Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Myeloid-Specific SIRT6 Deletion Protects Against Particulate Matter (PM2.5)-Induced Airway Inflammation
Authors Chen S, Wu M, Xiong Z, Huang J, Lv Y, Li Y , Zeng M, Lai T
Received 23 November 2022
Accepted for publication 30 April 2023
Published 10 June 2023 Volume 2023:18 Pages 1135—1144
DOI https://doi.org/10.2147/COPD.S398796
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Shaopeng Chen,1,2,* Mindan Wu,3,* Zhilin Xiong,1,* Jiewen Huang,1 Yingying Lv,1 Yuyan Li,4 Minjuan Zeng,5 Tianwen Lai1
1Institute of Respiratory Diseases, The First Dongguan Affiliated Hospital of Guangdong Medical University, Dongguan, People’s Republic of China; 2Blood Donation Service Department, Zhanjiang Blood Center, Zhanjiang, People’s Republic of China; 3Department of Pulmonary and Critical Care Medicine, Shantou Central Hospital, Shantou, People’s Republic of China; 4Department of Pulmonary and Critical Care Medicine, Dongguan Hospital of Southern Medical University, Dongguan, People’s Republic of China; 5Laboratory Animal Center, Guangdong Medical University, Zhanjiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tianwen Lai; Minjuan Zeng, Email [email protected]; [email protected]
Purpose: Particulate matter (PM2.5) is a common risk factor for airway inflammation. Alveolar macrophages play a critical role in airway inflammation. Sirtuin 6 (SIRT6) is a class Ill histone deacetylase that exerts an anti-inflammatory effect in airway diseases. However, the role of SIRT6 on PM2.5-induced airway inflammation in macrophages remains unclear. We aimed to determine whether SIRT6 protects against PM2.5-induced airway inflammation in macrophages.
Methods: The effect of SIRT6 on PM2.5-induced airway inflammation was assessed by using THP1 cells or bone marrow-derived macrophages (BMDMs) exposed to PM2.5 in vitro and myeloid cell-specific SIRT6 conditional knockout mice (Sirt6fl/fl-LysMCre) in vivo.
Results: PM2.5 increased SIRT6 expression in THP1 cells, but SIRT6 gene silencing decreased PM2.5 induced inflammatory cytokines in THP1 cells. Moreover, the expression of SIRT6 and inflammatory cytokines was also decreased in BMDMs with myeloid-specific deletion of SIRT6 after stimulation of PM2.5. In vivo, Sirt6fl/fl-LysMCre mice substantially decreased airway inflammation in response to PM2.5 exposure.
Conclusion: Our results revealed that SIRT6 promotes the PM2.5-induced airway inflammation in macrophages and indicated that inhibition of SIRT6 in macrophages may represent therapeutic strategy for airway disorders induced by airborne particulate pollution.
Keywords: particulate matter, SIRT6, macrophage, lung inflammation
Introduction
Airborne particulate matter (PM2.5) pollution exposure is a major risk factor for global public health and known to cause many adverse health effects.1 Epidemiological studies have documented that there is a close correlation between PM2.5 exposure and increased incidence of respiratory diseases, such as asthma and chronic obstructive pulmonary disease.2 PM2.5 with aerodynamic diameter ≤ 2.5μm (PM2.5) is a leading contributor to pulmonary inflammation because its major target organ is lung and can penetrate deep into the alveolar regions. PM2.5-induced pulmonary inflammation might be seen as a critical process in mediating systemic adverse effects.3 Therefore, it is urgently needed to explore the molecular mechanisms responsible for PM2.5-induced airway inflammation.
As a fundamental component of the respiratory immune system, alveolar macrophages (AMs), which reside at the boundary between the human body and outside world, are the most abundant macrophage in the lung. They clear dead cells and foreign antigen or airborne particles through the release of anti-inflammatory cytokines and the high phagocytic activity. Thus, AMs play a critical role in host defense, tissue homeostasis and control of airway inflammation.4–6 However, the underlying mechanisms of AMs in regulation of PM2.5-induced airway inflammatory remain unknown.
Acetylation is a widely occurring epigenetic modification of proteins that are involved in diverse biological processes.7,8 In recent years, the physiological functions of the sirtuin deacetylase family (SIRT1-SIRT7) have been studied. Among the seven sirtuins, SIRT6 plays a leading role in regulating aging, inflammation, cancer and metabolic homeostasis.9 Accumulating evidences have revealed that SIRT6 widely participates in respiratory diseases, such as allergic airway inflammation,10 acute respiratory distress syndrome (ARDS),11 chronic obstruction pulmonary diseases,9 and lung fibrosis.12 Moreover, SIRT6 regulated macrophage polarization by activating the AMPK pathway and subsequent autophagy.13,14 Although AMs are the first responders to ambient air pollution exposure in the airway, the possible role of SIRT6 in airway inflammation of macrophages following PM2.5 exposure remains unclear.
In the present study, we sought to determine the underlying mechanisms of SIRT6 in the regulation of PM2.5-induced airway inflammation in macrophages. Our findings demonstrated that myeloid-specific SIRT6 deletion protects against PM2.5-induced airway inflammation and might suggest that inhibition of SIRT6 could prevent airway disorders induced by PM2.5.
Materials and Methods
Animal Studies
The LysMcre mice were kindly donated by Dr G. Feng (University of California at San Diego, CA, USA). Using Sirt6fl/fl mice, obtained from Jax Lab., we developed Myeloid cell-specific Sirt6 conditional knockout mice (Sirt6fl/fl-LysMCre) by crossing Sirt6fl/fl mice with the LysMCre mice. The primer sequences for the LysMCre and Sirt6 genes are shown in Table 1. A specific pathogen-free environment was maintained in the Laboratory Animal Center of Guangdong Medical University for all mice. A mouse model for short-term exposure to PM2.5 was established according to a previous study.8 Standard reference airborne PM2.5 was purchased from National Institute of Standards and Technology (NIST) Company. Briefly, PM2.5 was suspended and sonicated in saline at 100 μg PM (in 50 μL saline) per day by intratracheal instillation for 3 days. The same volume of saline was given to control mice. Experiments were conducted under protocols using experimental procedures and anesthesia methods according to the ethical review of laboratory animal welfare People’s Republic of China National Standard GB/T 35892–2018. Experiments were also conducted under protocols using experimental procedures and anesthesia methods approved by the Animal Ethical Committee of Guangdong Medical University (No. GDY2003027).
 |
Table 1 Sequence of Primers Were Used in This Study |
Cell Cultures
Isolation and culture of BMDMs were carried out similarly to previous study.7 Briefly, from mice aged 6–8 weeks, BM cells were harvested from femurs and tibiae with PBS. To promote differentiation of bone marrow-derived macrophages, BM cells were cultured in DMEM medium supplemented with 10% FBS (vol/vol) and 10 ng/mL recombinant murine M-CSF for 7 days.
The human monocyte-derived macrophages cell line, THP1 cells were purchased from the Cell Bank of the Chinese Academy of Science (Shanghai, China). THP-1 cells were cultured in RPMI-1640 medium supplemented with 10% FBS (vol/vol) and 20 nM PMA over 48 h as primary human macrophages. Water-saturated atmosphere containing 5% CO2 was routinely maintained at 37°C for the cell cultures above. PM2.5, which contains polycyclic aromatic hydrocarbons, was purchased from National Institute of Standards and Technology (NIST) Company. We suspended and sonicated PM2.5 in PBS or saline at a final concentration of 2 μg/μL (mass/vol), harvested BMDMs and THP-1 cells were treated with four concentrations of PM2.5 (25, 50, 75, 100 μg/mL) for 24 h or with PM2.5 (100 μg/mL) for four times (3, 6, 12, 24 h).
Collection of Bronchoalveolar Lavage Fluid (BALF)
BALF was performed according to our previous study.7 In brief, the lungs were instilled with 0.8 mL PBS after exsanguination. Cytospin slides were prepared by Wright-Giemsa staining, and cell counts in BALF were counted under the microscope in a blinded method.
RT-PCR Analysis
Total RNA of lung tissue or cells was isolated using the Trizol reagent (Invitrogen). Reverse transcription was performed via PrimeScript RT reagent Kit with gDNA Eraser (TAKARA) according to the manufacturer’s instructions. Quantitative PCR was carried out with SYBR Green PCR Master Mix (TAKARA). The primers are presented in Table 1.
Western Blot
Cells were lysed in RIPA buffer. Proteins in lysates of cells were separated by SDS-PAGE. Western Blot (WB) analyses were performed as described with antibody to SIRT6 (Santa Cruz), antibody to GAPDH, Tubulin and β-actin (Beyotime Biotechnology, China).
Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of mouse IL-6, TNF-α, CXCL1, and CXCL2 in lung homogenate were measured using ELISA kit (Elabscience).
siRNA Studies
siRNA for knockdown of Sirt6 was synthesized by GenePharma, and the sequence (5’-3’) was as follows: UCCAUCACGCUGGGUACAUTT. siRNA was transfected to cells using lipofectamine®3000 (Invitrogen), according to the manufacturer’s protocols with the following minor modifications. After 4–6 h of siRNA transfection, the transfected cells were exposed to PM2.5.
Statistical Analysis
Results are presented as mean with SEM. We used the GraphPad Prism software (version 8.0, San Diego, CA) for all calculations and graphing. Comparisons between two groups were made using the Student’s t-test or Mann–Whitney U-test. One-way ANOVA was used for comparisons between more than two groups. Statistical significance was defined as a value of P less than 0.05.
Results
SIRT6 Expression is Increased in Macrophages Following PM2.5 Exposure
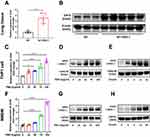
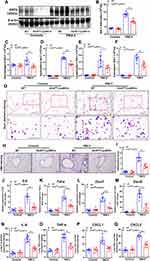
To address the possible role of SIRT6 in PM2.5-induced airway inflammation, we first assessed SIRT6 expression in the lung tissue of mice exposed to PM2.5. A mouse model for short-term exposure to PM2.5 was established as described in method. We found that the expression of SIRT6 was significantly increased in lung tissue of mice following PM2.5 exposure compared with those in control groups (Figure 1A and Figure 1). To identify whether SIRT6 is macrophage specific, we first examined the expression of SIRT6 in THP1 derived macrophages exposed to PM2.5. We found that treating THP1 cells with PM2.5 resulted in a dose- or time-dependent increase of SIRT6 (Figure 1C–). Furthermore, we generated bone marrow–derived macrophages (BMDMs) from Sirt6fl/fl mice. Consistent with the above findings, the expression of SIRT6 was significantly increased in PM2.5-induced BMDMs in a dose- and time-dependent manner (Figure 1F–). Collectively, these findings suggested that macrophages are involved in SIRT6-mediated airway inflammation induced by PM2.5.
Inhibition of SIRT6 Attenuated PM2.5-Induced Inflammatory Cytokines in vitro
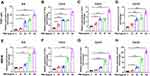
Previous studies showed that PM2.5 induces inflammatory cytokine secretion. In the present study, we also found that mRNA levels of tumor necrosis-alpha (TNF-α), interleukin 6 (IL6), C-X-C ligand 1 (CXCL1), and CXCL2 were significantly increased in PM2.5-induced THP1 cells and BMDMs (Figure 2). We further investigated the role of SIRT6 gene silencing in THP1 cells on PM2.5-induced inflammatory cytokines (Figure 3A and Figure 3). SIRT6 gene silencing further increased PM2.5 induced inflammatory cytokines compared with the control cells that received control siRNA (Figure 3C–).
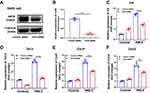
To further confirm this effect, we generated mice with myeloid conditional deletion of Sirt6 (Sirt6fl/fl-LysMCre). The genotyping results were determined by WB and RT-PCR analysis (Figure 4A and Figure 4). Representative genotyping results as shown in Supplementary Figure 1A. Moreover, double staining of macrophage (F4/80) and SIRT6 was also performed. Compared with the Sirt6fl/fl mice, the expression of SIRT6 in macrophages was significantly decreased in PM2.5-exposed Sirt6fl/fl-LysMCre mice (Supplementary Figure 1B and C). BMDMs were isolated from Sirt6fl/fl-LysMCre and Sirt6fl/fl mice. Consistent with the above findings, we found that the levels of IL-6, TNF-α, CXCL1, and CXCL2 were significantly decreased in Sirt6-deficient BMDMs compared with the control group (Figure 4C–). Collectively, these findings imply that the loss of SIRT6 in macrophages attenuated PM2.5-induced inflammatory cytokine.
Sirt6fl/Fl-LysMCre Mice Attenuated PM2.5-Induced Airway Inflammation
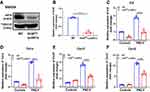
Next, we further elucidate the role of SIRT6 in PM2.5-induced airway inflammation in vivo. As described in method, a mouse model for short-term exposure to PM2.5 was established using Sirt6fl/fl littermates and Sirt6fl/fl-LysMCre mice. As described in Figure 5A, compared with PM2.5-induced Sirt6fl/fl mice, SIRT6 expression in lung tissue was significantly decreased in Sirt6fl/fl-LysMCre mice after PM2.5 exposed. The number of total cells, macrophage, neutrophil, lymphocytes and eosinophil were significantly decreased in BALF of PM2.5-induced Sirt6fl/fl-LysMCre mice compared with those in Sirt6fl/fl-LysMCre mice after PM2.5 exposed (Figure 5B–). Moreover, airway inflammation was significantly decreased in Sirt6fl/fl-LysMCre mice exposed to PM2.5 (Figure 5H and Figure 5). Compared with the Sirt6fl/fl mice, both mRNA and protein expression of IL-6, TNF-α, CXCL1, and CXCL2 in the lung tissue were significantly decreased in PM2.5-exposed Sirt6fl/fl-LysMCre mice (Figure 5J and Figure 5).
Discussion
In the present study, we showed that SIRT6 expression was increased in PM2.5-induced BMDMs, but Sirt6-deficient BMDMs decreased cytokine secretion when treated with PM2.5. Moreover, mice with myeloid cells specific knockdown of SIRT6, display decreased airway inflammation. Collectively, our data suggested that inhibition of SIRT6 in macrophages may represent therapeutic strategy for PM2.5-induced airway inflammation.
Sirtuin family, type III histone deacetylase (HDAC), consists of seven members. They participate in aging, inflammation, cancer and metabolic homeostasis by regulating cell cycle, cell differentiation, cell metabolism, cell senescence and death. Previous study revealed that SIRT6 might act as a double-edged sword in respiratory inflammatory diseases. SIRT6 has been found to play a protective role in allergen-induced inflammation by inhibiting IL-4-associating TH2 immune response, attenuating inflammatory cell recruitment, decreasing mucin production.10,15 To identify whether SIRT6 is macrophage specific, we first examined the expression of SIRT6 in THP1 derived macrophages exposed to PM2.5. Furthermore, we generated bone marrow–derived macrophages (BMDMs) from Sirt6fl/fl mice. The expression of SIRT6 was significantly increased in PM2.5-induced BMDMs and THP1 cells in a dose- and time-dependent manner. Double staining of macrophage (F4/80) and SIRT6 was also performed and confirmed that SIRT6 gene knockout is macrophage-specific. We found that inflammatory cytokines were decreased in BMDMs with myeloid-specific deletion of SIRT6 after stimulation of PM2.5. In vivo, Sirt6fl/fl-LysMCre mice substantially decreased airway inflammation in response to PM2.5 exposure. According to previous studies, we found that the profiles of sirtuin deacetylase family in the myeloid cells are different in different diseases. Some sirtuin such as SIRT1, SIRT4, SIRT5, and SIRT7protect again airway inflammation, hepatocellular carcinoma development, pro-inflammatory response, or mycobacterial clearance in macrophages.7,16–18 However, some sirtuin such as SIRT2 and SIRT3 promote chronic staphylococcal infection or inflammasome activation in macrophages.19,20 Therefore, the exact functions and mechanisms of sirtuin members in different diseases need to be further elucidated.
Limitations
Our study does have some limitations. First, the use of simulated PMs in our study was purchased from National Institute of Standards and Technology (NIST) Company, which do not represent airborne PMs that contain more chemicals. Although we found that PMs could induce airway inflammation in vivo and in vitro experiments, it is true that the use of simulated PMs in our study does not represent airborne PMs that contain more chemicals. Second, macrophages are highly phagocytic that may affect the experimental results in our study. Therefore, the role of SIRT6 on PM2.5-induced airway inflammation in macrophages should be interpreted cautiously due to limitations in our study.
Conclusion
Collectively, our study indicated that SIRT6 promoted PM2.5-induced airway inflammation in macrophages and suggested that inhibition of SIRT6 in macrophages might be a strategy for treating the airway inflammation induced by PM2.5.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (82170030; 81873404), Guangdong Basic and Applied Basic Research Foundation (2020B1515020004; 2020A1515110804), Guangdong Provincial Key Laboratory of Autophagy and Major Chronic Non-Communicable Diseases (2022B1212030003).
Disclosure
The authors declare no competing interests.
References
1. Cohen AJ, Brauer M, Burnett R, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389(10082):1907–1918. doi:10.1016/s0140-6736(17)30505-6
2. Guan W-J, Zheng X-Y, Chung KF, Zhong N-S. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet. 2016;388(10054):1939–1951. doi:10.1016/s0140-6736(16)31597-5
3. Li Z, Wu Y, Chen HP, et al. MTOR suppresses environmental particle-induced inflammatory response in macrophages. J Immunol. 2018;200(8):2826–2834. doi:10.4049/jimmunol.1701471
4. Mu X, Li Y, Fan GC. Tissue-resident macrophages in the control of infection and resolution of inflammation. Shock. 2021;55(1):14–23. doi:10.1097/SHK.0000000000001601
5. Shi T, Denney L, An H, Ho LP, Zheng Y. Alveolar and lung interstitial macrophages: definitions, functions, and roles in lung fibrosis. J Leukoc Biol. 2021;110(1):107–114. doi:10.1002/jlb.3ru0720-418r
6. Forrester MA, Wassall HJ, Hall LS, et al. Similarities and differences in surface receptor expression by THP-1 monocytes and differentiated macrophages polarized using seven different conditioning regimens. Cell Immunol. 2018;332:58–76. doi:10.1016/j.cellimm.2018.07.008
7. Lai T, Su G, Wu D, et al. Myeloid-specific SIRT1 deletion exacerbates airway inflammatory response in a mouse model of allergic asthma. Aging. 2021;13(11):15479–15490. doi:10.18632/aging.203104
8. Lai T, Wen X, Wu D, et al. SIRT1 protects against urban particulate matter-induced airway inflammation. Int J Chron Obstruct Pulmon Dis. 2019;14:1741–1752. PMCID: PMCPMC6689129. doi:10.2147/copd.s202904
9. Zhang XY, Li W, Zhang JR, Li CY, Zhang J, Lv XJ. Roles of sirtuin family members in chronic obstructive pulmonary disease. Respir Res. 2022;23(1):66. doi:10.1186/s12931-022-01986-y
10. Jang HY, Gu S, Lee SM, Park BH. Overexpression of sirtuin 6 suppresses allergic airway inflammation through deacetylation of GATA3. J Allergy Clin Immunol. 2016;138(5):1452–5.e13. doi:10.1016/j.jaci.2016.05.019
11. Wang QL, Yang L, Liu ZL, et al. Sirtuin 6 regulates macrophage polarization to alleviate sepsis-induced acute respiratory distress syndrome via dual mechanisms dependent on and independent of autophagy. Cytotherapy. 2022;24(2):149–160. doi:10.1016/j.jcyt.2021.09.001
12. Mazumder S, Barman M, Bandyopadhyay U, Bindu S. Sirtuins as endogenous regulators of lung fibrosis: a current perspective. Life Sci. 2020;258:118201. doi:10.1016/j.lfs.2020.118201
13. Kugel S, Mostoslavsky R. Chromatin and beyond: the multitasking roles for SIRT6. Trends Biochem Sci. 2014;39(2):72–81. doi:10.1016/j.tibs.2013.12.002
14. Chanput W, Mes JJ, Wichers HJ. THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol. 2014;23(1):37–45. doi:10.1016/j.intimp.2014.08.002
15. Ma K, Lu N, Zou F, Meng FZ. Sirtuins as novel targets in the pathogenesis of airway inflammation in bronchial asthma. Eur J Pharmacol. 2019;865:172670. doi:10.1016/j.ejphar.2019.172670
16. Wang F, Wang K, Xu W, et al. SIRT5 desuccinylates and activates pyruvate kinase M2 to block macrophage IL-1β production and to prevent DSS-induced colitis in mice. Cell Rep. 2017;19(11):2331–2344. doi:10.1016/j.celrep.2017.05.065
17. Zhang S, Liu Y, Zhou X, et al. Sirtuin 7 regulates nitric oxide production and apoptosis to promote mycobacterial clearance in macrophages. Front Immunol. 2021;12:779235. doi:10.3389/fimmu.2021.779235
18. Li Z, Li H, Zhao ZB, et al. SIRT4 silencing in tumor-associated macrophages promotes HCC development via PPARδ signalling-mediated alternative activation of macrophages. J Exp Clin Cancer Res. 2019;38(1):469. doi:10.1186/s13046-019-1456-9
19. Ciarlo E, Heinonen T, Théroude C, et al. Sirtuin 2 deficiency increases bacterial phagocytosis by macrophages and protects from chronic staphylococcal infection. Front Immunol. 2017;8:1037. doi:10.3389/fimmu.2017.01037
20. Guan C, Huang X, Yue J, et al. SIRT3-mediated deacetylation of NLRC4 promotes inflammasome activation. Theranostics. 2021;11(8):3981–3995. doi:10.7150/thno.55573
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.