Back to Journals » Journal of Inflammation Research » Volume 17
N-Ethyl-N-Nitrosourea Induced Leukaemia in a Mouse Model: Protective Effect of Icaritin via Inhibition of IL-6/JAK2/STAT3 Pathway Causes Apoptosis
Authors Hou X, Han Y, Hirad AH, Alarfaj AA, Liu L
Received 23 September 2023
Accepted for publication 20 January 2024
Published 7 February 2024 Volume 2024:17 Pages 777—790
DOI https://doi.org/10.2147/JIR.S441755
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Adam D Bachstetter
Xinjun Hou,1 Yanhui Han,2 Abdurahman Hajinur Hirad,3 Abdullah A Alarfaj,3 Linxiang Liu4
1Department of Traditional Chinese Medicine Hematology, Xin’an People’s Hospital, Luoyang, Henan, 471000, People’s Republic of China; 2Department of Internal Medicine of Traditional Chinese Medicine, Xin’an the Second People’s Hospital, Luoyang, Henan, 471000, People’s Republic of China; 3Department of Botany and Microbiology, College of Science, King Saud University, Riyadh, 11451, Saudi Arabia; 4Department of Hematology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, 450052, People’s Republic of China
Correspondence: Linxiang Liu, Department of Hematology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, People’s Republic of China, Email [email protected]
Background: The present study aimed to investigate the protective effect of icaritin (ICT) on ENU-induced leukemia in male mice.
Methods: The mice received intraperitoneal injections of 80 mg/kg ENU twice a week for three months for induction of leukemia. Blood smears from these mice showed blast cells, confirming the presence of leukemia. After confirming leukemia, mice were divided into control, ENU-induced leukemia, and leukemia groups (10 mg/kg bw and 20 mg/kg bw) were treated with ICT for 35 days. Blood, spleen, and liver samples were collected for analysis. The expression of IL-6, JAK2, STAT3, as well as inflammatory, pro-apoptotic (Bax), and anti-apoptotic (Bcl-2) proteins were evaluated using qPCR, immunohistochemistry, and immunofluorescence techniques.
Results: The study found that ICT inhibited inflammation and the IL-6/JAK2/STAT3 pathway in ENU-induced mice. ICT treatment induced apoptosis in the spleen and liver by activating Bax and downregulating Bcl-2. The findings provide novel evidence that ICT acts as a dual inhibitor of IL-6/JAK2/STAT3 signaling, promoting apoptosis and playing an essential role in anti-leukemic activity.
Conclusion: These results suggest that ICT has potential as a therapeutic target for treating leukemia, offering a novel approach to leukemia treatment through inhibiting the IL-6/JAK2/STAT3 pathway and induction of apoptosis.
Keywords: N-ethyl-n-nitrosourea, leukaemia, ICT, IL-6/JAK2/STAT3, inflammation, apoptosis
Introduction
Leukemia is characterized by the accumulation of immature leukocytes, also known as blast cells, in organs and blood. Blast cells are early-stage blood cells that have not fully matured into their specific types (such as red blood cells, white blood cells, or platelets). The presence of blast cells in leukemia can disrupt normal blood cell functions and increase the risk of infection.1 Chronic lymphocytic leukemia (CLL), chronic myeloid leukemia (CML), and acute myeloid leukemia (AML) are all included.2 The differentiation of leukemia types is often associated with abnormalities in neutrophil counts, which are a type of white blood cell. In acute leukemia patients, there is a characteristic decrease in neutrophil counts in the blood. On the other hand, CML patients may exhibit irregular neutrophils, indicating alterations in the appearance or function of these cells.3
Teratogenic N-ethyl-N-nitrosourea (ENU) can potentially cause congenital disabilities or abnormalities in developing organisms, including humans. ENU specifically is known to cause central nervous system (CNS) tumors and genetic disorders.4 ENU administration in these models can help simulate and study tumor development, including leukemia.5 Studies have shown that intravenous administration of ENU during pregnancy, at doses ranging from 40 to 80 mg/kg, increases the risk of CNS malignancies.6 Notably, the administration of ENU through transplacental injections during the second-to-last week of gestation (E15 to E21) or the first week of postnatal development (PN7) has been found to lead to the development of cancer in most children.7 In experiments with pregnant Sprague Dawley rats, a dose of 80 mg/kg ENU was administered on embryonic day 15, resulting in the induction of gliomas and schwannomas.8 It is important to consider that the specific dosage, frequency of administration, and treatment technique employed can significantly influence leukemia induction and impact the target cell and affected organ.9
Inflammatory reactions and leukemia both include the IL-6/JAK2/STAT3 signaling pathway, which has recently become prominent.10 The IL-6 cytokine activates the JAK2 kinase, which, phosphorylates and activates STAT3. Translocating to the nucleus upon activation, STAT3 controls the transcription of genes involved in cellular proliferation, survival, and immune response.11 Dysregulation of this pathway can contribute to leukemogenesis and provide potential targets for therapeutic interventions.12 This pathway is particularly active in multiple myeloma (MM).13 Bone marrow contains cytokines that are necessary for the survival and multiplication of MM cells. Interleukin-6 (IL-6), insulin-like growth factor 1, and tumor necrosis factor-alpha are examples of cytokines that promote MM cell survival and proliferation.14 Leukemia treatments encompass various approaches, such as radiation, bone marrow transplants, and surgery.15 However, while rapid restorative therapies, such as targeted molecular therapies or immune-based treatments, can lead to near-complete remission, they may also result in disease recurrence, resistance, progression, and ultimately death. Radiation and chemotherapy, commonly employed in leukemia treatment, carry risks, including potential side effects such as bone marrow suppression, increased susceptibility to infections, hair loss, fatigue, and gastrointestinal disturbances.16 Consequently, there is a growing interest in natural medicine therapies. Cutting-edge cancer treatments incorporate natural compounds like lycopene, curcumin, epigallocatechin gallate (EGCG), and resveratrol. These natural compounds have shown a potential to inhibit cancer cell growth, promote apoptosis, and modulate signaling pathways involved in tumor development and progression (Lüthi et al, 2021). Recent studies have shown that these natural compounds can influence various cellular processes involved in cancer, such as receptor binding, growth signaling, and the pro-apoptosis pathway, without significantly harming normal cells. For instance, curcumin has been found to modulate the activation of growth factor receptors (eg, EGFR), inhibit the PI3K/Akt signaling pathway, and promote apoptosis through mechanisms involving caspase activation.17,18 Traditional Chinese medicine has also contributed to the development of modern anticancer drugs. For instance, Chansu and Huachansu injections have been used in China for many years as anticancer treatments.19
Icaritin (ICT), derived from the traditional Chinese herbal medicine icariin, exhibits various beneficial effects in different biological systems. Studies have investigated the impact of ICT in cancer cells, immune modulation, bone health, and hormonal regulation, among other areas of research,20 promote neuronal growth,21 and facilitate cardiac differentiation in mouse embryonic stem cells.22 Moreover, ICT has been shown to induce apoptosis in human endometrial cancer cells by modulating apoptotic proteins and inhibiting specific signaling pathways, such as the PI3K/Akt and MAPK pathways.23 Additionally, in vitro and in vivo studies have demonstrated that ICT can suppress leukemia’s MAPK, AKT, and JAK2/STAT3 signaling pathways.24 Although previous studies have explored the effects of ICT in various biological systems and its potential as a therapeutic agent for leukemia, there is a lack of research specifically focusing on the efficacy of ICT in ENU-induced leukemia and its relationship with the IL-6/JAK2/STAT3 signaling pathway. Therefore, the objective of the present study was to comprehensively evaluate the therapeutic benefits of ICT against ENU-induced leukemia, including assessing its effects on leukemia cell survival, proliferation, and apoptosis. Additionally, the study aimed to investigate the underlying mechanism associated with the IL-6/JAK2/STAT3 signaling pathway, with the hypothesis that ICT exerts its anti-leukemic effects, at least in part, by modulating this pathway. By elucidating the specific objectives and hypotheses, this study aims to contribute to understanding ICT as a potential therapeutic agent for ENU-induced leukemia and provide valuable insights into its molecular mechanisms of action.
Materials and Methods
Cayman Chemical supplied the Icaritin (ICT; cat. no. Cay20236-500, Purity > 98%), and Sigma Aldrich supplied the N-ethyl-N’-nitrosourea (ENU; St. Louis, MO, USA).
Maintenance of Animals
The Zhejiang University Animal Center in China provided Swiss albino male mice weighing 18–20 g. These mice were 6–8 weeks old. The mice lived under sterile settings at Zhengzhou University’s First Affiliated Hospital. The temperature in the housing was kept at 25°C ± 2°C, the humidity at 45 ± 5%, and the day/night cycle was held at 12 hours. The mice could eat and drink as much as they wanted.
The Induction of Leukemia
Leukemia was induced intraperitoneally administering ENU twice a week for three months to the mice at a dosage of 80 mg/kg body weight via the intraperitoneal route.25 One gram of ENU was first dissolved in 10 mL of 95% ethanol to make the solution. Prior to injection, this solution was diluted with 90 mL of phosphate citrate buffer (0.2 M Na2HPO4, 0.1 M citric acid, pH 5.0). After three months, blood smears were examined for blast cells to confirm leukemia induction. Splenomegaly and a significant presence of blast cells were observed as hallmarks of leukemic animals.
Animal Groups
The experiment consisted of four groups, six animals in each.
Group-1: Normal control
Group-2: Leukemic control
Group-3: Leukemic + 10 mg/kg bw ICT treatment
Group-4: Leukemic + 20 mg/kg bw ICT treatment
ICT Treatment and Sample Collection
Mice received ICT injections, i.p. once daily at 10 and 20 mg/kg body weight for 35 days. The DMSO was dissolved in corn oil.26 Following treatment, heart puncture samples of blood were taken to isolate the serum. The spleen and liver were removed and stored in 4% paraformaldehyde for histological exams and at −80°C for molecular study.
Organ Index Calculation
Following sacrifice, the spleen and liver were weighed, and tissue indices (TI) were calculated using the formula: TI = tissue weight (mg)/ mouse weight (g) × 100%.
Hematology Analysis
Blood samples were subjected to hematological analysis using an automated hematology analyzer (Mindray, Shenzhen, China) to determine red blood cell (RBC, x10^12 /L) count, white blood cell (WBC, x10^9 /L) count, platelet count (x10^9/L), and hemoglobin (Hb, g/dL) concentration.
Enzyme-Linked Immunosorbent Assays (ELISA)
Blood was centrifuged at 2000 rpm after 30 minutes at room temperature. Samples of serum were taken and kept cold at −80°C. The levels of NF-κB p65 (E-EL-M0838; Labscience Biotechnology Co., Ltd. Wuhan, Hubei, China), TNF-α (Cat No: 560478), IFN-γ (Cat No: 558258) and IL-1β (559603) (BD Biosciences, Franklin Lakes, New Jersey, USA) were measured in the serum using commercially available ELISA kits according to the manufacturer’s protocol.
Histopathology
To prepare the tissue samples for histopathological examination, small pieces of the spleen and liver were first sectioned and then preserved in a 10% formalin solution for a minimum of 48 hours. Following fixation, the tissue samples underwent a series of steps for processing and staining. An automatic tissue processor was used to dehydrate the tissues for embedding in paraffin wax. We sectioned the paraffin-embedded tissue blocks using a microtome into 5 µm thick sections. Using a hotplate, these pieces were delicately attached to glass slides. In that order, the mounted pieces were given a 2-minute soaking in solutions of 100%, 90%, and 70% ethanol. After 30 minutes at room temperature, blood was centrifuged at 2000 rpm. To monitor and assess the tissue morphology, the stained tissue sections were seen at 400x magnification with a light microscope (Nikon, Chiyoda-ku, Tokyo, Japan).
Immunohistochemistry Staining
The 5µm thick tissue sections were first subjected to deparaffinization and rehydration for immunohistochemistry staining. Antigen retrieval was performed using citrate buffer at pH 6.0 to enhance the accessibility of the target antigens. After cooling, the sections were treated with hydrogen peroxide to block endogenous peroxidase activity. Subsequently, the sections were incubated overnight at 4°C with primary antibodies against NF-κB p65 and Bax (Cell Signaling Technology (CST), Danvers, Massachusetts, USA) at a dilution of 1:1000. The next day, the sections were washed and incubated with secondary antibodies conjugated to horseradish peroxidase (HRP) at room temperature for one hour. The presence of the target antigens was visualized by incubating the sections with 3.3’-diaminobenzidine (DAB, Vector Laboratories, USA) for 3–5 minutes, resulting in a brown color reaction. Finally, the sections were counterstained with hematoxylin to provide contrast. After dehydration and clearance, the sections were mounted with DPX mounting medium for preservation.
Immunofluorescence Experiments
To perform immunofluorescence experiments, the 5µm thick tissue sections were incubated with primary antibodies against JAK2, p-JAK2, STAT3, p-STAT3 (Cell Signaling Technology (CST), Danvers, Massachusetts, USA) at a dilution of 1:1000 for one hour at room temperature. After washing with PBS, the sections were incubated with fluorescently labeled secondary antibodies, such as FITC (fluorescein isothiocyanate) or PE (phycoerythrin), at a dilution of 1:200. This allowed for the specific visualization of the target antigens using fluorescence microscopy. The sections were counterstained with DAPI (4’,6-diamidino-2-phenylindole), a blue fluorescent dye that binds to DNA to visualize the nuclei. The sections were then examined and imaged using confocal microscopy, which provides high-resolution three-dimensional images of the labeled antigens and cellular structures.
Real-Time qPCR Analysis
To analyze mRNA expression levels, RNA was isolated from the spleen and liver tissues using Trizol (Tiangen, Beijing, China), a commonly used reagent for RNA extraction. Subsequently, cDNA synthesis was performed using the iScript cDNA Synthesis kit (Cat No. 170-8891; Bio-Rad, USA). The cDNA served as a real-time quantitative PCR (qPCR) analysis template. The mRNA expression levels of target genes, such as Il-6, Jak2, Stat3, Bcl-2, and Bax, were determined using an SYBR Green kit (Tiangen, Beijing, China). The 2−ΔΔCT method was employed for data analysis, with GAPDH serving as an internal control. The primer sequences used for amplification of the target genes were as follows: IL-6 forward ACCTTCCAGGATGAGGACATGA, reverse CTAATGGGAACGTCACACACCA; Jak2 forward AGGCGACGGGAACAAGATG, reverse TTCAGAACATTGGCCTTCGC; STAT3 forward GAAGCCGACCCAGGTAGTG, reverse CTGCAGGTCGTTGGTGTCA, Bcl-2 forward GCTACCGTCGTGACTTCGCA, reverse CATCCCAGCCTCCGTTATCC; BAX forward GCCTTTTTGCTACAGGGTTTCAT, reverse TATTGCTGTCCAGTTCATCTCCA; actin forward TGCCTTCGGGTTTGCATTTG, reverse AAGATCAC CCCCAAGATGACAC.
Statistical Analysis
Mean and standard deviation were used to summarize the experimental results. One-way ANOVA and Student’s t-tests were used for statistical analyses, and a p-value ≤ 0.05 was considered statistically significant.
Results
Effects of ICT on Blast Cells and Splenic Enlargement
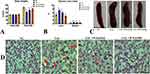
The study examined the effects of ICT on blast cells and splenic enlargement. Blast cell count in peripheral blood is an important factor in diagnosing and monitoring leukemia, as well as assessing the efficacy of anti-leukemia treatments. The results showed that ENU-injected mice experienced significant weight loss compared to normal control mice. Still, this weight loss was prevented in leukemic groups treated with ICT (Figure 1A). Leukemic mice had significantly larger liver (Figure 1B) and spleen (Figure 1C) sizes than control mice. In contrast, ICT-treated leukemic animals had significantly smaller spleens. Blood smears revealed that the leukemic control group had significantly more circulating blast cells than the normal control group. Conversely, ICT-treated leukemic groups had significantly fewer blast cells than the leukemic control group. These findings suggest that ICT treatment prevented weight loss, reduced splenic enlargement, and decreased blast cell count in leukemic mice. (Figure 1D).
Effects of ICT on Blood Parameters
The effects of ICT on blood parameters, histopathological changes, and hematopoietic cytokines were investigated. The leukemic control group’s total white blood cell counts considerably increased, whereas hemoglobin concentration, platelet count, and total red blood cell count dropped. In ICT-treated leukemic mice, RBC count, platelet count, and Hb concentration rose while WBC count dropped. This indicates that ICT treatment improved these blood parameters in leukemic mice (Figure 2A–D).
Effects of ICT on Histopathological Changes
Histopathological changes were observed in the spleen and liver. The untreated control group exhibited normal cells, while the leukemic control group showed an abundance of irregularly shaped cancerous cells in the spleen, leading to the disappearance of sinusoids. However, mice treated with ICT displayed fewer cancer cells in their spleen compared to the untreated group (Figure 3A). In the liver, the leukemic control mice had pale bone marrow, swollen liver, and spleen filled with lymphoblasts. Additionally, their livers exhibited signs of damage, including disrupted hepatic cords, increased cell spaces, inflammation, red blood cell infiltration, and fat accumulation. These liver abnormalities were less severe in the ICT-treated group, indicating improvement with treatment (Figure 3B).
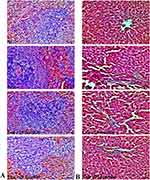 |
Figure 3 Effect of ICT on histopathological changes in ENU-induced leukemic mice. Representative H&E-stained (A) Spleen and (B) liver tissue section images. Scale bar= 50 µm, Magnification= 400 ×. |
Effects of ICT on Hematopoietic Cytokines
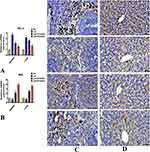
Hematopoietic cytokines, including NF-κB p65, TNF-α, IL-1β, and IFN-γ, were measured in the spleen and liver tissue homogenate. The leukemic control group showed significantly higher levels of NF-κB p65, TNF-α, and IL-1β than the normal control group. However, leukemic mice treated with ICT exhibited lower levels of NF-κB p65 (Figure 4A), TNF-α (Figure 4B), and IL-1β (Figure 4C) after treatment. The level of IFN-γ was significantly lower in the leukemic control group but significantly higher in the ICT-treated leukemic group (Figure 4D). Immunohistochemistry analysis showed higher levels of NF-κB p65 expression in the liver and spleen of leukemic control mice compared to normal control mice. Still, ICT treatment reduced NF-κB p65 expression in these tissues. (Figure 5A and B)
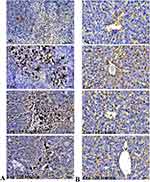 |
Figure 5 Immunohistochemistry results show protein distribution of NF-κB p65 in the (A) spleen and (B) liver of ENU-induced leukemic mice. Scale bar= 50 µm, Magnification= 400 ×. |
Effects of ICT on the IL-6/JAK2/STAT3 Pathway in the Spleen and Liver
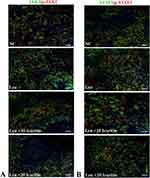
The effects of ICT on the IL-6/JAK2/STAT3 pathway in the spleen and liver were investigated. In the leukemic group, the mRNA levels of IL-6 were increased in both spleen and liver tissues. However, in the ICT-treated leukemic group, the expression of IL-6 was decreased (Figure 6A). The mRNA levels of JAK2 (Figure 6B) and STAT3 (Figure 6C), which are transcription factors associated with the IL-6 pathway, were significantly increased in the spleen and liver of the leukemic group. In contrast, the ICT-treated leukemic group showed a significant decrease in JAK2 and STAT3 mRNA levels compared to the leukemic group. Immunofluorescence results confirmed that the expression levels of JAK2, p-JAK2 (phosphorylated JAK2), STAT3, and p-STAT3 were higher in the spleen of the leukemic group but decreased in the ICT-treated leukemic group. (Figure 7A and B)
Effects of ICT on Cell Apoptosis and Autophagy
The impacts of ICT on cell apoptosis and autophagy were also examined. Levels of the anti-apoptotic protein Bcl2 in the spleen and liver were considerably greater in the leukemic group. In contrast, the ICT-treated leukemic group showed significantly lower Bcl2 mRNA levels (Figure 8A). On the other hand, the mRNA levels of the pro-apoptotic marker Bax (Figure 8B) were significantly lower in the spleen and liver of the leukemic group but significantly higher in the ICT-treated leukemic group. Immunohistochemical results confirmed that the distribution of Bax protein was lower in the spleen and liver of the leukemic group but increased in the ICT-treated leukemic group.
The findings suggest that ICT modulates the IL-6/JAK2/STAT3 pathway, leading to decreased IL-6 expression and reduced activation of JAK2 and STAT3 in the spleen and liver. Moreover, ICT influences cell apoptosis by altering the expression of Bcl2 and Bax, promoting pro-apoptotic markers and inhibiting anti-apoptotic markers (Figure 8C and D).
Discussion
Leukemia is a medical condition characterized by an abnormal increase in the number of immature leukocytes, which compromises immune function and makes individuals more susceptible to infections.27 The exact causes of leukemia are still not fully understood, although exposure to alkylating agents, toxins, and ionizing radiation has been identified as potential contributors.28 These agents can cause DNA damage and chromosome abnormalities, leading to the development of leukemia.29 Specifically, nitrosoureas like n-ethyl-n-nitrosourea (ENU) have been found to induce leukemia by alkylating nucleobases, disrupting DNA, and resulting in bone marrow suppression and the formation of leukemic cells.30,31 ENU has also been observed to generate malignant cells in the brain and reproductive system in animal models.4,32 Any disruption in the signaling cascade that regulates differentiation and proliferation can lead to hematological abnormalities, resulting in a buildup of cancerous cells in the bone marrow and blood.
In the present study involved mice, the administration of ENU led to a persistent decrease in body weight gain, indicating that the chemical or leukemia burden affected the animals’ appetite or metabolism. Additionally, ENU-treated mice exhibited increased spleen and liver weights, possibly due to leukemic cell infiltration or metastasis.33 This finding aligns with a previous study that reported increased liver and spleen weights in ENU-treated SD rats.25 Moreover, Total leukocyte counts were more significant in the ENU group, whereas RBC counts, platelet counts, and hemoglobin levels were lower.34 Immature precursors accumulated because hematopoietic stem cells failed to differentiate properly, hampered hemopoiesis normal progression. According to Abou Elazab, Elbaiomy, Ahmed, Alsharif, Dahran, Elmahallawy and Mokhbatly35 study on leukemic rats exposed to benzene also reported higher white blood cell (WBC) counts than controls. Consistent with our research, Aliyu, Shaari, Ahmad Sayuti, Reduan, Sithambaram, Noordin, Shaari and Hamzah25 found that ENU-treated mice had more blast cells in peripheral blood smears, indicating active leukemia. However, the administration of ICT, a potential therapeutic agent, reduced blast and WBC cells, suggesting anti-leukemic action.24 Leukemic cells produce cytokines that promote their growth and survival in the bone marrow while inhibiting normal hematopoiesis.36 Imbalanced cytokine expression can lead to increased WBC production, decreased RBC production, and the development of leukemia. After ICT therapy, several hematopoietic growth factors and cytokines were suppressed in the ENU-induced leukemic mouse model, potentially counteracting the abnormal cytokine production.
There were increased IL-1β, IL-6, and TNF-α levels in leukemic mice.37 Abnormal production of these cytokines, resulting from genetic flaws or signaling errors, can promote leukemia development.38 IL-1β, for instance, induces the synthesis of GM-CSF, facilitating the development of leukemic cell colonies.39 Leukemic cells also produce high amounts of IL-6, which inhibits lymphoid tissue and promotes chronic myeloid leukemia.40 Similarly, ICT treatment has been shown to reduce proinflammatory cytokine levels in other animal models, such as transgenic adenocarcinoma mouse prostate (TRAMP) mice.41 Increased levels of the transcription factor NF-κB were detected in the livers and spleens of leukemic control mice, supporting its role in leukemia development.42 However, ICT treatment suppressed NF-κB expression, reducing hematological and inflammatory cytokine levels. According to our findings, NF-κB suppression reduced hematological and inflammatory cytokines.43 However, in a social defeat paradigm in mice, ICT treatment lowers neuroinflammation by blocking NF-κB signaling in the hippocampus44 and inflammatory model using lipopolysaccharide and C57BL/6 J mice.44
The IL-6/JAK2/STAT3 pathway is critical in leukemia pathogenesis.45 This signaling cascade promotes the proliferation and survival of plasma cells. In the present study, ICT modulated the JAK2/STAT3 pathway by reducing the expressions of JAK2, p-JAK2, STAT3, and p-STAT3 in spleen and liver tissues. Previous studies have also demonstrated that ICT impairs the Jak2/Stat3/Akt signal pathway and induces growth arrest in leukemia cells.46 ICT has shown promise as a potential treatment for renal cell cancer by reducing STAT3 activity.26
Leukemia can also alter the expression of Bcl-2 and Bax, which regulate mitochondrial function.47 ICT has been found to modulate the expression of these proteins, increasing Bax expression and decreasing Bcl-2 expression. This leads to a reduced Bcl-2/Bax ratio and may contribute to apoptosis induction. Similar effects have been observed in other cancer cell lines, such as endometrial cancer and breast cancer cells, where ICT treatment increased Bax expression and decreased Bcl-2 expression.48 In addition, ICT decreased Bcl-2 expression in MDA-MB-453 and MCF7 breast cancer cells.49 ICT has been found to modulate the expression of these proteins, increasing Bax expression and decreasing Bcl-2 expression. This leads to a reduced Bcl-2/Bax ratio and may contribute to apoptosis induction. Similar effects have been observed in other cancer cell lines, such as endometrial and breast cancer cells, where ICT treatment increased Bax expression and decreased Bcl-2 expression.
Conclusion
In conclusion, the study investigated the effects of ICT in ENU-induced leukemic mice. ICT demonstrated anti-inflammatory effects at 10 and 20 mg/kg doses and exhibited potential therapeutic effects against leukemia by promoting apoptosis and inhibiting the IL-6/JAK2/STAT3 signaling cascades. Validation of the anti-inflammatory effects of ICT at these levels in leukemia patients requires more study. ICT, a compound isolated from the Epimedium genus, has demonstrated anticancer effects in several neoplasms, including hematological malignancies like leukemia, lymphoma, and multiple myeloma. It has cytotoxic effects by inducing apoptosis, stopping the cell cycle, blocking proliferation, encouraging differentiation, and reducing metastasis and infiltration. Critical cell signaling pathways, such as PI3K/Akt, JAK/STAT3, and MAPK/ERK/JNK, are involved in the underlying mechanisms. Icariin and its metabolite ICT have also demonstrated anti-inflammatory and immune-regulating effects, making them potential therapeutic agents for various diseases. Further research on drug delivery systems and treatment methods involving ICT and is warranted to explore their clinical applications.
Declaration of Generative AI and AI-Assisted Technologies in the Writing Process
Authors disclose that no use of generative AI and AI-assisted technologies have been used in the writing process.
Data Sharing Statement
The relevant author can receive requests for the datasets utilized in this inquiry and/or analyzed therewith.
Ethics Approval and Consent to Participate
The First Affiliated Hospital Ethics Committee of Zhengzhou University ensured that the Declaration of Helsinki was followed throughout this investigation. Before having their samples obtained, all individuals gave their informed consent. The Animal Ethics Committee approved all animal experiments, which followed NIH guidelines for laboratory animal care, with measures taken to minimize animal suffering (Approval no. 251111005).
Acknowledgments
This study was supported by the First Affiliated Hospital of Zhengzhou University and King Saud University for providing excellent Services.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R98), King Saud University, Riyadh, Saudi Arabia for financial support.
Disclosure
The authors have no competing interests to disclose in this work.
References
1. Prakash G, Bhattacharjee U, Ck D. Chronic myeloid leukemia blast crisis: an emergency. Onco-Critical Care. 2022;74:271–277.
2. Haferlach C, Rieder H, Lillington DM, et al. Proposals for standardized protocols for cytogenetic analyses of acute leukemias. Chronic Lymphocytic Leukemia. 2007;46(5):494–499.
3. Rausch CR, DiPippo AJ, Jiang Y, et al. Comparison of mold active triazoles as primary antifungal prophylaxis in patients with newly diagnosed acute myeloid leukemia in the era of molecularly targeted therapies; 2022.;
4. Nath P, Modak S, Akter T, Maiti DJIJOP, Sciences A. Effects of N Ethyl N′ nitrosourea in mice brain in time fashion: effect of ENU in brain. Indian J Physiol. 2022;74:1.
5. Vohra N, Chavez T, Troncoso JR, et al. Mammary tumors in Sprague Dawley rats induced by N-ethyl-N-nitrosourea for evaluating terahertz imaging of breast cancer. J Med Im. 2021;8(2):023504. doi:10.1117/1.JMI.8.2.023504
6. Martinez A, Merchan J, Sala M, et al. Carcinogénesis y nitrosoamidas. Patolog. 1974; 7:225–230.
7. Slikker III W, Mei N, Chen TJTS. N-ethyl-N-nitrosourea (ENU) increased brain mutations in prenatal and neonatal mice but not in the adults. Toxicol Sci. 2004;81(1):112–120.
8. Bulnes S, Bermúdez G, Lafuente JVJO. Association of Notch-1. Steo Stem-Like Cells ENU-Glioma Malig Proc. 2018;9(59):31330.
9. Kumar CC. Genetic abnormalities and challenges in the treatment of acute myeloid leukemia. Genes Cancer. 2011;2(2):95–107. doi:10.1177/1947601911408076
10. Huang B, Lang X, Li X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front Oncol. 2022;12:1023177. doi:10.3389/fonc.2022.1023177
11. Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809. doi:10.1038/nrc2734
12. Wang Y, Zheng N, Sun T, Zhao H, Chen Y, Liu CJMMR. Role of TGM2 in T‑cell lymphoblastic lymphoma via regulation of IL‑6/JAK/STAT3 signalling. Mol Med Rep. 2022;25(3):1–9.
13. Xu L, Yao Y, Lu T, LJJom J. interactions n. miR-451a targeting IL-6R activates JAK2/STAT3 pathway, thus regulates proliferation and apoptosis of multiple myeloma cells. Journal of Musculoskeletal & Neuronal Interactions. 2022;22(2):251–260.
14. Wu X-Y, Tian F, Su M-H, et al. BF211, a derivative of bufalin, enhances the cytocidal effects in multiple myeloma cells by inhibiting the IL-6/JAK2/STAT3 pathway. Int Immunopharmacol. 2018; 64(24–32):1.
15. Sheikh IN, Ragoonanan D, Franklin A, et al. Cardiac relapse of acute lymphoblastic leukemia following hematopoietic stem cell transplantation: a case report and review of literature. Cancers. 2021;13(22):5814. doi:10.3390/cancers13225814
16. Allodji RS, Tucker MA, Hawkins MM, et al. Role of radiotherapy and chemotherapy in the risk of leukemia after childhood cancer: an international pooled analysis. International Journal of Cancer. 2021;148(9):2079–2089. doi:10.1002/ijc.33361
17. Piktel D, Nair RR, Rellick SL, et al. Pitavastatin is anti-leukemic in a bone marrow microenvironment model of b-lineage acute lymphoblastic Leukemia. Cancers. 2022;14(11):2681. doi:10.3390/cancers14112681
18. Bukhari S, Siddique MH, Naeem A, et al. Combined efficacy of Cinnamomum zeylanicum and doxorubicin against leukemia through regulation of TRAIL and NF-kappa B pathways in rat model. Mol Biol Rep. 2022; 49:1–13.
19. Maher T, Ahmad Raus R, Daddiouaissa D, et al. Medicinal Plants with anti-leukemic effects: a review. Molecules. 2021;26(9):2741. doi:10.3390/molecules26092741
20. Gao L, Zhang SQ, Effects A. Pharmacokinetics, and drug delivery systems of icaritin: advances and prospects. Pharmaceuticals. 2022;154: 1. doi:10.3390/ph15040397
21. Liu S, Liu C, Xiong L, et al. Icaritin alleviates glutamate-induced neuronal damage by inactivating GluN2B-containing NMDARs through the ERK/DAPK1 pathway. Front Neurosci. 2021;15:525615. doi:10.3389/fnins.2021.525615
22. Wo Y, Zhu D, Hu Y, Wang ZQ, Liu J, Lou Y. Reactive oxygen species involved in prenylflavonoids, icariin and icaritin, initiating cardiac differentiation of mouse embryonic stem cells. J Cellular Bioche. 2008;103(5):1536–1550. doi:10.1002/jcb.21541
23. Liu S, Guo Y, Wang J, et al. A novel anticancer agent SNG1153 inhibits growth of lung cancer stem/progenitor cells. Oncotarget. 2016;7(29):45158. doi:10.18632/oncotarget.9783
24. Zhu J, Li Z, Zhang G, et al. Icaritin shows potent anti-leukemia activity on chronic myeloid leukemia in vitro and in vivo by regulating MAPK/ERK/JNK and JAK2/STAT3/AKT signalings. PLoS One. 2011;6(8): e23720. doi:10.1371/journal.pone.0023720
25. Aliyu A, Shaari MR, Ahmad Sayuti NS, et al. N-Ethyl-n-Nitrosourea induced Leukaemia in a mouse model through upregulation of vascular endothelial growth factor and evading apoptosis. Cancers. 2020;12(3):678. doi:10.3390/cancers12030678
26. Li S, Priceman SJ, Xin H, et al. Icaritin inhibits JAK/STAT3 signaling and growth of renal cell carcinoma. PLoS One. 2013;8(12): e81657. doi:10.1371/journal.pone.0081657
27. Kayser S, Levis M. Updates on targeted therapies for acute myeloid leukaemia. British J Haematol. 2022;196(2):316–328. doi:10.1111/bjh.17746
28. Shallis RM, Wang R, Davidoff A, Ma X, Zeidan A. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Reviews. 2019;36:70–87. doi:10.1016/j.blre.2019.04.005
29. Bhatia S. Therapy-Related Myelodysplasia and Acute Myeloid Leukemia. Elsevier; 2013:666–675.
30. Aliyu A, Shaari MR, Mustapha NM, et al. Some chemical carcinogens for leukaemia induction and their animal models. Annual Res. 2019; 2019:1–7.
31. Nath P, DJJoB M. A review of the mutagenic potential of N‐ethyl‐N‐nitrosourea (ENU) to induce hematological malignancies. J Biochem Molecu Toxicol. 2022;36(7): e23067. doi:10.1002/jbt.23067
32. Kennedy CL, MKJHru O. N-ethyl-N-nitrosourea (ENU) mutagenesis and male fertility research. Human Reprod Update. 2006;12(3):293–301. doi:10.1093/humupd/dmk004
33. Wang J, Liu X, Heflich RH, Tjts C. Time course of cII gene mutant manifestation in the liver, spleen, and bone marrow of N-ethyl-N-nitrosourea-treated big blue transgenic mice. Toxicol Sci. 2004;82(1):124–128. doi:10.1093/toxsci/kfh234
34. Rathkolb B, Klempt M, Sabrautzki S, et al. Screen for alterations of iron related parameters in N-ethyl-N-nitrosourea-treated mice identified mutant lines with increased plasma ferritin levels. Biometals. 2015;28(2):293–306. doi:10.1007/s10534-015-9824-1
35. Abou Elazab MF, Elbaiomy AE, Ahmed MS, et al. Ameliorative effects of bovine lactoferrin on benzene-induced hematotoxicity in albino rats. Veteri Sci. 2022; 9(5): doi:10.3390/vetsci9050240
36. Van Etten RA. Aberrant cytokine signaling in leukemia. Oncogene. 2007;26(47):6738–6749. doi:10.1038/sj.onc.1210758
37. Kartikasari AE, Huertas CS, Mitchell A, Plebanski MJFi O. Tumor-induced inflammatory cytokines and the emerging diagnostic devices for cancer detection and prognosis. Front Oncol. 2021; 11.
38. Zhang CC, Lodish HF. Cytokines regulating hematopoietic stem cell function. Current Opinion Hematol. 2008;15(4):307–311. doi:10.1097/MOH.0b013e3283007db5
39. Parajuli B, Sonobe Y, Kawanokuchi J, et al. GM-CSF increases LPS-induced production of proinflammatory mediators via upregulation of TLR4 and CD14 in murine microglia J Neuroinflam. 2012;9(1):1–12.
40. Reynaud D, Pietras E, Barry-Holson K, et al. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell. 2011;20(5):661–673. doi:10.1016/j.ccr.2011.10.012
41. Hu J, Yang T, Xu H, Hu M, Wen H, Jiang H. A novel anticancer agent icaritin inhibited proinflammatory cytokines in TRAMP mice. Int Urol Nephrol. 2016;48(10):1649–1655. doi:10.1007/s11255-016-1341-9
42. Zhou J, Ching YQ, Chng WJ. Aberrant nuclear factor-kappa B activity in acute myeloid leukemia: from molecular pathogenesis to therapeutic target. Oncotarget. 2015;6(8):5490–5500. doi:10.18632/oncotarget.3545
43. Zhang T, Ma C, Zhang Z, Zhang H, Hu H. NF-κB signaling in inflammation and cancer. Med Comm. 2021;2(4):618–653. doi:10.1002/mco2.104
44. Liu L, Zhao Z, Lu L, et al. Icariin and icaritin ameliorated hippocampus neuroinflammation via inhibiting HMGB1-related pro-inflammatory signals in lipopolysaccharide-induced inflammation model in C57BL/6 J mice. Int Immunol. 2019;68:95–105. doi:10.1016/j.intimp.2018.12.055
45. Johnson DE, Ra O, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15(4):234–248. doi:10.1038/nrclinonc.2018.8
46. Yang J-G, Lu R, X-J Y, Zhang J, Tan Y-Q, Zhou G. Icaritin reduces oral squamous cell carcinoma progression via the inhibition of STAT3 signaling. Int J Mol Sci. 2017;18(1):132. doi:10.3390/ijms18010132
47. Park C, Moon D-O, Rhu C-H, et al. Beta-sitosterol induces anti-proliferation and apoptosis in human leukemic U937 cells through activation of caspase-3 and induction of Bax/Bcl-2 ratio. Bio Phar Bulle. 2007;30(7):1317–1323. doi:10.1248/bpb.30.1317
48. Tong J-S, Zhang Q-H, Huang X, et al. Icaritin causes sustained ERK1/2 activation and induces apoptosis in human endometrial cancer cells. PLoS One. 2011;6(3): e16781. doi:10.1371/journal.pone.0016781
49. Guo Y, Zhang X, Meng J, Wang Z-Y. An anticancer agent icaritin induces sustained activation of the extracellular signal-regulated kinase (ERK) pathway and inhibits growth of breast cancer cells. Eur J Pharmacol. 2011;658(2–3):114–122. doi:10.1016/j.ejphar.2011.02.005
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.