Back to Journals » Infection and Drug Resistance » Volume 16
Relationship Between Drug Resistance Characteristics and Biofilm Formation in Klebsiella Pneumoniae Strains
Authors Dan B, Dai H, Zhou D, Tong H, Zhu M
Received 16 November 2022
Accepted for publication 4 February 2023
Published 17 February 2023 Volume 2023:16 Pages 985—998
DOI https://doi.org/10.2147/IDR.S396609
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Binzhi Dan,* Heping Dai,* Dangui Zhou, Hongfang Tong, Mei Zhu
Department of Clinical Laboratory, the Affiliated Chaohu Hospital of Anhui Medical University, Chaohu, Anhui, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mei Zhu, Tel +86 551 8232 4254, Email [email protected]
Objective: To conduct epidemiological analysis of Klebsiella pneumoniae (K. pneumoniae) with hypervirulence, and to investigate its drug resistance phenotype, Extended-spectrum β-lactamase (ESBLs) gene, virulence factor, capsular serotype and biofilm formation, so as to provide theoretical basis for further understanding of the drug resistance mechanism of K. pneumoniae with hypervirulence.
Methods: K. Pneumoniae were isolated from clinical samples collected from inpatients. All strains were identified by VITEK2 Compact using fully automatic microbial analyzer, the minimal inhibitory concentration (MIC) of antibiotics was determined by microbroth dilution test. The double disk diffusion method was used to detect the production of ESBLs, modified carbapenem inactivation method (mCIM) was used to detect the production of carbapenemase, and hypermucoviscosity phenotype was detected by wire drawing test. PCR was used to detect ESBLs gene, virulence factor and capsular serotype. Crystal violet staining was used to detect the ability of biofilm formation.
Results: The ESBLs genes detected in this study included strains blaTEM 35 (36.5%), blaSHV 51 (53.1%), and blaCTX-M 49 (51.0%). Most strains carried multiple ESBLs genes, but not all of them produce ESBLs. K1 and K2 accounted for 14.6% and 11.5% respectively. Most (91.7%) strains carried the fimH gene, and the other virulence genes were ybtS (53.1%), entB (46.9%), rmpA (41.7%), aerobactin (32.3%), allS (15.6%), kfu (15.6%). Of all the Klebsiella pneumoniae strains, 33 (34.4%) exhibited ESBLs phenotype, 16 (16.7%) were carbapenemase-producing, and 20 (20.8%) with ESBLs phenotype tested were resistant to all four drugs. The correlation between ESBLs-producing strains and biofilm formation was significantly increased compared to strains without ESBLs phenotype (P=0.035).
Conclusion: Compared to hypervirulent Klebsiella pneumoniae (hvKP), classical Klebsiella pneumoniae (cKP) has a tendency to acquire antibiotic resistance. Our study showed that genes encoding rmpA, K1 or K2, and kfu were highly associated with hvKP.
Keywords: Klebsiella pneumoniae, antimicrobial resistance, hypermucoviscosity, virulence factors, epidemiology
Introduction
In 1920s, it was discovered that certain microorganisms inhibit the growth and reproduction of other microorganisms, a phenomenon known as antibiotics. Later, people extracted substances with antibiotic effect from certain microorganisms, namely antibiotics. There are many types of antibiotics in clinical use, and the common ones are β-lactams, aminoglycosides, quinolones, sulfonamides, nitrofurans, etc. The bactericidal effect of β-lactam antibiotics is achieved by inhibiting the activity of bacterial peptidoglycan transpeptidase, and it is usually classified into five groups, namely penicillins, carbapenems, cephalosporins, clavams and monolactams.1 Aminoglycosides are naturally occurring antibiotics with oligosaccharide structures whose bactericidal effect is a result of their linkage to specific conserved sequences of 16SrRNA leading to some changes in the 16SrRNA spatial conformation, which leads to misinterpretation of the genetic code by the associated transfer RNA (tRNA). Quinolones are broad-spectrum antibiotics used to treat infectious diseases caused by Gram-negative bacteria. Quinolones target type II bacterial topoisomerases, which inactivate the processes of DNA replication, DNA repair, transcription, and superhelical topology, resulting in rapid bacterial cell death. Sulfonamides are synthetic broad-spectrum antibiotics that achieve antibacterial effects by inhibiting the formation of folic and nucleic acids. Nitrofuran antibiotics are a combination of nitro chemical groups and heterocycles that kill bacteria by inactivating enzymes that contribute to DNA and/or RNA and/or protein and/or cell wall biosynthesis. Over eighty years ago, antimicrobial agents began to be used for the treatment of different infectious diseases, such as urinary tract infections (UTIs). However, soon after the introduction of antibiotics there was an increase in the number of drug-resistant strains, leading to the worldwide spread of multidrug and pan-drug strains (MDR and PDR, respectively), with a projected 10,000,000 deaths per year by 2050, which has serious economic consequences for public health and governments.2
Klebsiella pneumoniae is a gram-negative bacillus that can cause serious infections such as liver abscesses, pneumonia, bacteremia and urinary tract infections (UTIs) in young people and other healthy people.3 Currently, pathogenic K. pneumoniae can be divided into hvKP and cKP, with cKP and hvkp pathogenic forms being difficult to treat due to their antimicrobial resistance genes. At the same time, K. pneumoniae is highly predisposed to biofilm formation, making this challenging infection even more severe.4 It is well known that hospital infections often involve highly biofilm-forming pathogens, such as Escherichia coli, Pseudomonas aeruginosa, and K. pneumoniae.5 Biofilms are most notorious for their high level of resistance to antibiotics.6 Due to the high resistance of biofilms to antibiotics, infections associated with biofilm formation isolates are very difficult to treat. And the concern about biofilm formation of K. pneumoniae is related to the colonization of the organism in many medical conditions, while certain virulence factors are also closely related to the ability of biofilm formation.7 Therefore, we should continue to focus on the virulence profile of multi-drug resistant K. pneumoniae during biofilm formation and raise clinical awareness of this new serious threat. In this study, we examined and analyzed the virulence, drug resistance and biofilm formation ability of the isolated K. pneumoniae to provide a theoretical reference for further understanding of the drug resistance mechanism of K. pneumoniae.
Materials and Methods
Source of Strains
Ninety-six non-replicated strains of K. pneumoniae were isolated from sputum, urine, blood, secretions, pleural and ascitic fluid and bile specimens collected at a university teaching hospital (The Affiliated Chaohu Hospital of Anhui Medical University), from August 2018 to June 2019, A total of 96 inpatients, 64 males and 32 females, aged 23 to 93 years, with a mean of 65 years and an interquartile range of 53–76 years, were included in the observation. Blood samples were first tested for bacteria in blood cultures by the Bact/ALERT®3D system. Sputum, urine, blood culture positive samples and other samples were placed on blood agar plates and incubated at 37 °C for 16–18 h for bacterial isolation. All strains were identified using a Vitek II Compact fully automated microbiology analyzer. Ethical approval was granted by Affiliated Chaohu Hospital of Anhui Medical University Ethics Committee.
Instruments and Reagents
Vitek II Compaet fully automatic microbiological analyzer, turbidimeter (bioMérieux, France); 96-well polystyrene plate (Costar, USA); crystalline violet staining solution (Beijing Solabao Technology Co., Ltd.); MH agar dry powder, drug-sensitive paper sheets (Oxoid, UK); PCR reaction reagent, DNA Marker, agarose (Shanghai Biotech Biological Co., Ltd.); electrophoresis instrument (Beijing Liuyi Instrument Factory). Gel imaging system (BioRad, USA).
Antibiotic Susceptibility Testing
The antibiotic susceptibility testing was performed using the micro-broth dilution method with reference to the 2019 Clinical and Laboratory Standards Institute (CLSI) guidelines for drug sensitivity analysis, the minimum inhibitory concentration (MIC) of the antimicrobial drug is detected. The MIC is the lowest concentration at which an antimicrobial drug can completely inhibit bacterial growth.8 The specific drugs are as follows: piperacillin, ampicillin/sulbactam, cefazolin, ceftazidime, ceftriaxone, cefotaxime, cefepime, cefoxitin, aminoglutethimide, gentamicin, tobramycin, ciprofloxacin, cotrimoxazole, furantoin (Chinese Institute for the Control of Pharmaceutical Products), imipenem, meropenem (Merck & Co.). E. coli ATCC 25922 was used as the quality control strain for the drug sensitivity test.
β-Lactamase Identification and String Test
The double disk diffusion method was used to detect whether ESBLs were produced and the modified carbapenem inactivation method (mCIM) was used to detect whether carbapenemases were produced,9,10 referring to the 2019 CLSI operating standard for the specific test methods and procedures. Hypermucosity is a typical characteristic of hvKP strains and is commonly used to define hvKP. Strains with hypermucoviscous phenotype were positive, defined as hvKP; those that are negative were defined as classical K. pneumoniae.11 Hypermucoviscousity (String Test): fresh colonies cultured overnight on blood agar plates were stretched outward by gently touching them with an inoculating ring, and if there was mucus filament formation and the length was greater than 5 mm, the strain were judged as positive for the HM phenotype, ie the strain was hypermucoviscous.12 E. coli ATCC 25922 was used as the quality control strain for the above test.
Extraction of Genomic DNA
Pick a single colony from Mueller-Hinton agar plate (MH plate) with an inoculation loop and place it in an autoclaved EP tube with 1mL of sterile distilled water, mix well, then place it on a foam plate and boil it in boiling water for 10 min (lysis of bacteria to release DNA), after boiling, mix the top and bottom upside down, immediately put it into −20 °C refrigerator and freeze it for 10 min, then put the Ep tube into a centrifuge at 12,000 rpm for 10 min and the supernatant was aspirated as the amplification template and stored at −20 °C for backup.13
Molecular Detection of ESBLs Genes and Virulence Genes
PCR was used to detect common ESBLs genes (blaTEM, blaSHV, blaCTX-M), capsular serotypes (K1, K2, K5, K20 and K57) and virulence-related genes including mucus phenotype genes (rmpA), type 1 and 3 adhesins (fimH, mrkD), aerobactin Aerobactin, enterobactin (entB) and Yersinabactin (ybtS), allantoin regulatory factor (allS), iron uptake system (kfu), primer sequences were synthesized by Shanghai Bioengineering Co. The total reaction system was 50 μL: Taq PCR Master Mix 25 μL, bacterial DNA template 1 μL, upstream primers and downstream primers 2 μL each, ddH2O 20 μL. PCR reaction conditions: pre-denaturation at 94 °C for 4 min; denaturation at 94 °C for 30 s, primer specific annealing temperature 30 s, extension at 72 °C for 40 s, 32 cycles; extension at 72 °C for 10 min. Amplification products were amplified in a 1.2% agarose gel containing ethidium bromide at 120 V. After electrophoresis for 40 min, the results were observed by gel imager, and the detection (predicted size band) of the target band was considered positive for the tested gene. Samples were amplified using specific primers (Extend, Sao Paulo, Brazil), as in Table 1.
 |
Table 1 Primer Sequences Used in This Study |
Assessing Biofilm Formation
The biofilm formation ability was detected by crystalline violet staining method.15 The strains were inoculated on MH plates and incubated at 37 °C for 24 h, and 0.5 McFarland’s bacterial suspension was prepared and added to 96-well polystyrene plates with 10μL bacterial suspension and 190 μL sterile LB broth per well, and three replicate wells were set up with 200 μL sterile LB broth as a negative control. The 96-well plate was incubated at 37 °C for 24 h and then removed, washed 3 times with 200 μL sterile distilled water to remove the floating bacteria, added 200 μL of 99% methanol for 15 min, aspirated and discarded the methanol, dried and added 200 μL of 1% crystalline violet dye for 20 min, washed 3 times with sterile distilled water, dried at room temperature and then added 200 μL of 95% ethanol for 10 min to fully dissolve crystalline violet. The absorbance (OD) of each well was read at 570 nm using an enzyme marker and measured three times, and the ODc value (mean OD of negative control + 3×standard deviation) was used as the threshold value. If the ODc value is greater than this value, the biofilm is determined to be formed. Referring to the literature criteria,19 the biofilm forming ability of strains can be classified into 4 categories, OD≤ODc as negative (-), ODc<OD≤2ODc as weak positive (+), 2ODc<OD≤4ODc as moderate positive (++), and OD>4ODc as strong positive (+++).
Data and Statistical Analysis
The correlation test was performed using the chi-square test (χ2). Therefore, correlations between antibiotics and bacterial resistance, virulence genes and two types of strains, hvKP strains and ESBL-producing strains, ESBLs genes and resistance phenotypes and biofilm formation were calculated by the chi-square (χ2) independence test in SPSS version 22.0. Spearman correlation analysis was used to calculate the correlation between biofilm formation levels and cephalosporin resistance. Indeed, the chi-square (χ2) test was suitable to determine the significance of the correlation between two nominal parameters.20 A P value of <0.05 was considered statistically significant.
Results
Analysis of Strain Sources and Their Biofilm Characteristics
The strains in this study were mainly derived from sputum (54, 56.2%), urine (15, 15.6%), blood (11, 11.5%), secretions (9, 9.4%), thoraco-abdominal fluid (4, 4.2%) and bile (3, 3.1%). In comparison with isolates from different sources, the biofilm formation value was higher for isolates from secretions than from sputum, urine, blood, thoracic and ascitic fluid and bile, but the difference was not statistically significant (P > 0.05) (Figure 1).
 |
Figure 1 The value of biofilm formation of strains from different sources. |
Antibiotic Resistance Characteristics and Hypervirulent Strains
In this study, microbroth dilution method was used to determine the MIC value of the selected antibiotics against pathogenic bacteria (Table 2). As shown in Table 3, except for ceftazidime, cefoxitin, aminotrans, imipenem, and meropenem, cKP was significantly more resistant to the above antimicrobial drugs than hvKP. MDR strains are bacteria that are simultaneously insensitive to at least one of the three or more classes of antimicrobial drugs.16 In this study, 53 (55.2%) isolates were classified as MDR.
 |
Table 2 Distribution of the Lowest Inhibitory Concentrations of 96 Strains of Klebsiella Pneumoniae to 16 Antibiotics |
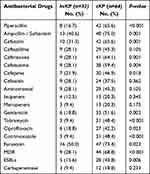 |
Table 3 Resistance Rates of hvKP Vs cKP, n=96 |
Toxicity Genes and Their Distribution Characteristics in hvKP and cKP
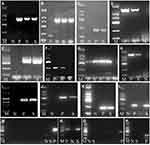
Only one of all 96 K. pneumoniae strains did not carry the mrkD gene, most (91.7%) strains carried the fimH gene, and the other virulence genes were ybtS (53.1%), entB (46.9%), rmpA (41.7%), aerobactin (32.3%), allS (15.6%), kfu (15.6%). K1 and K2 were the most common capsular serotypes, accounting for 14.6% and 11.5%, respectively; no K54 was found. More than half (61.5%) of the strains did not belong to the common capsular serotypes of K1, K2, K5, K20, K54, and K57. As shown in Table 4, the virulence genes (entB, rmpA, aerobactin, kfu) and capsular serotypes (K1, K2, K57) of hvKP isolates were significantly higher than those of cKP (P < 0.05). However, fimH, mrkD, ybtS genes and K5, K20 capsular serotypes did not differ between hvKP and cKP strains. Agarose gel electrophoresis of ESBLs genes and virulence factors are shown in Figure 2.
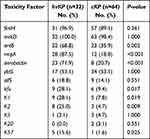 |
Table 4 Distribution of Virulence Genes in cKP Strains and hvKP Strains, n=96 |
The genes of ESBLs detected in this study including strain blaTEM 35 (36.5%), strain blaSHV 51 (53.1%), and strain blaCTX-M 49 (51.0%), most of cKP strains carried multiple ESBL genes, and all hvKP strains carried ESBL genes (Table 5).
 |
Table 5 Distribution of ESBLs Genes in ESBL-Positive cKP Strains and hvKP Strains, n=54 |
Correlation of hvKP Strains with ESBL-Producing Strains
The correlation of hvKP strains with ESBL-producing strains are shown in Table 6. blaTEM, blaSHV, and blaCTX-M were all negatively associated with high mucus phenotype (P=0.001) and also highly negatively associated with entB, rmpA (P<0.001). Except for blaTEM, blaSHV, and blaCTX-M were significantly negatively associated with allS, K1 and K2 genes as well.
 |
Table 6 Correlation of hvKP Strains with ESBL-Producing Strains |
Biofilm Formation Characteristics of hvKP and cKP Strains
According to Table 7, PCR was used to detect common ESBLs genes (blaTEM, blaSHV, blaCTX-M) and the double disk diffusion method was used to detect whether ESBLs were produced and the modified carbapenem inactivation method (mCIM) was used to detect whether carbapenemases were produced (Figure 3), the absorbance (OD) of each pore and the formation of the biofilm were read at 570 nm using an enzyme marker, thirty-three (63%) strains of K. pneumoniae exhibited ESBLs phenotype. Sixteen of the strains were carbapenemase-producing and most ESBLs phenotype tested were resistant to all four drugs conclusively. Not all ESBL-positive strains produce ultra-broad-spectrum β-lactamases. The correlation between ESBLs-producing strains and biofilm formation was significantly increased compared to strains without ESBLs phenotype (P = 0.035). In addition, MDR strains were also more likely to form biofilms than sensitive strains (P = 0.013). ESBLs-producing cKP strains were significantly more correlated with biofilm formation compared to cKP (P = 0.021).
 |
Table 7 Statistics of the Relationship Between the Genes Carrying Different ESBLs and Biofilm Formation |
The difference in biofilm formation between hvKP strains and cKP strains was not statistically significant (P = 0.134). Strains carrying blaSHV and blaCTX-M were significantly associated with biofilm formation (P = 0.007 and P = 0.003, respectively). However, strains carrying the blaTEM were associated with biofilm formation, and the difference was not statistically significant (P = 0.178) (Table 7). The level of biofilm formation was significantly and negatively correlated with cephalosporin resistance (P = 0.034) (Table 8).
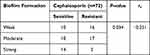 |
Table 8 Statistics on the Relationship Between Biofilm Formation Level and Drug Resistance |
Statistical processing showed no significant correlation between sex and urinary tract infection (P = 1.00). Age was significantly associated with infection (P = 0.006). There was no significant relationship between age and sex (P = 0.794). Age was not significantly correlated with urinary tract infection (P = 0.927), neither mrkD (P = 0.333) nor FimH (P = 0.602) was significantly related to gender.
Discussion
cKP causes severe infections when infecting immunocompromised individuals,21 and the carriage and expression of its drug resistance does not enhance the virulence of K. pneumoniae, although it increases the difficulty of treatment. However, hvKP is a hypervirulent pathogen capable of infecting both healthy and immunocompromised populations, causing purulent liver abscesses, endophthalmitis, and meningitis in a variety of serious infections.22 hvKP is rarely resistant to commonly used antimicrobial agents, except for intrinsic resistance to ampicillin.23 Our data showed that among 16 antibiotics, cKP strains were significantly more resistant to 11 antibiotics compared to hvKP (P < 0.05) and 68.8% of cKP were MDR strains, while only 28.1% of hvKP strains were MDR strains, suggesting a propensity for cKP to acquire antibiotic resistance.24 Previous studies have shown an increased propensity for hvKP strains to acquire antimicrobial resistance, although cKP is more resistant to multiple antibiotics than hvKP. The present study also compared the phenotypic characteristics of ESBL of hvKP and cKP isolates, which is consistent with previous reports.25,26 hvKP strains produced significantly less ESBL than cKP strains because ESBL is a class of β-lactamases that hydrolyze penicillins, cephalosporins, and monocyclic antibiotics, which explains the aforementioned higher resistance of cKP to most antibiotics than hvKP. It is also important to note, according to Table 7, thirty-three (63%) strains of K. pneumoniae exhibited ESBLs phenotype. Sixteen of the strains were carbapenemase-producing and most ESBLs phenotype tested were resistant to all four drugs conclusively. Not all ESBL-positive strains produce ultra-broad-spectrum β-lactamases. The reason is that a biological structural gene, consisting of several coding and non-coding regions separated from each other but continuously embedded, can be translated into a complete protein composed of continuous amino acids after the coding region is removed. In other words, the sequence within a structural gene that codes for an amino acid (called an exon) is discontinuous and separated by some non-coding sequence (called an intron), which is called a break gene, some ESBL positive strains did not produce ESBL enzyme because the truncated gene could not encode the protein. The nine hvKP strains found to be MDR strains in our study, all carrying two or more ESBL genes, may have evolved because cKP strains acquired virulence factors through horizontal (lateral) gene transfer by phage, plasmids or transposons,27 or hvKP acquired mobile progenitors of resistance determinants.28
In our study, the prevalence of podocyst serotypes K1, K2, K57 and the virulence gene rmpA was found to be significantly higher in hvKP strains than in cKP strains, which is consistent with previous results in which genes encoding rmpA, K1 or K2 were highly associated with hypervirulent (highly adherent) variants of K. pneumoniae (hvKP).29,30 Most of the hypervirulent K. pneumoniae podocyst serotypes are K1, K2, K5, K20 and K57.31 K1 and K2 capsular types are considered to be highly pathogenic to humans.32 To date, 70%–80% of reported hvKP strains belong to K1 and K2 types of K. Pneumoniae.33 The gene rmpA is the regulatory gene, which has been identified to be closely associated with the hyperviscosity phenotype, commonly detectable in hvKP.34 Other virulence genes entB, aerobactin, and kfu were also significantly increased in hvKP strains. The iron carrier-related entB mainly mediates iron transport in Gram-negative bacteria and is widely spread in K. pneumoniae strains. Kfu is the gene encoding the iron uptake system. Our study showed that the kfu gene was significantly increased in strains with high mucilage. This finding is consistent with previous studies, which showed that the kfu gene was associated with a high-mucus phenotype.35 Previous studies have shown that aerobactin (aerobic actin) gene and aerobic actin production are more common in hvKP strains than in cKP strains. hvKP strains produce aerobactin more commonly than cKP strains, as demonstrated by cross-feeding assays (Yu et al, 2007). This analysis suggests that hvKP strains may be more likely to acquire iron than cKP strains.36,37 It has been shown that aerobic actin is the main virulence factor for increased iron carrier production in hvKP isolates and was used to define hvKP.38
K. pneumoniae contains plasmid-mediated β-lactamases with broad-spectrum hydrolytic activity,39 carrying multiple antimicrobial drug resistance genes, including ESBLs and carbapenemases.40 In Table 7, some strains produce carbapenase, carbapenemase includes A, B, C and D of β-lactam group classified by Ambler, class A carbapenems belong to the 2f group in Bush, including KPC, SME, NMC-A, IMI, GES, SFC-1, etc. As one of the most important carbapenemases at present, the emergence of KPC-producing carbapenemase is the main reason for increasing the resistance to carbapenems. This enzyme can hydrolyze all β-lactamases including carbapenems, penicillin, and cephalosporins of the 1st to 4th generation, but is sensitive to monocylactam. Class B carbapenemase is a kind of metal β-lactamase, its activity needs the assistance of metal ion zinc, referred to as metal enzyme, most by plasmid coding, its genes are located in a class of integron, integron integration into the transposon or transfer plasmid, resulting in the spread of IMP gene between the same or different strains, so that the strains that were sensitive to antibiotics become resistant, increased resistance to hydrocarbansene antibiotics, especially imipenem was enhanced. Category C is rarely reported. Most of class D carbapenems have weak hydrolysis ability to carbapenems and ultra-broad spectrum β-lactam.41 Factors affecting biofilms include capsular polysaccharides (capsule k antigen) lipopolysaccharide extracellular DNA and adhesion factors (including adhesion proteins and pili structures). It can be known from reading a large number of literatures that the types and abilities of the four types of antibiotics aimed at hydrolyzing are different, which have nothing to do with the formation of biofilms. In other words, the influencing factors of biofilm formation have nothing to do with carbapenase. Statistics of carbapenase and biofilm formation were calculated in the supplementary data in Table 7, and it was concluded that (P = 0.417) was not statistically significant, which was consistent with the conclusion mentioned above, that is carbapenase production had no direct relationship with biofilm formation.42 Microorganisms that produce ESBLs are clinically relevant and remain an important cause of cephalosporin treatment failure.40 There are hundreds of variants of ESBLs, mainly including the blaTEM, blaSHV and blaCTX-M families. In this study, ESBL genes were negatively correlated with hvKP (P = 0.001). The reason for this difference is unclear, but it can be speculated that hvKP strains do not have access to plasmids associated with drug resistance or that some resistance genes are lost when they become hyper-virulent.24 The strains in our collection carry multiple virulence genes and some of them coexist with drug resistance genes. Also virulence genes were associated with ESBL resistance genes. blaTEM, blaSHV and blaCTX-M were highly negatively correlated with entB, rmpA and rmpA (P < 0.001), except for blaTEM, blaSHV and blaCTX-M were significantly negatively correlated with allS, K1 and K2. Highly mucoid Klebsiella pneumoniae strains (HMV) are early associated with hypervirulence, and mutations leading to the loss of this HMV phenotype also lead to reduced virulence. hvKP strains with a high mucoid phenotype produce significantly less ESBL than cKP strains as shown by the above experimental results, and it has been shown that the virulence of K. pneumoniae strains is associated with the usual virulence factors (entB, rmpA and rmpA) and capsular serotypes (K1 and K2) positively correlated.
Biofilms are microbial communities consisting of cellular aggregates in a matrix composed of surface polysaccharides, proteins and DNA that are attached by bacteria to living or non-living surfaces. The ability to produce biofilms results in increased resistance to host defense factors and antimicrobial agents, and is considered an important virulence property. The biofilm formation ability of strains with different antibiotic resistance is also different, according to the relationship between biofilm production and drug resistance of K. pneumoniae clinically isolated from Beijing Shijitan Hospital affiliated to Capital Medical University. Among 140 strains, 52 strains were positive for biofilm, accounting for 37.1% of all strains. The sensitive rates of biofilm-positive strains to amikacin, gentamicin, ceftazidime, cefepime and amtrannan were 44.2%, 15.4%, 28.8%, 30.8% and 11.5%, respectively. The sensitivity rates of biofilm-negative bacteria to amikacin, gentamicin, ceftazidime, cefepime and amtriaxam were 80.6%, 26.1%, 35.2%, 37.5% and 35.2%, respectively. The sensitivity rate of positive and negative biofilm bacteria to the above-mentioned drugs was statistically significant.43 The correlation between ESBLs-producing strains and biofilm formation was significantly increased compared to strains without ESBLs phenotype (P = 0.035), and it has been suggested that this may be due to (1) the fact that biofilm is a multi-species microbial community and these species can share their genetic material in high proportions; (2) low concentrations of antibiotics can induce ESBLs, which is one of the reasons for reduced penetration of one of the reasons for this.24 Strains carrying blaSHV and blaCTX-M were significantly associated with biofilm formation (P = 0.007 and P = 0.003, respectively). According to the name Proteomic and Transcriptomic Analyses Indicate Reduced Biofilm-Forming Abilities in Cefiderocol-resistant K. pneumoniae, it was found that drug resistance was closely related to the decreased biofilm formation ability, and the downregulation of hdeB, stpA, yhjQ, fba, bcsZ, uvrY, bcsE, bcsC and ibpB was the main factor. In addition, downregulation of ferritic transporters, including efeO, Tonb-dependent receptor fecA and ABC transporter fbpA, may be one of the determining factors that promote reduced biofilm formation ability and lead to cephalosporin resistance, the results are consistent with those in Table 8.44 Several studies have shown that ESBL-producing K. pneumoniae form heavier biofilms than non-ESBL-producing K. pneumoniae, and another recent study showed that serum-resistant strains of ESBL-producing K. pneumoniae are more prevalent than non-ESBL K. pneumoniae.24 However, strains carrying the blaTEM gene were associated with biofilm formation, and the difference was not statistically significant (P = 0.178). This suggests that blaSHV and blaCTX-M are associated with biofilm formation. It has been proposed that (I) the production of ESBLs encoded by resistance plasmid increases when adhesion factors and production of extra-envelope polysaccharides are increased in hvKP strains. Thus strains carrying the blaTEM may lack adhesion factors or have other factors in combination that affect biofilm formation. In vitro studies have demonstrated the ability of cKP strains to produce biofilms, with type 3 hairs, podocyanin polysaccharide (CP) and lipopolysaccharide (LPS), amino acid synthesis genes, l-arabinose metabolism, sugar phosphotransferase system, type 2 population-sensing regulatory system, Lys-R-type regulator oxR and a possible cell surface protein identified as contributing factors. hvKP strains NTUH- K2044 and KpL1 were also shown to produce biofilms, and studies on hvKP strain hvKP1 showed that genes encoding glutamine synthetase, the alpha subunit of succinyl coenzyme A synthase and the glycerol uptake manipulator transcriptional repressor were associated with biofilm formation.45
Conclusion
In conclusion, with the spread of drug-resistant K. pneumoniae, clinical treatment will face serious challenges. Therefore, we need to make continuous efforts to control the spread of drug-resistant K. pneumoniae and prevent a full-scale outbreak in the future.
Ethical Conduct of Research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Acknowledgments
The authors wish to thank colleagues who gave precious suggestions to this study. The authors also acknowledge Meiyi Lu and Ying Lu for their significant contributions to the manuscript for revising and language polishing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study received funding from the Scientific Research Project of the Health Commission of Anhui Province (grant no. AHWJ2021a013), Major Project of Humanities and Social Sciences Research in Anhui Universities (grant no. SK2021ZD0032), Teaching and Research Project of Anhui Province (grant no. 2019jyxm1020) and Research Fund of Anhui Medical University (grant no. 2020xkj216). The authors have no relevant affiliations or financial involvement with any of these organizations. No writing assistance was utilized in the production of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Issakhanian L, Behzadi P. Antimicrobial agents and urinary tract infections. Curr Pharm Des. 2019;25(12):1409–1423. doi:10.2174/1381612825999190619130216
2. Sarshar M, Behzadi P, Ambrosi C, et al. FimH and anti-adhesive therapeutics: a disarming strategy against uropathogens. Antibiotics. 2020;9(7):58. doi:10.3390/antibiotics9070397
3. Ahmadi Z, Noormohammadi Z, Ranjbar R, et al. Prevalence of Tetracycline Resistance Genes tet (A, B, C, 39) in Klebsiella pneumoniae Isolated from Tehran, Iran. Iranian J Med Microbiol. 2022;16(2):141–147. doi:10.30699/ijmm.16.2.141
4. Cusumano JA, Caffrey AR, Daffinee KE, et al. Weak biofilm formation among carbapenem-resistant Klebsiella pneumoniae. Diagn Microbiol Infect Dis. 2019;95(4):114877. doi:10.1016/j.diagmicrobio.2019.114877
5. Khan F, Pham DTN, Oloketuyi SF, et al. Antibiotics Application Strategies to Control Biofilm Formation in Pathogenic Bacteria. Curr Pharm Biotechnol. 2020;21(4):270–286. doi:10.2174/1389201020666191112155905
6. Piperaki ET, Syrogiannopoulos GA, Tzouvelekis LS, et al. Klebsiella pneumoniae: virulence, Biofilm and Antimicrobial Resistance. Pediatr Infect Dis J. 2017;36(10):1002–1005. doi:10.1097/INF.0000000000001675
7. Surgers L, Boyd A, Girard PM, et al. Biofilm formation by ESBL-producing strains of Escherichia coli and Klebsiella pneumoniae. Int J Med Microbiol. 2019;309(1):13–18. doi:10.1016/j.ijmm.2018.10.008
8. Bellich B, Lagatolla C, Tossi A, et al. Influence of Bacterial Biofilm Polysaccharide Structure on Interactions with Antimicrobial Peptides: a Study on Klebsiella pneumoniae. Int J Mol Sci. 2018;19(6):989. doi:10.3390/ijms19061685
9. Salvia T, Dolma KG, Dhakal OP, et al. Phenotypic Detection of ESBL, AmpC, MBL, and Their Co-occurrence among MDR Enterobacteriaceae Isolates. J Lab Physicians. 2022;14(3):329–335. doi:10.1055/s-0042-1744239
10. Rizvi M, Sami H, Azam M, et al. Reliability of carbapenem inactivation method (CIM) and modified carbapenem inactivation method (mCIM) for detection of OXA-48-like and NDM-1. Indian J Med Microbiol. 2021;39(4):451–456. doi:10.1016/j.ijmmb.2021.07.004
11. Zhu J, Wang T, Chen L, et al. Virulence Factors in Hypervirulent Klebsiella pneumoniae. Front Microbiol. 2021;12:642484. doi:10.3389/fmicb.2021.642484
12. Zhan L, Wang S, Guo Y, et al. Outbreak by Hypermucoviscous Klebsiella pneumoniae ST11 Isolates with Carbapenem Resistance in a Tertiary Hospital in China. Front Cell Infect Microbiol. 2017;7:182. doi:10.3389/fcimb.2017.00182
13. Zhao Y, Zhang S, Fang R, et al. Dynamic Epidemiology and Virulence Characteristics of Carbapenem-Resistant Klebsiella pneumoniae in Wenzhou, China from 2003 to 2016. Infect Drug Resist. 2020;13:931–940. doi:10.2147/IDR.S243032
14. Dallenne C, Da Costa A, Decre D, et al. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J Antimicrob Chemother. 2010;65(3):490–495. doi:10.1093/jac/dkp498
15. Briñas L, Moreno MA, Zarazaga M, et al. Detection of CMY-2, CTX-M-14, and SHV-12 beta-lactamases in Escherichia coli fecal-sample isolates from healthy chickens. Antimicrob Agents Chemother. 2003;47(6):2056–2058. doi:10.1128/AAC.47.6.2056-2058.2003
16. Ferreira RL, Da Silva BCM, Rezende GS, et al. High Prevalence of Multidrug-Resistant Klebsiella pneumoniae Harboring Several Virulence and beta-Lactamase Encoding Genes in a Brazilian Intensive Care Unit. Front Microbiol. 2018;9:3198. doi:10.3389/fmicb.2018.03198
17. Yan Q, Zhou M, Zou M, et al. Hypervirulent Klebsiella pneumoniae induced ventilator-associated pneumonia in mechanically ventilated patients in China. Eur J Clin Microbiol Infect Dis. 2016;35(3):387–396. doi:10.1007/s10096-015-2551-2
18. Fu L, Huang M, Zhang X, et al. Frequency of virulence factors in high biofilm formation bla(KPC-2) producing Klebsiella pneumoniae strains from hospitals. Microb Pathog. 2018;116:168–172. doi:10.1016/j.micpath.2018.01.030
19. Stepanovic S, Vukovic D, Dakic I, et al. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40(2):175–179. doi:10.1016/s0167-7012(00)
20. Khonsari MS, Behzadi P, Foroohi F. The prevalence of type 3 fimbriae in Uropathogenic Escherichia coli isolated from clinical urine samples. Meta Gene. 2021;28. doi:10.1016/j.mgene.2021.100881
21. Russo TA, Marr CM. Hypervirulent Klebsiella pneumoniae. Clin Microbiol Rev. 2019;32(3). doi:10.1128/CMR.00001-19
22. Yang X, Wai-Chi Chan E, Zhang R, et al. A conjugative plasmid that augments virulence in Klebsiella pneumoniae. Nat Microbiol. 2019;4(12):2039–2043. doi:10.1038/s41564-019-0566-7
23. Caneiras C, Lito L, Melo-Cristino J, et al. Community- and Hospital-Acquired Klebsiella pneumoniae Urinary Tract Infections in Portugal: virulence and Antibiotic Resistance. Microorganisms. 2019;7(5):138. doi:10.3390/microorganisms7050138
24. Shah RK, Ni ZH, Sun XY, et al. The Determination and Correlation of Various Virulence Genes, ESBL, Serum Bactericidal Effect and Biofilm Formation of Clinical Isolated Classical Klebsiella pneumoniae and Hypervirulent Klebsiella pneumoniae from Respiratory Tract Infected Patients. Pol J Microbiol. 2017;66(4):501–508. doi:10.5604/01.3001.0010.7042
25. Su SC, Siu LK, Ma L, et al. Community-acquired liver abscess caused by serotype K1 Klebsiella pneumoniae with CTX-M-15-type extended-spectrum beta-lactamase. Antimicrob Agents Chemother. 2008;52(2):804–805. doi:10.1128/AAC.01269-07
26. Derakhshan S, Najar Peerayeh S, Bakhshi B. Association Between Presence of Virulence Genes and Antibiotic Resistance in Clinical Klebsiella Pneumoniae Isolates. Lab Med. 2016;47(4):306–311. doi:10.1093/labmed/lmw030
27. Lev AI, Astashkin EI, Kislichkina AA, et al. Comparative analysis of Klebsiella pneumoniae strains isolated in 2012-2016 that differ by antibiotic resistance genes and virulence genes profiles. Pathog Glob Health. 2018;112(3):142–151. doi:10.1080/20477724.2018.1460949
28. Walker KA, Miller VL. The intersection of capsule gene expression, hypermucoviscosity and hypervirulence in Klebsiella pneumoniae. Curr Opin Microbiol. 2020;54:95–102. doi:10.1016/j.mib.2020.01.006
29. Shen P, Berglund B, Chen Y, et al. Hypervirulence Markers Among Non-ST11 Strains of Carbapenem- and Multidrug-Resistant Klebsiella pneumoniae Isolated From Patients With Bloodstream Infections. Front Microbiol. 2020;11:1199. doi:10.3389/fmicb.2020.01199
30. Dong N, Yang X, Chan EW, et al. Klebsiella species: taxonomy, hypervirulence and multidrug resistance. EBioMedicine. 2022;79:103998. doi:10.1016/j.ebiom.2022.103998
31. Catalán-Nájera JC, Garza-Ramos U, Barrios-Camacho H. Hypervirulence and hypermucoviscosity: two different but complementary Klebsiella spp. phenotypes? Virulence. 2017;8(7):1111–1123. doi:10.1080/21505594.2017.1317412
32. Chew KL, Lin RTP. Klebsiella pneumoniae in Singapore: hypervirulent Infections and the Carbapenemase Threat. Front Cell Infect Microbiol. 2017;7:515. doi:10.3389/fcimb.2017.00515
33. Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the Offense with a Strong Defense. Microbiol Mol Biol Rev. 2016;80(3):629–661. doi:10.1128/MMBR.00078-15
34. Hsieh PF, Lin TL, Lee CZ, et al. Serum-induced iron-acquisition systems and TonB contribute to virulence in Klebsiella pneumoniae causing primary pyogenic liver abscess. J Infect Dis. 2008;197(12):1717–1727. doi:10.1086/588383
35. Compain F, Babosan A, Brisse S, et al. Multiplex PCR for detection of seven virulence factors and K1/K2 capsular serotypes of Klebsiella pneumoniae. J Clin Microbiol. 2014;52(12):4377–4380. doi:10.1128/JCM.02316-14
36. Jung SW, Chae HJ, Park YJ, et al. Microbiological and clinical characteristics of bacteraemia caused by the hypermucoviscosity phenotype of Klebsiella pneumoniae in Korea. Epidemiol Infect. 2013;141(2):334–340. doi:10.1017/S0950268812000933
37. Kim D, Park BY, Choi MH, et al. Antimicrobial resistance and virulence factors of Klebsiella pneumoniae affecting 30 day mortality in patients with bloodstream infection. J Antimicrob Chemother. 2019;74(1):190–199. doi:10.1093/jac/dky397
38. Lin ZW, Zheng JX, Bai B, et al. Characteristics of Hypervirulent Klebsiella pneumoniae: does Low Expression of rmpA Contribute to the Absence of Hypervirulence? Front Microbiol. 2020;11:436. doi:10.3389/fmicb.2020.00436
39. Mbelle NM, Feldman C, Sekyere JO, et al. Pathogenomics and Evolutionary Epidemiology of Multi-Drug Resistant Clinical Klebsiella pneumoniae Isolated from Pretoria, South Africa. Sci Rep. 2020;10(1):1232. doi:10.1038/s41598-020-58012-8
40. Pitout JD, Nordmann P, Laupland KB, et al. Emergence of Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs) in the community. J Antimicrob Chemother. 2005;56(1):52–59. doi:10.1093/jac/dki166
41. Xiaoyu Q, Qi L. Mechanism of carbapenem-resistant drugs in Klebsiella pneumoniae. J Clin Pediatrics. 2015;33(10):907–911.
42. Li XU, Bei LI. Research progress on the formation mechanism of Klebsiella pneumoniae biofilm[J]. Chine J Pathogen Biol. 2016;11(11):1056–1059. doi:10.13350/j.cjpb.161123
43. Duo Y, Dongyuan M, Songxue W. Relationship analysis between Klebsiella pneumoniae biofilm and bacterial resistance. Chine J Hospital Infectol. 2013;23(12):2785–2786+2851.
44. Bao J, Xie L, Ma Y, et al. Proteomic and Transcriptomic Analyses Indicate Reduced Biofilm-Forming Abilities in Cefiderocol-Resistant Klebsiella pneumoniae. Front Microbiol. 2021;12:778190. doi:10.3389/fmicb.2021.778190
45. Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4(2):107–118. doi:10.4161/viru.22718
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.