Back to Journals » Infection and Drug Resistance » Volume 15
Myrtenol Inhibits Biofilm Formation and Virulence in the Drug-Resistant Acinetobacter baumannii: Insights into the Molecular Mechanisms
Authors Liu L, Liu B, Li L, He MX, Zhou XD, Li Q
Received 20 June 2022
Accepted for publication 17 August 2022
Published 2 September 2022 Volume 2022:15 Pages 5137—5148
DOI https://doi.org/10.2147/IDR.S379212
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Lei Liu,1,2,* Bin Liu,1,2,* Liang Li,1,2 Ming-Xin He,2 Xiang-Dong Zhou,1,2 Qi Li1,2
1Department of Respiratory Medicine, the First Affiliated Hospital of Hainan Medical University, Haikou, Hainan, People’s Republic of China; 2NHC Key Laboratory of Tropical Disease Control, Hainan Medical University, Haikou, Hainan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qi Li, Email [email protected]
Background: blaNDM-1-producing Acinetobacter baumannii (BP-AB) remains a critical problem in nosocomial infections, because of its resistance mediated by the biofilm formation and virulence factors. No studies have confirmed myrtenol’s efficacy in inhibiting the biofilm formation and virulence associated with biofilm of BP-AB.
Methods: The tested concentrations of myrtenol were wild type (A), 50 μg/mL (B), 100 μg/mL (C), 200 μg/mL (D), 250 μg/mL (E), and 300 μg/mL (F).
Results: The BP-AB biofilm inhibition was significantly higher in the D, E, and F groups than in the A, B, and C groups. Myrtenol significantly reduced the air-liquid interface ring formation in glass tubes. It also effectively inhibited the attachment of BP-AB strains on polystyrene surfaces as shown by crystal violet staining. Microscopy showed a significant reduction in biofilm formation with dispersed BP-AB strains. The confocal laser scanning microscopy analysis showed a significant reduction in the biofilm’s biomass, covered surface area, and thickness. The scanning electron microscopy analysis revealed significantly fewer BP-AB aggregates on the coverslip surface. In the CompStat analysis, the biofilm’s biomass, maximum thickness, and surface-to-volume ratio were significantly reduced. The qPCR analysis revealed a significant down-regulation of bfmR, bap, csuA/B, and ompA expression, which positively correlated with the biofilm’s biomass, maximum thickness, and surface-to-volume ratio in BP-AB strains. Myrtenol significantly improved the susceptibility of BP-AB to the antibiotics amikacin, piperacillin/tazobactam, cefoperazone/sulbactam, and ceftazidime.
Conclusion: Myrtenol attenuates the BP-AB biofilm formation and virulence by suppressing the expression of bfmR, bap, csuA/B, and ompA.
Keywords: Acinetobacter baumannii, myrtenol, biofilm, virulence factors, resistance
Introduction
Acinetobacter baumannii is a major opportunistic pathogen that causes a variety of nosocomial infections, constituting a worldwide critical problem in healthcare settings despite the implementation of infection control practices.1 A. baumannii infections are often difficult to treat because of intrinsic and acquired resistance mechanisms associated with poor clinical outcomes,2 with carbapenems being the mainstream treatment for multidrug resistant (MDR) A. baumannii.3
The clinically important enzyme New Delhi metallo-β-lactamase 1 (NDM-1) was first reported in A. baumannii in India, and blaNDM-1-producing A. baumannii (BP-AB) has since been reported worldwide.4,5 BP-AB makes several antibiotics such as carbapenems and other beta-lactam antibiotics ineffective.6 BP-AB is an MDR pathogen of concern and has been categorized as of high priority by the World Health Organization.7 In recent years, the number of BP-AB infections has been climbing.8 Due to the extremely limited number of antibiotics to treat BP-AB infections, mortality has increased.8
One of the major and pivotal characteristics of BP-AB is the ability to form biofilms on biotic and abiotic surfaces, which is the primary cause of its resistance and persistence.9 BP-AB biofilms are made of self-produced extracellular polymeric molecules such as polysaccharides, proteins, and eDNA.8,10 BP-AB has multiple cell surfaces that secrete virulence factors, such as biofilm-associated protein (bap), two-component system (bfmS/bfmR), chaperon-usher pilus (csuA/BABCDE), poly-β-(1,6)-N-acetyl glucosamine, and outer membrane protein A (ompA), that are principally involved in adherence and persistence.8,10 The biofilm protects A. baumannii from the host’s innate immune response and acts as a barrier against the entry of antibiotics.11 Targeting the biofilm formation of A. baumannii is an effective way to control antibiotic resistance and biofilm-associated infections.11 The target of novel therapies is to inhibit the biofilm formation and the production of virulence factors instead of killing the bacteria, thereby, diminishing the selection pressure on bacteria and preventing the development of resistance.11,12
Recently, a study revealed myrtenol’s sarA-mediated antibiofilm and antivirulence potential against methicillin-resistant staphylococcus aureus (MRSA).12 Another study demonstrated that myrtenol improved the susceptibility of A. baumannii strains to conventional antibiotics such as amikacin, ciprofloxacin, gentamicin, and trimethoprim, also presenting a therapeutic potential against biofilm-associated infections.11
Different A. baumannii strains have distinct abilities of adhesion and biofilm formation.11 However, no studies have confirmed the efficacy of myrtenol against BP-AB. The objective of this study was to evaluate myrtenol’s effect on BP-AB biofilm formation, virulence factor production, and antibiotic susceptibility profile.
Methods
Phytochemical
Myrtenol of 95% purity was purchased from Sigma Aldrich (Gillingham, UK). The myrtenol concentrations used in all the experiments were 0 μg/mL(wild type, A), 50 μg/mL (B), 100 μg/mL (C), 200 μg/mL (D), 250 μg/mL (E), and 300 μg/mL (F).
Bacterial Strains and Culture Conditions
The bacterial strains used in this study are as follows: the clinically isolated BP-AB strain and the AB reference strain ATCC-19606. The bacterial strains were cultivated in tryptic soy broth supplemented with 1% sucrose and 0.5% yeast extract (TSBSY) to boost biofilm formation and were incubated in an orbital shaker (160 rpm) at 37 °C for 24 h.
Determination of the Minimum Biofilm Inhibitory Concentration (MBIC)
A overnight culture of BP-AB was applied to inoculate 1 mL of 1%TSBSY in a 24-well plate incubated with or without myrtenol for 24 h at 37 °C. The cell density was determined at 600 nm after incubation, and the planktonic cells were subsequently removed. Then, the plate was washed using sterile phosphate buffered saline (PBS) to discard unbound cells and was immediately air-dried. Next, the plate was stained with 0.4% crystal violet solution (CVS) for 10 min and washed with phosphate-buffered saline to discard the excess stain. The plate was destained with 30% glacial acetic acid solution and observed at 570 nm. The rate of biofilm inhibition was determined using the formula: Rate = [(ControlOD570nm − TreatedOD570nm)/ControlOD570nm]×100.11 The density of cell or concentration of starter culture was 1×108 CFU/mL for MBIC determination method.
Determination of the Minimum Inhibitory Concentration (MIC)
Myrtenol-induced MIC against BP-AB was determined using the microtiter plate method.11 The cell density of the culture after incubation with myrtenol was determined at 600 nm using Spectramax M3 (Molecular Devices, USA). The density of cell or concentration of starter culture was 1×108 CFU/mL for MIC determination method.
Determination of Ring Formation with the Air-Liquid Interface Assay in Glass Tubes
The influence of myrtenol on biofilm formation of the BP-AB strains was evaluated with the air-liquid interface assay as previously described.11 Briefly, the overnight cultures were incubated with 2 mL TSBSY with or without myrtenol in glass test tubes at 37 °C for 48 h. Subsequently, the test tubes were washed 3 times with PBS and immediately stained with 0.4% CVS.11
Microscopy Analyses
The biofilm assay was performed on 24-well plates including glass slides incubated with or without myrtenol at 37°C for 24 h. Then, the glass slides were washed with sterile PBS and immediately air-dried.
For the light microscopy analysis, the slides were stained with 0.4% CVS and subsequently observed at a magnification of 400× using a Nikon Eclipse Ti-S microscope (Tokyo, Japan).
For the confocal laser scanning microscopy analysis,11 bacterial cells from the biofilm and pellicle were fixed with 2% glutaraldehyde in 0.1 M cacodylate buffer for 16 h and 1% osmium tetroxide in 0.1 M phosphate for 1 h. The samples were washed with 0.05 M cacodylate buffer and dehydrated in a series of ethanol washes (50%, 75%, 90%, 95%, and 100% for 20 min each) before being dried with a critical point dryer (Hitachi, Tokyo, Japan). The dried specimens were coated with platinum/palladium and were observed(25nm) using a Hitachi E-1030 ion sputter. The prepared specimens were observed under a Hitachi S-4300 scanning electron microscopy (SEM) operating at 15 kV. CompStat 2 was used to calculate the BP-AB biofilm’s biomass, thickness, and surface-to-volume ratio grown with or without myrtenol.
Antibiotic Sensitivity Analysis
To evaluate the efficacy of myrtenol in improving the susceptibility of BP-AB to the conventional antibiotics amikacin, meropenem, piperacillin/tazobactam, cefoperazone/sulbactam, and ceftazidime, antibacterial agents’ MIC was measured by performing the microbroth dilution method. The associative effectiveness of myrtenol against the antibacterial agents was evaluated by growing BP-AB strains with or without antibiotics. Briefly, 200 μL of TSBSY broth was placed in each well of a 96-well plate in the presence of a 1% overnight culture of BP-AB treated with or without myrtenol and the antibacterial agents for 24 h at 37 °C and read at 600 nm. The disc diffusion analysis was executed as per the Clinical and Laboratory Standards Institute guidelines to ascertain the sensitivity of BP-AB to antibiotics after treatment with or without myrtenol.
Quantitative Real-Time PCR
Total RNA from control and myrtenol-treated BP-AB cultures was isolated with an RNeasy Mini Kit (Qiagen, Valencia, CA, USA). cDNA was synthesized from the total RNA using a PrimeScript™ RT Master Mix (TakaRa, Japan). Real-time PCR amplification of the cDNA was performed on a Thermal Cycler Dice® Real Time System (TaKaRa, Japan) using an SYBR Premix Ex Taq (TaKaRa, Japan) and a pair of specific primers (Table 1).
 |
Table 1 List of Primers Used for qPCR Analysis |
The qPCR analysis was performed on the real-time PCR 7500 Sequence Detection System (Applied Biosystems Inc. Foster, CA, USA) for the biofilm-associated virulence genes bfmR, bap, csuA/B, ompA, pgaA, pgaC, katE, and rplB using a PCR mix (SYBR Green kit, Applied Biosystems, United States) at a predefined ratio. The relative expression level of the target genes was calculated using the 16S rRNA expression levels as an internal reference for normalization. The fold change was determined using the 2(−ΔΔCt) method.
Statistical Analysis
There were five independent experiments in each method. Average values and standard errors of the means were calculated from at least three independent experiments. Quantitative variables are expressed as means ± standard deviation and were compared using the t-test. Spearman’s rank-order correlation was used to study the correlations between the expression levels of genes and biofilm of BP-AB isolates. The differences between experimental groups were considered significant for P-values < 0.05. All statistical tests were performed on SPSS 17.0 (SPSS Inc., Chicago, IL)
Results
BP-AB Growth and Biofilm Formation
After 4 h of culture, BP-AB entered the exponential growth period. After 14 h of culture, BP-AB entered the platform stage (Figure 1). The biofilm formation entered a rapid growth period after 18 h of culture. After 36 h of culture, the BP-AB biofilm formation entered the platform stage (Figure 1).
 |
Figure 1 Growth and biofilm formation of BP-AB assessed by curve analysis. |
Effect of Myrtenol on the BP-AB Biofilm Growth and Formation
The biofilm formation entered a rapid growth period after 18 h of culture in all the groups. After 36 hours of culture, the biofilm formation of BP-AB entered the platform stage. However, the biofilm formation was significantly higher in groups A, B, and C than in groups D, E, and F(P<0.01) (Figures 2 and 3).
 |
Figure 2 Inhibitory curve with various concentrations of myrtenol treatment on biofilm formation of BP-AB. |
 |
Figure 3 Antibiofilm efficacy comparison with various concentrations of myrtenol treatment on biofilm formation of BP-AB. Note: ****Represents P<0.01. |
Effect of Myrtenol on MIC and MBIC of BP-AB Strains
The microtiter plate method and the crystal violet quantification assay were performed to determine MIC and MBIC of myrtenol-treated BP-AB strains, respectively. MBIC is defined as the minimum concentration of myrtenol that manifests greater than 50% biofilm inhibition without influencing growth. Group D showed strong antibiofilm activity against BP-AB (85%), therefore, 200 μg/mL of myrtenol was fixed as the MBIC for the BP-AB strains. Myrtenol’s MIC was 600 μg/mL for the BP-AB strain.
Effect of Myrtenol on BP-AB Ring Formation in Glass Test Tubes
The BP-AB biofilm’s ability to form rings at the air-liquid interface assay by adhering to the top portion of the liquid medium was significantly higher in groups A, B, and C than in groups D, E, and F(P<0.01). Therefore, myrtenol reduced the ring formation at the highest myrtenol concentrations (Figure 4A).
Microscopic Visualization of the BP-AB Biofilm
Well-structured and highly aggregated biofilm formation was observed in groups A, B, and C. However, a significant reduction in biofilm formation with dispersed BP-AB strains was observed in groups D, E, and F(P<0.01) (Figure 4B). Confocal laser scanning microscopy was performed to assess the effect of myrtenol on the three-dimensional biofilm architecture of BP-AB. Multi-layered biofilms of high biomass were observed in groups A, B, and C. Nonetheless, a significant reduction in biofilm’s biomass, surface-covered area, and thickness was observed in groups D, E, and F(P<0.001) (Figure 4C). The SEM analysis indicated that BP-AB was aggregated on the polystyrene coverslip and semi-submerged in the culture; these aggregates were extensively spread throughout the coverslip in groups A, B, and C (Figure 4D). In contrast, significantly fewer BP-AB aggregates were found on the coverslip surface in groups D, E, and F. In the CompStat analysis, the biofilm’s biomass, maximum thickness, and surface-to-volume ratio were significantly reduced in groups D, E, and F(P<0.001) than groups A, B, and C. However, these parameters were not notably reduced in groups A, B, and C (Figure 5).
 |
Figure 5 Comstat analysis of biofilms formed by BP-AB strains with various concentrations of myrtenol treatment. Note: *Represents P<0.001. |
Myrtenol Regulates the Expression of Biofilm-Associated Genes in the BP-AB Strain
The qPCR analysis revealed a significant reduction in the expression levels of bfmR, bap, csuA/B, and ompA in the D, E, and F groups than groups A, B, and C (P<0.01). A non-significant reduction in the levels of bfmR, bap, csuA/B, and ompA was also found in groups A, B, and C (Figure 6). The bfmR, bap, csuA/B, and ompA genes are the key regulators of the transition from biofilm formation to maturation in the BP-AB strain (Figure 7).
 |
Figure 6 Effect of various concentrations of myrtenol treatment on expression profile of genes involved in biofilm formation in BP-AB. Note: *Represents P<0.01. |
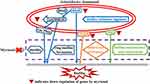 |
Figure 7 A schematic diagram representing the effect of myrtenol treatment on biofilm associated genes expression in BP-AB. |
Myrtenol Improves the Antibiotic Susceptibility of BP-AB
The antibiotic susceptibility of BP-AB was evaluated with or without myrtenol, for which the MIC of the antibiotics amikacin, meropenem, piperacillin/tazobactam, cefoperazone/sulbactam, and ceftazidime was determined by the microbroth dilution method. Myrtenol at the highest concentrations (groups D, E, and F) improved the sensitivity of amikacin, piperacillin/tazobactam, cefoperazone/sulbactam, and ceftazidime against BP-AB. In the case of groups A, B, and C, the variation of the sensitivity was non-significant. These results indicate that myrtenol improves the susceptibility of BP-AB strains to the antibiotics amikacin, piperacillin/tazobactam, cefoperazone/sulbactam, and ceftazidime but not meropenem (Table 2).
 |
Table 2 Antibiotic MIC Values of blaNDM-1-Producing Acinetobacter Baumannii Strains in the Absence and Presence of Myrtenol |
Gene Expression Correlates with BP-AB Biofilm’s Parameters
The biofilm’s biomass, maximum thickness, and surface-to-volume ratio positively correlated with the expression levels of bfmR (r = 0.81; p = 0.022), bap (r = 0.86; p = 0.018), csuA/B (r = 0.89; p = 0.015), and ompA (r = 0.79; p = 0.025) in the BP-AB strain.
Discussion
BP-AB has emerged as an important nosocomial pathogen causing mainly meningitis, peritonitis, bacteremia, and pneumonia among other diseases.1 Multidrug resistance may complicate the treatment of serious BP-AB infections, leading to a lack of therapeutic options and subsequently poor clinical outcomes.2 Therefore, the need for a novel therapeutic strategy to combat BP-AB infections has gained more attention.11 Its biofilm-forming ability and the secreted virulence factors are the major components of drug resistance.9 Almost all bacteria can form biofilms. Because the drug resistance of bacteria with biofilm is multifold compared to those without biofilm, it causes refractory infections in the clinic.13 The bacterial biofilm is a dense complex attached to the surface of objects where bacteria are tightly connected and wrapped by an extracellular matrix composed of carbohydrates, nucleic acids, proteins, and other molecules.12 The formation of biofilms is additionally an important component for the long-term survival of BP-AB in the hospital environment causing persistent infections.14
Antibiofilm agents attenuate the adherence and the secretion of virulence factors of pathogens instead of affecting their growth, leading to lower resistance development.15 In addition, this therapy enhances the sensitivity of bacteria to antibiotics and the host immune system.11 This indicates the necessity of antibiofilm therapy and the importance of the discovery of novel antibiofilm agents. Blocking the pathogenic virulence factors of the BP-AB biofilm can be an effective method to treat BP-AB infections in the future.
In this context, medicinal plants are some of the largest sources of bioactive molecules for antibacterial and antifungal purposes.9–11 Products of natural origin can show considerable efficacy against biofilms formed by different types of bacteria.9 Previously, numerous antibiofilm agents from natural sources, such as chimeric peptides, curcumin, magainin, honokiol, and magnolol have been explored against A. baumannii biofilms.11 Myrtenol is derived from Myrtus communis, Cyperus rotundus, Tanacetum vulgare, Dendroctonus armandi, and Pinus armandii.11,12 In traditional medicine, myrtenol (PubChem CID: 10582) has been explored for the treatment of inflammations and infections.11,12 It is essential to analyze their cytotoxicity to ensure pharmaceutical safety as a therapeutic candidate.16
It has been shown that myrtenol inhibits biofilm formation and virulence factors without affecting MRSA growth and viability.12 Another study showed that myrtenol has antibiofilm and antivirulence effects against A. baumannii.11 Biofilm formation and virulence factors’ inhibition by myrtenol in BP-AB strains remain to be explored. In this study, we explored the mechanism and efficacy of myrtenol in the BP-AB biofilm formation and the inhibition of virulence factors, alongside the genetic environment of clinical MDR BP-AB strains carrying the NDM-1 gene and determined the in vitro effects of various antibiotics.
Here, we showed that BP-AB entered the exponential growth period after 4 h of culture and the plateau period after 14 h of culture, consistent with previous literature on the growth curve of BP-AB.17 A minor discrepancy was found for the plateau period (14-h culture here instead of the previously reported 16 h), which may be due to the different average time of culture. Our results showed that BP-AB biofilm formation entered a rapid growth period after 18 h of culture. After 36 h of culture, the biofilm formation entered the platform stage. Our results are consistent with previous studies.17 This is in line with the law of BP-AB growth and biofilm formation, that is after the BP-AB population reaches a certain growth, the biofilm formation follows.
We confirmed the previously reported broadness of myrtenol’s antibiofilm efficacy against A. baumannii isolates11 with further assays for BP-AB strains. Myrtenol’s MBIC was 200 μg/mL without affecting the growth of BP-AB strains, consistent with previous results.11 The growth curve analysis of BP-AB treated with 200 µg/mL of myrtenol demonstrated the non-antibacterial nature of myrtenol. Myrtenol’s MIC was 600 μg/mL against the BP-AB strains, a value slightly higher than previous results,11 probably because BP-AB biofilm inhibition requires higher concentrations of myrtenol.
Ring formation on the surface of the biofilm as evaluated by the air-liquid interface assay on a glass surface was highly diminished with 200 μg/mL, 250 μg/mL, and 300 μg/mL of myrtenol. These treatments also displayed significantly fewer bacterial aggregates on the coverslip surface than the control, 50 μg/mL, and 100μg/mL myrtenol groups. As revealed by the SEM analysis, BP-AB aggregated on the biofilm, and these aggregates were extensively spread throughout the coverslip in the control group, and the groups of 50 μg/mL and 100 μg/mL myrtenol treatment. In contrast, myrtenol at 200 μg/mL, 250 μg/mL, and 300 μg/mL displayed significantly fewer bacterial aggregates on the coverslip surface.
To study the molecular mechanism of myrtenol, the gene expression profiles of control and myrtenol-treated BP-AB strains were evaluated using qPCR. The levels of bfmR, bap, csuA/B, and ompA, key genes in the regulation of BP-AP biofilm formation and maturation, were down-regulated after myrtenol treatment.
The regulation system of pili synthesis encoded by the csuA/BABCDE gene directly plays a role in the early stage of biofilm formation.18 The csuA/BABCDE assembly system mediates the synthesis and adhesion of pili products to abiotic surfaces and the formation of colonies, which is the first step of biofilm formation.18 This system is regulated by the bfmR system,19 which is the response regulatory component of the two-component system bfmRS that regulates the csu operon and is related to cilia assembly and biofilm formation.20 The biofilm-associated protein bap is necessary for the interaction among bacteria and participates in the formation and maturation of the biofilm.21 BP-AB ompA is found in outer-membrane vesicles and plays a role in the adhesion to and invasion of epithelial cells.22 OmpA also promotes BP-AB’s survival in the environment by assisting in the formation of the biofilm.23 The present study showed that the expression levels of bfmR, bap, csuA/B, and ompA positively correlated with the biofilm’s biomass, maximum thickness, and surface-to-volume ratio in BP-AB strains. Therefore, myrtenol affects BP-AB biofilm and virulence by possibly suppressing the bfmR, bap, csuA/B, and ompA expression (Figure 7).
Previous studies reported that antibiofilm agents increased the sensitivity of chronic infection-causing bacteria towards antibiotics.11 Hence, the influence of myrtenol treatment on the BP-AB sensitivity to antibiotics was evaluated. Myrtenol treatment improved the susceptibility of BP-AB to the conventional antibiotics amikacin, piperacillin/tazobactam, cefoperazone/sulbactam, and ceftazidime, but not meropenem. Our results are almost consistent with previous reports.11 However, the effect of myrtenol on meropenem may be mediated by ompA which plays an important role in the resistance of BP-AB to this agent.3 Myrtenol’s ability to enhance the antibacterial activity of commonly used antibiotics is crucial in the clinical context.
The present study is the first to demonstrate the antibiofilm and antivirulence factors efficacy of myrtenol against BP-AB strains. Nevertheless, there are a few limitations to our study. First, due to limited funding, not all virulence factors were tested. This will be the focus of our future study. Second, considering that it has been previously confirmed that myrtenol does not affect A. baumannii and MRSA growth and viability,11,12 the relevant tests of BP-AB growth and viability were not repeated. Finally, myrtenol treatment improved the susceptibility of BP-AB strains to only conventional antibiotics. Therefore, it is still necessary to test the drug sensitivity of BP-AB to other antibiotics.
Conclusions
The present study revealed myrtenol’s antibiofilm and antivirulence efficacy against BP-AB strains. Myrtenol effectively reduced the biofilm-forming ability of BP-AB strains. Simultaneously, it suppressed the expression of key genes in biofilm formation and maturation, namely bfmR, bap, csuA/B, and ompA. The levels of said genes positively correlated with the biofilm’s biomass, maximum thickness, and surface-to-volume ratio in BP-AB strains. Myrtenol possibly inhibits the BP-AB biofilm and virulence capacity by suppressing the bfmR, bap, csuA/B, and ompA expression. Furthermore, myrtenol treatment improved the susceptibility of BP-AB strains to the conventional antibiotics amikacin, piperacillin/tazobactam, cefoperazone/sulbactam, and ceftazidime but not meropenem.
Abbreviations
bap, Biofilm-associated protein; bfmS/bfmR, two-component system; BP-AB, blaNDM-1-producing A. baumannii; csuA/BABCDE, chaperon-usher pilus; CVS, crystal violet solution; MDR, multidrug-resistant; MBIC, minimum biofilm inhibitory concentration; MIC, minimum inhibitory concentration; MRSA, methicillin-resistant staphylococcus aureus; NDM-1, New Delhi metallo-β -lactamase 1; ompA, outer membrane protein A; PBS, phosphate buffered saline; SEM, scanning electron microscopy; TSBSY, tryptic soy broth supplemented with 1% sucrose and 0.5% yeast extract.
E-Mails of Authors
Lei Liu: [email protected]; Bin Liu: [email protected]; Liang Li: [email protected]; Mingxin He: [email protected]; Xiangdong Zhou: [email protected]; Qi Li: [email protected].
Data Sharing Statement
The data will be made available upon reasonable request to the corresponding author after the publication. The availability of data also needs to be approved by the institutional ethics board of the Hainan Medical University.
Compliance with Ethics Guidelines
The institutional ethics board of the Hainan Medical University provided ethical approval (ethical approval number: HYLL-2021-273) for this project.
Consent for Publication
All the authors have been involved in this study and will hold themselves responsible for its content.
Acknowledgments
We would like to thank the Heilongjiang Provincial Key Laboratory of Veterinary Medicine, the Harbin Institute of Veterinary Medicine, and the Chinese Academy of Agricultural Sciences for their technical support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The present study was supported by Hainan Provincial Natural Science Foundation of China, project number:822MS176. The project supported by the Education Department of Hainan Province, project number: Hnky2022ZD-11. This study was supported by the Open Foundation of NHC Key Laboratory of Tropical Disease Control, Hainan Medical University (2021NHCTDCKFKT21008), the project supported by Hainan Province Clinical Medical Center.
Disclosure
We have no conflicts of interest to declare.
References
1. Chatterjee S, Mondal A, Mitra S, Basu S. Acinetobacter baumannii transfers the blaNDM-1 gene via outer membrane vesicles. J Antimicrob Chemother. 2017;72(8):2201–2207. doi:10.1093/jac/dkx131
2. Leão AC, Menezes PR, Oliveira MS, Levin AS. Acinetobacter spp. are associated with a higher mortality in intensive care patients with bacteremia: a survival analysis. BMC Infect Dis. 2016;16:386. doi:10.1186/s12879-016-1695-8
3. Nowak P, Paluchowska P. Acinetobacter baumannii: biology and drug resistance - role of carbapenemases. Folia Histochem Cytobiol. 2016;54(2):61–74. doi:10.5603/FHC.a2016.0009
4. Jaidane N, Naas T, Oueslati S, et al. Whole-genome sequencing of NDM-1-producing ST85 Acinetobacter baumannii isolates from Tunisia. Int J Antimicrob Agents. 2018;52(6):916–921. doi:10.1016/j.ijantimicag.2018.05.017
5. Pritsch M, Zeynudin A, Messerer M, et al. First report on bla NDM-1-producing Acinetobacter baumannii in three clinical isolates from Ethiopia. BMC Infect Dis. 2017;17(1):180. doi:10.1186/s12879-017-2289-9
6. Agoba EE, Govinden U, Peer AKC, Osei Sekyere J, Essack SY. ISAba1 regulated OXA-23 carbapenem resistance in Acinetobacter baumannii strains in Durban, South Africa. Microb Drug Resist. 2018;24(9):1289–1295. doi:10.1089/mdr.2017.0172
7. Dortet L, Poirel L, Nordmann P. Worldwide dissemination of the NDM-type carbapenemases in Gram-negative bacteria. Biomed Res Int. 2014;2014:1–12. doi:10.1155/2014/249856
8. Aliramezani A, Soleimani M, Fard RMN, Nojoomi F. Virulence determinants and biofilm formation of Acinetobacter baumannii isolated from hospitalized patients. Germs. 2019;9(3):148–153. doi:10.18683/germs.2019.1171
9. Villacís JE, Bovera M, Romero-Alvarez D, et al. NDM-1 carbapenemase in Acinetobacter baumannii sequence type 32 in Ecuador. N Microbes New Infect. 2019;29:100526. doi:10.1016/j.nmni.2019.100526
10. Eze EC, Chenia HY, El Zowalaty ME. Acinetobacter baumannii biofilms: effects of physicochemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infect Drug Resist. 2018;15(11):2277–2299. doi:10.2147/IDR.S169894
11. Selvaraj A, Valliammai A, Sivasankar C, Suba M, Sakthivel G, Pandian SK. Antibiofilm and antivirulence efficacy of myrtenol enhances the antibiotic susceptibility of Acinetobacter baumannii. Sci Rep. 2020;10(1):21975. doi:10.1038/s41598-020-79128-x
12. Selvaraj A, Jayasree T, Valliammai A, Pandian SK. Myrtenol attenuates MRSA biofilm and virulence by suppressing sarA expression dynamism. Front Microbiol. 2019;4(10):2027. doi:10.3389/fmicb.2019.02027
13. Figueiredo AMS, Ferreira FA, Beltrame CO, Côrtes MF. The role of biofilms in persistent infections and factors involved in ica-independent biofilm development and gene regulation in Staphylococcus aureus. Crit Rev Microbiol. 2017;43(5):602–620. doi:10.1080/1040841X.2017.1282941
14. Jayathilaka EH, Rajapaksha DC, Nikapitiya C, De Zoysa M, Whang I. Antimicrobial and anti-biofilm peptide octominin for controlling multidrug-resistant Acinetobacter baumannii. Int J Mol Sci. 2021;22(10):5353. doi:10.3390/ijms22105353
15. Kim HR, Shin DS, Jang HI, Eom YB. Anti-biofilm and anti-virulence effects of zerumbone against Acinetobacter baumannii. Microbiology. 2020;166(8):717–726. doi:10.1099/mic.0.000930
16. Cordeiro L, Figueiredo P, Souza H, et al. Antibacterial and antibiofilm activity of myrtenol against Staphylococcus aureus. Pharmaceuticals. 2020;13(6):133. doi:10.3390/ph13060133
17. Guo HN, Chen Z, Xiang J. [Influence of abaR gene knockout on growth metabolism and biofilm formation of Acinetobacter baumannii]. Zhonghua Shao Shang Za Zhi. 2020;36(1):32–36. Chinese. doi:10.3760/cma.j.issn.1009-2587.2020.01.006
18. Ramezanalizadeh F, Owlia P, Rasooli I. Type I pili, CsuA/B and FimA induce a protective immune response against Acinetobacter baumannii. Vaccine. 2020;38(34):5436–5446. doi:10.1016/j.vaccine.2020.06.052
19. Krasauskas R, Skerniškytė J, Armalytė J, Sužiedėlienė E. The role of Acinetobacter baumannii response regulator BfmR in pellicle formation and competitiveness via contact-dependent inhibition system. BMC Microbiol. 2019;19(1):241. doi:10.1186/s12866-019-1621-5
20. Schweppe DK, Harding C, Chavez JD, et al. Host-microbe protein interactions during bacterial infection. Chem Biol. 2015;22(11):1521–1530. doi:10.1016/j.chembiol.2015.09.015
21. Yang CH, Su PW, Moi SH, Chuang LY. Biofilm formation in Acinetobacter baumannii: genotype-phenotype correlation. Molecules. 2019;24(10):1849. doi:10.3390/molecules24101849
22. Nie D, Hu Y, Chen Z, et al. Outer membrane protein A (OmpA) as a potential therapeutic target for Acinetobacter baumannii infection. J Biomed Sci. 2020;27(1):26. doi:10.1186/s12929-020-0617-7
23. Clemmer KM, Bonomo RA, Rather PN. Genetic analysis of surface motility in Acinetobacter baumannii. Microbiology. 2011;157(Pt 9):2534–2544. doi:10.1099/mic.0.049791-0
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

