach C. [Cyclooxygenase 2 and carcinogenesis]. Bull Cancer. 2004;91(Suppl 2):S61–76. French.
29. Tong D, Liu Q, Wang LA, et al. The roles of the COX2/PGE2/EP axis in therapeutic resistance. Cancer Metastasis Rev. 2018;37(2–3):355–368. doi:10.1007/s10555-018-9752-y
30. Bos PD, Zhang XHF, Nadal C, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459(7249):1005–1009. doi:10.1038/nature08021
31. Nandi P, Girish GV, Majumder M, Xin X, Tutunea-Fatan E, Lala PK. PGE2 promotes breast cancer-associated lymphangiogenesis by activation of EP4 receptor on lymphatic endothelial cells. BMC Cancer. 2017;17(1):11. doi:10.1186/s12885-016-3018-2
32. Kochel TJ, Reader JC, Ma X, Kundu N, Fulton AM. Multiple drug resistance-associated protein (MRP4) exports prostaglandin E2 (PGE2) and contributes to metastasis in basal/triple negative breast cancer. Oncotarget. 2017;8(4):6540–6554. doi:10.18632/oncotarget.14145
33. Li S, Wei X, He J, Tian X, Yuan S, Sun L. Plasminogen activator inhibitor-1 in cancer research. Biomed Pharmacother. 2018;105:83–94. doi:10.1016/j.biopha.2018.05.119
34. Duffy MJ, McGowan PM, Harbeck N, Thomssen C, Schmitt M. uPA and PAI-1 as biomarkers in breast cancer: validated for clinical use in level-of-evidence-1 studies. Breast Cancer Res. 2014;16(4):428. doi:10.1186/s13058-014-0428-4
35. Bhatelia K, Singh K, Singh R. TLRs: linking inflammation and breast cancer. Cell Signal. 2014;26(11):2350–2357. doi:10.1016/j.cellsig.2014.07.035
36. Chow MT, Luster AD. Chemokines in cancer. Cancer Immunol Res. 2014;2(12):1125–1131. doi:10.1158/2326-6066.CIR-14-0160
37. Chang SH. T helper 17 (Th17) cells and interleukin-17 (IL-17) in cancer. Arch Pharm Res. 2019;42(7):549–559. doi:10.1007/s12272-019-01146-9
38. Song Y, Yang JM. Role of interleukin (IL)-17 and T-helper (Th)17 cells in cancer. Biochem Biophys Res Commun. 2017;493(1):1–8. doi:10.1016/j.bbrc.2017.08.109
39. Lim KH, Staudt LM. Toll-like receptor signaling. Cold Spring Harb Perspect Biol. 2013;5(1):a011247. doi:10.1101/cshperspect.a011247
40. Echizen K, Hirose O, Maeda Y, Oshima M. Inflammation in gastric cancer: interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways. Cancer Sci. 2016;107(4):391–397. doi:10.1111/cas.12901
41. Javaid N, Choi S. Toll-like receptors from the perspective of cancer treatment. Cancers (Basel). 2020;12(2):297. doi:10.3390/cancers12020297
42. van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11(4):397–408.
43. Cruceriu D, Baldasici O, Balacescu O, Berindan-Neagoe I. The dual role of tumor necrosis factor-alpha (TNF-alpha) in breast cancer: molecular insights and therapeutic approaches. Cell Oncol (Dordr). 2020;43(1):1–18. doi:10.1007/s13402-019-00489-1
44. Ple C, Fan Y, Ait Yahia S, et al. Polycyclic aromatic hydrocarbons reciprocally regulate IL-22 and IL-17 cytokines in peripheral blood mononuclear cells from both healthy and asthmatic subjects. PLoS One. 2015;10(4):e0122372. doi:10.1371/journal.pone.0122372
45. Ryu H, Chung Y. Regulation of IL-17 in atherosclerosis and related autoimmunity. Cytokine. 2015;74(2):219–227. doi:10.1016/j.cyto.2015.03.009
46. Qian XL, Xu P, Zhang YQ, et al. Increased number of intratumoral IL-17+ cells, a harbinger of the adverse prognosis of triple-negative breast cancer. Breast Cancer Res Treat. 2020;180:311–319. doi:10.1007/s10549-020-05540-6
47. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22(2):240–273, Table of Contents.
48. Schwartz AL, Dickerson E, Dagia N, Malgor R, McCall KD. TLR signaling inhibitor, phenylmethimazole, in combination with tamoxifen inhibits human breast cancer cell viability and migration. Oncotarget. 2017;8(69):113295–113302. doi:10.18632/oncotarget.10358
49. Xu M, Li D, Yang C, Ji JS. MicroRNA-34a inhibition of the TLR signaling pathway via CXCL10 suppresses breast cancer cell invasion and migration. Cell Physiol Biochem. 2018;46(3):1286–1304. doi:10.1159/000489111
50. Zhang Z, Lin G, Yan Y, et al. Transmembrane TNF-alpha promotes chemoresistance in breast cancer cells. Oncogene. 2018;37(25):3456–3470. doi:10.1038/s41388-018-0221-4
51. Ma Y, Ren Y, Dai ZJ, Wu CJ, Ji YH, Xu J. IL-6, IL-8 and TNF-alpha levels correlate with disease stage in breast cancer patients. Adv Clin Exp Med. 2017;26(3):421–426. doi:10.17219/acem/62120
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




