Back to Journals » International Journal of Nanomedicine » Volume 17
Mycology-Nanotechnology Interface: Applications in Medicine and Cosmetology
Authors Dhanjal DS, Mehra P, Bhardwaj S, Singh R, Sharma P, Nepovimova E, Chopra C, Kuca K
Received 20 February 2022
Accepted for publication 29 April 2022
Published 2 June 2022 Volume 2022:17 Pages 2505—2533
DOI https://doi.org/10.2147/IJN.S363282
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Daljeet Singh Dhanjal,1,* Parul Mehra,2,* Sonali Bhardwaj,1,* Reena Singh,1 Parvarish Sharma,3 Eugenie Nepovimova,4 Chirag Chopra,1 Kamil Kuca4,5
1School of Bioengineering and Biosciences, Lovely Professional University, Phagwara, Punjab, India; 2Central Research Institute, Kasauli, Himachal Pradesh, India; 3School of Pharmaceutical Sciences, Lovely Professional University, Phagwara, Punjab, India; 4Department of Chemistry, Faculty of Science, University of Hradec Kralove, Hradec Kralove, 50003, Czech Republic; 5Biomedical Research Center, University Hospital Hradec Kralove, Hradec Kralove, 50005, Czech Republic
*These authors contributed equally to this work
Correspondence: Kamil Kuca, Department of Chemistry, Faculty of Science, University of Hradec Kralove, Hradec Kralove, 50003, Czech Republic, Tel +420 603 289 166, Email [email protected] Chirag Chopra, School of Bioengineering and Biosciences, Lovely Professional University, Jalandhar-Delhi GT Road, Phagwara, Punjab, 144411, India, +91-7837815676, Email [email protected]
Abstract: In today’s time, nanotechnology is being utilized to develop efficient products in the cosmetic and pharmaceutical industries. The application of nanotechnology in transforming bioactive material into nanoscale products substantially improves their biocompatibility and enhances their effectiveness, even when used in lower quantities. There is a significant global market potential for these nanoparticles because of which research teams around the world are interested in the advancements in nanotechnology. These recent advances have shown that fungi can synthesize metallic nanoparticles via extra- and intracellular mechanisms. Moreover, the chemical and physical properties of novel metallic nanoparticles synthesised by fungi are improved by regulating the surface chemistry, size, and surface morphology of the nanoparticles. Compared to chemical synthesis, the green synthesis of nanoparticles offers a safe and sustainable approach for developing nanoparticles. Biosynthesised nanoparticles can potentially enhance the bioactivities of different cellular fractions, such as plant extracts, fungal extracts, and metabolites. The nanoparticles synthesised by fungi offer a wide range of applications. Recently, the biosynthesis of nanoparticles using fungi has become popular, and various ways are being explored to maximize nanoparticles synthesis. This manuscript reviews the characteristics and applications of the nanoparticles synthesised using the different taxa of fungi. The key focus is given to the applications of these nanoparticles in medicine and cosmetology.
Keywords: fungi, biological application, biosynthesis, nanoparticles, nanotechnology
Introduction
The advancement in nanotechnology has enabled the creation and control of the nano-sized features in devices and materials to develop novel products in agricultural, cosmetic, environmental, food, medical device, personal care, and pharmaceutical industries. The emerging nanotechnology applications rely on the cost-effective synthesis and nanocomposites or nanoparticles with a size less than 100 nm.1 In naïve conditions, the bulk material tends to have consistent physical properties. However, during synthesis, the nanoparticles undergo various changes in chemical, electrical, magnetic, and optical properties due to the changes in particle size and shape. The published literature has revealed that silver and gold nanomaterials are extensively used to synthesize cosmeceutical and medical products.2 Although inert, silver nanoparticles (Ag-NPs) work effectively with antimicrobial compounds as they elicit the production of reactive oxygen species like hydrogen peroxide, which improves the antimicrobial activity.3 Owing to this ability, these nanoparticles can be used as antimicrobial agents to decrease pathogen infections during surgery and can help overcome antimicrobial resistance. Lately, it has been discovered that Ag-NPs also exhibit anti-angiogenic, anti-permeability, and anti-inflammatory potential, which has made them an effective entity for healthcare industries.4 Gold Nanoparticles (Au-NPs) also show antimicrobial and anticancer potential, and they are extensively used for treating numerous diseases. Progressive development in nanotechnology has enabled us to develop highly stable Au-NPs with suitable electrical, photothermal and optical properties for different applications in medical diagnostics and the health care industry.5 Apart from these two metals, other metals like cadmium, iron, platinum, and zinc are also used for synthesizing metallic nanoparticles via green technology.6
Biosynthetic Zinc oxide (ZnO) NPs have been reported to show antimicrobial activity and UV-blocking properties.7 Fungal-synthesized Maghemite NPs showed good properties of decolorizing textile effluents and removing different heavy metals.8 Fouda et al biosynthesized nanocomposites consisting of Cupric oxide and zinc oxide from Penicillium that could degrade methylene blue dye. Fungal-synthesized cupric oxide NPs have also been shown to have insecticidal activity against wheat-grain insects.9 These applications notarize the real potential of biosynthetic nanoparticles with multifunctional properties. Syed and Ahmad have reported the potential of Fusarium oxysporum for the extracellular synthesis of platinum nanoparticles. Their research reports the conversion of hexachloroplatinic acid into NPs, which showed suitable characteristics upon analysis using X-ray diffraction, transmission electron microscopy (TEM), and x-ray photoelectron spectroscopy (XPS).10
The conventional chemical methods of nanoparticle synthesis impose various negative impacts like the emission of toxic residues in the environment, which affects the health of both animals and humans, and improper disposal also results in other environmental issues.11 Additionally, these methods require high-end, sophisticated instrumentation and high energy costs for effective synthesis at a commercial scale. Reports show the benefit of chemically synthesised nanoparticles with antibacterial activities. One such report on curcumin silver nanoparticles showed antimicrobial activity of these NPs.12 However, the nanoparticles are of chemical origin and have some inherent disadvantages, including low biocompatibility and toxic effects of chemicals. The development of novel approaches using plants and microbes has emerged as an effective alternative for NPs synthesis. This is referred to as the green synthesis of nanoparticles. This approach has added advantages like non-toxic end-product, higher energy efficiency, and lower cost.13 The significant advantage of the green synthesis is that nanoparticle characteristics can be customized by regulating their composition, shape, morphology, and particle size, which directs their optical properties, chemical properties, catalytic properties, and anti-microbial potential, among other properties.14,15 Owing to these properties, the fungal synthesised nanoparticles find tremendous applications in the medicine and cosmetics industry. In the medicine and therapeutics industry, the fungal synthesized nanoparticles can potentially be used as antivirals, antibacterial, antifungals, anticancer drugs, drug delivery systems (DDS), and wound-healing agents, among others.16,17 For most antimicrobial activities, the size range of the nanoparticles is kept between 4–30 nm, which is confirmed through different analytical methods such as EDS (energy dispersive spectroscopy) and SAED (selected area electron diffraction).18 However, the expected size range for antivirals is much smaller, ie, between 1–10 nm.19 The field of cosmetology also finds several applications for these nanoparticles, such as preservative agents, antimicrobial agents, anti-inflammatory and antioxidants, among others.20
Since cosmetics work mainly on the skin, therefore, the size of the nanoparticles expected to work on the skin is 10–60 nm. However, there are concentration-dependent effects on the skin because of which the concentration of these nanoparticles is kept very low and close to 1ppm only.21,22 The fungal biomass provides the necessary capping agents, which are organic (usually proteinaceous) and biologically originated.23 As a result, the nanoparticles show high biocompatibility as a general feature. Biocompatibility is one of the critical features of green-synthesized nanoparticles because of ease of binding and internalization whenever required. The potential of biosynthetic NPs is also reviewed by Alavi and Rai (2021).24 This review highlights the present knowledge regarding nanoparticles synthesis via fungal species, their market potential, physio-chemical characteristics, and applications in medicine and cosmetology.
Global Market Potential of Nanoparticle Products
Nanomaterials are ultrafine materials, ranging from 1–100 nm in size. They can be synthesized through two processes, ie, biogenic or physiochemical. There are beyond 1000 products based on nanotechnology that can currently be procured from the market. Bruker Nano GmbH, Karlsruhe, Germany (https://www.bruker.com/en/landingpages/bna/bruker-nano-analytics.html), Advanced Diamond Technologies, Romeoville, IL, USA (http://www.thindiamond.com/company/team/); Altair Nanotechnologies, Reno, NV, USA (https://altairnano.com/), Nanophase Technologies Corporation, Romeoville, IL, USA (http://nanophase.com/), Nanosys, Milpitas, CA, USA (https://nanosys.com/) and are a few dominant players in the field of nanotechnology who market products entailing nanotechnology.25 Some noteworthy mentions of nanotechnology could be made in biomedical diagnostics, healthcare, textile, and food processing industries. An investigative report presented by the BBC considered the international trade for nanotechnology-based commodities and remarked that the value of these nano-tech products in 2013 was $US22.9 billion, that expanded to approximately $US26 billion in 2014. By 2019, this marketplace was anticipated to stretch to around $US64.2 billion.
The stated growth statistics show that 19.8% was the CAGR (compound annual growth rate) from 2014 to 2019.26,27 In a study conducted by Grand View Research in 2015, there would be an expected upsurge in the global capitalism curve regarding Ag-NPs by 2022 to ~ $US2.54 billion. The statistics uncover that the CAGR will exceed 25% forecast by 2022, along with the global capitalism that surpassed $US1.30 billion in 2014. Contributing beyond 30% of the Ag-NP world market, the health care industry marked the largest implementation of NP technologies in 2014.28 With the inflating demands in technology and related commodities due to the booming research and development in North America and the European region, these countries dominate the global NP technology, as stated by the Grand View Research. The standard-bearer in research innovation and the NP market is the United States. A recent increase in expenditure on R&D in biotechnological industries of Asian countries, India and China, in particular, anticipates fortifying the progress in the international Nanotechnology market in the Asian continent. The research and development expenses of Asian manufacturers are likely to increase as a consequence of trying to acquire a more capitalistic advantage in the worldwide Nanoparticle technology market in the upcoming years.29 Increased expenditure on R&D by companies will expand the augmentation of novel Nanoparticle production techniques involving harmless microbes. Additionally, further investigations would enhance the development of unprecedented Nano-tech-based products, particularly in the field of cosmeceutical and medical industries.30,31
Mechanisms of Synthesis: Extracellular and Intracellular Biosynthesis
Fungi produce nanoparticles as a defensive response against the hazardous contaminants such as heavy metals found in the environment. When they encounter toxic metal ions in their surroundings, fungi synthesize and secrete various metabolites like protein, enzymes, or cell membrane-bound molecules that reduce the metal ions into metal nanoparticles. This occurs via multiple mechanisms such as immobilization, complexation, bio-coupling, precipitation, biosorption, co-precipitation, and ion-form modification (Figure 1). After the reduction of metal ions, they are precipitated either extracellularly or intracellularly based on the synthesis mechanism (Figure 2).32 The following section describes the salient features of the extracellular and intracellular mechanisms of nanoparticle synthesis.
 |
Figure 1 Basic mechanism flowchart of fungal-based biosynthesis of nanoparticles. |
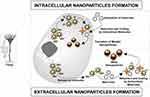 |
Figure 2 Schematic representation of intracellular and extracellular nanoparticle formation by fungi. |
Extracellular Fungal Biosynthesis of Metal Nanoparticles (MNPs)
Cell membranes of fungi display various molecules such as polysaccharides, quinones, peptides, oxidoreductases, proteins, etc., that are involved in reducing metal ions and precipitating them into MNPs, thus playing a vital role in the extracellular production of MNPs. Extracellular reductases are considered the chief enzymes accountable for the synthesis of MNPs.33 A study established that F. oxysporum cells produce sulphite and nicotinamide adenine dinucleotide phosphate (NADPH)- dependent nitrate reductases that synthesize Au-NPs and Ag-NPs, respectively.34 Moreover, nitrate reductases, quinones (naphthoquinones and anthraquinones), flavin adenine dinucleotide (FAD)-dependent glutathione reductase, quinine derivatives, and hydrogenases have been reported to contribute to the reduction process during fungal synthesis of MNPs. In another study by Siddiqui et al, Au-NPs synthesized using Fusarium oxysporum showed anti-proliferative activity against breast cancer and Burkitt’s lymphoma, in-vitro.35 Mukherjee et al also reported the potential of Fusarium oxysporum for extracellular synthesis of Au-NPs.36 MNPs biosynthesis by fungi through reductases needs an electron shuttle to reduce the metal ions. Research showed that fungi overexpressed some metalloproteins when exposed to a high concentration of heavy metal ions. These metalloproteins were believed to assist in the reduction of metal ions. The surface proteins bound on cell membranes of fungi have also been reported to carry out the synthesis of MNPs extracellularly.37 An investigation of R. oryzae and Coriolus versicolor revealed that the surface proteins embedded in the mycelia of these fungi assist in the reduction and stabilization of Ag ions and Au ions to biosynthesize Ag-NPs and Au-NPs.38
Intracellular Fungal Biosynthesis of Metal Nanoparticles
Enzymes such as hydrogenases and cellular ATPases are primarily responsible for the intracellular production of MNPs by fungi. Research on F. oxysporum showed the biosynthesis and aggregation of Au-NPs in the vacuoles present in the cytoplasm of fungal cells. Further analysis showed that plasma membrane-ATPase, glyceraldehyde-3-phosphate dehydrogenase, and 3-glucan binding protein regulated the intracellular synthesis of Au-NPs. Metallothionein and phytochelatin (metal-binding proteins), along with glutathione, are involved in the metal detoxification in yeasts leading to the reduction of metal ions to MNPs as they possess specific nucleophilic and redox properties.39 Fungi also use their antioxidants to precipitate these metal ions into MNPs to prevent cell damage through oxidation by these toxic metal ions. Toxic metal ions can also penetrate the cytosol of fungal cells through transporter proteins and membrane channels present in the cell membrane of fungi. Upon attack by metal ions, the fungal cells obstruct or eradicate such transport systems through which metal ions can enter. However, this defensive response of fungal cells affects the ongoing cellular processes, and some metal ions still penetrate the cytoplasmic space via multiple transport systems. In such a scenario, the intracellular fungal enzymes react with these metal ions to precipitate them into MNPs.40
Green Synthesis of Metal-Based Nanoparticles
The widespread application of MNPs in the medical sector has made it necessary to develop sustainable ways to produce MNPs that circumvent the usage of hazardous chemicals. The advancements in nanotechnology have enabled the evolution of traditional methods to more sustainable and versatile methods to produce Ag-NPs with controlled and explicit properties.41 Usually, NPs are synthesized either by the “top-down method” or “bottom-up method” (Figure 3).
 |
Figure 3 Diagrammatic illustration of top-down method” and “bottom-up method. |
In the bottom-up method, there is a homogeneous system wherein the catalytic agents such as enzymes and reducing agents control the catalysis of the synthesis of NPs. At the same time, appropriate pH, exposure time and temperature, and stabilizer provide the necessary environment for efficient bioprocessing of NPs. Moreover, reaction conditions and precursor concentrations determine the physicochemical attributes of NPs like morphological properties and surface structure.42 On the other hand, the top-down method uses the material in bulk form, and the size is reduced to nanoscale by physical treatment, chemical treatment, or their combination. Size reduction by physical treatment drastically increases the energy consumption, and therefore, is generally not preferred. This drawback paves the way for the chemical treatment to be the most common synthetic pathway for synthesizing MNPs. Using these approaches, both negatively charged and positively charged Ag-NPs can be synthesized.43 Negatively charged Ag-NPs can be synthesized using sodium citrate acting as a reducing agent, while positively charged Ag-NPs can be produced from the process using ethylene diamine tetra acetic acid (EDTA), branched polyethyleneimine, citrate, formaldehyde, hydrazine, alkali metals in ammonia, acetonitrile, inorganic and organic borohydrides, polyols, ascorbic acid, mono-alcohols or free radicals. A serious biological and environmental risk is posed by the top-down method as it uses various harmful solvents, reducing agents, and additives that are even unsuitable for medical use.44 Therefore, for the large-scale production of MNPs, it becomes essential to create eco-friendly, non-toxic precipitation processes. The green synthesis of MNPs by microbes focuses on increasing cell mass and synthesizing specific metabolites which act as reducing and capping agents. The primary purpose of this emphasis is to obtain homogeneous MNPs having uniform size, shape, and desirable optical properties such as the band gap energy, UV-visible absorption, and absorption coefficient, among others. Having desirable optical properties is essential for MNPs as they have to be employed for various purposes such as chemical, biological, medical, and electronic, among others.45
Applications of Microbial-Derived Nanoparticles
Much literature is available regarding the wet synthesis of MNPs by microbes. To survive in heavy metal contaminated areas, microbes naturally produce metal-binding peptides and proteins that bioremediate heavy metals. This characteristic of microbes gives an advantage to the microbial synthesis of MNPs over traditional MNP synthesis approaches. Microbes are called efficient bio-factories of NPs as they synthesize large quantities of enzymes, vitamins, polysaccharides, amino acids, and proteins which act as capping and reducing agents to reduce metal ions. Being potential bio-factories of MNPs, microbes can produce several NPs such as silver, titanium, gold, palladium, cadmium, platinum, etc. Among microbial species, bacteria, lower fungi, yeasts, edible mushrooms, and algae have been reported to synthesize MNPs. To prevent contamination by toxic metabolites, the microbe used for biosynthesis of MNPs must belong to either GRAS (generally regarded as safe) or Risk group 1 as classified by USFDA and WHO, respectively. Owing to this, much research has been carried out on edible mushrooms and lactic acid bacteria for the production of MNPs for use in food and nutraceutical industries. The following section discusses the applications of nanoparticles derived from different bacterial taxa.
Bacteria
Bacterial-synthesized nanoparticles have several reported potential applications that have been mentioned as follows. Green synthesised silver nanoparticles from different species of Streptomyces showed considerable antimicrobial and insecticidal activities.9 Ag-NPs derived from Bacillus sp. SBT8 showed antimicrobial activity.49 Pseudomonas aeruginosa-derived ZnO NPs showed significant antimicrobial activity against pathogens such as Candida albicans, E. coli (MIC 50ppm), and Staphylococcus aureus (MIC 200ppm).50 The extracellular biosynthesis of NPs is preferred over intracellular biosynthesis as it has simple and cheap downstream processing and does not require additional steps to optimize the process and break the cells to release NPs.51 Although bacteria have been reported to yield nanoparticles with different biological properties as reviewed above, fungal nanoparticles have been consistently gaining attention because of relatively higher biomass and convenient extraction of the filtrate.52
Fungi
Fungal NPs are produced extracellularly and offer certain advantages such as the production of stable NPs, synthesis of NPs is achieved in a single step, minimal use of complex chemicals, and no contamination by toxic metabolites.53 Botrytis cinerea is known to produce extracellular Au-NPs of varying shapes such as hexagonal, pyramidal, spherical, decahedral, and triangular. Studies have reported the production of both intracellular and extracellular NPs by Aspergillus oryzae var. viridis with sizes ranging between 10 and 60 nm.54 The biggest flex is that the fungal NPs biosynthesis can be carried out in a controlled manner allowing the production of NPs with desirable characteristics like shape, stability, size, and particle dispersity. Chemical stability, conductivity, antibacterial and catalytic activity of NPs is inversely related to their size, ie, the smaller the size of NPs higher will be the aforementioned properties. Small NPs with uniform dispersion have the advantage of possessing greater surface area, but the latter may also negatively affect the surface reactivity of NPs and reduce their stability in solutions. Attempts have been made to control the size of NP during biosynthesis, which usually involves optimization of pH and metal ion concentration at the start of the process.55 Ag-NPs derived from Penicillium italicum showed antioxidant activity (radical-scavenging) against DPPH and showed a dose-dependent antimicrobial activity against Candida tropicalis, Candida albicans, Staphylococcus aureus, and E. coli.56 Fungal synthesized Ag-NPs derived from Fusarium keratoplasticum showed significant growth inhibition of S. aureus, B. subtilis, P. aeruginosa, and E. coli when applied to cotton fabric in washing cycles.57 Fungal-derived Fe2O3 and MgO NPs (derived from Aspergillus carbonarius D-1) also showed biodegradation of different textile compounds in tannery effluent. The study also revealed immense potential in removing heavy metals from the effluent. Chromium was significantly removed from the water, whereas the MgO and Fe2O3 NPs were also effective in removing cadmium, lead, and nickel.58 MgO NPs synthesized from Penicillium chrysogenum could control the propagation of the malarial vector Anopheles stephensi through insecticidal activity against both larvae instar, pupal stages, and adult stages.59
Filamentous fungi have been widely studied for the biosynthesis of MNPs. Fungal synthesis of MNPs is considered an efficient and green approach for producing MNPs. Fungi are known for their ability to produce large quantities of extracellular metabolites, which play an important role in maintaining homeostasis and increasing their chances of survival during stress conditions like the presence of toxic contaminants or lack of nutrients. This ability of fungi benefits the NP biosynthesis process as the extracellular metabolites produced by fungi act as potential reducing agents.60 Extracellular proteins and enzymes secreted by fungi reduce metal ions into stable inorganic solid MNPs, which do not require external application capping agents.
Moreover, fungal MNPs exhibit good dispersion properties. Fungi can also tolerate high metal ion concentrations during the process, making them a suitable candidate for NP synthesis. Among microorganisms, fungi are preferred over other microorganisms owing to their low energy consumption, low process cost, high productivity, easy separation of biomass from broth, and simple downstream processing.61
A study by Balakumaran et al reported the production of extremely stable and well-dispersed spherical Ag-NPs by Guignardia mangiferae with particle size ranging between 5nm to 30 nm. This study synthesized Ag-NPs at pH 7 exhibited maximum antibacterial activity. Aureobasidium pullulans and Neurospora crassa have also been reported to produce Au-NPs but are intracellular; thus, they require additional steps for purification.62 Penicillium sp. has been shown to produce both extracellular and intracellular MNPs. Chan and Mat Don (2013) were successful in obtaining a yield of about 98.9% of Ag-NPs using Pycnoporus sanguineus. The metal ions from the broth were absorbed onto the cell surface of P. sanguineus by functional groups on the cell wall. The reducing sugars from the polysaccharide hydrolysates of the biomass reduced the metal ions into MNPs. The synthesized Ag-NPs were found to be spherical, with the diameter ranging between 52.8nm to 103.3 nm.63 A wide range of fungi like Aspergillus sydowii, Penicillium brevicompactum, Botrytis cinerea, Alternaria alternata, Trichoderma reesei, Fusarium semitectum, Aspergillus clavatus, Hormoconis resinae, Aspergillus fumigatus, Aspergillus terreus, Aspergillus oryzae var. viridis, etc. have been studied for the biosynthesis of Au-NPs and Ag-NPs. The primary focus has been on Au-NPs and Ag-NPs due to their widespread application in the cosmetic and medical sectors.64
Edible and Medicinal Mushrooms
A study showed that edible and medicinal mushrooms like Pleurotus florida, Agaricus bisporus, Ganoderma applanatum, Helvella lacunosa, and Fomes fomentarius are potent bio factories of Ag-NPs. Among all these mushrooms, Agaricus bisporus was found to be the most potent mushroom for the production of Ag-NPs. It has been observed that 75% of mushroom extracts are enriched proteins essential for the synthesis of MNPs. Moreover, they are also rich in volatile organic compounds like benzaldehyde, octanols, and octanones which work as reducing agents to reduce metal ions into MNPs.65 A study established the importance of enzymes and proteins secreted by Tricholoma matsutake in reducing metal ions. The protein and enzymes oxidized benzaldehyde into a carboxylic acid, which was confirmed by FTIR analysis. A band shift of carbonyl and hydroxyl groups was seen with the diminishing of existing carbonyl groups and the appearance of a new carbonyl peak. Ag ions bonded to hydroxyl groups reflected in a broad spectrum of the IR peak obtained at 3400 cm−1 in the FTIR spectrum of Ag-NPs. The presence of proteins during the biosynthesis and stabilization of Ag-NPs was confirmed when a band spectrum was detected at 1640 and 1550 cm−1 showing the extending vibrations of the secondary and primary amines.66
Many mushrooms show the potential to produce both extracellular and intracellular MNPs. Since Pleurotus sp. has GRAS status, it has been widely employed for the synthesis of non-toxic MNPs. Al Bahrani et al investigated the role of aqueous extract of the Pleurotus ostreatus in the synthesis of spherical Ag-NPs and found that it worked as a reducing and stabilizing agent during the synthesis process. It provided an eco-friendly and efficient system for the biosynthesis of Ag-NPs. Moreover, size and morphological analysis by transmission electron microscopy (TEM) revealed the sole presence of spherical Ag-NPs with particle size ranging between 10nm to 50 nm.67 Another study established that Pleurotus florida exhibits high potential for the production of Au-NPs using chloroauric acid (HAuCl4) with a glucan that acts as both a reducing agent as well as a stabilizing agent. P. florida exhibited size-controlled biosynthesis of Au-NPs.68 Moreover, studies have also shown intracellular production of stable Au-NPs in the mycelia of Flammulina velutipes upon incubation in chloroauric solution. These Au-NPs were found to possess catalytic activity against two major organic contaminants, 4-nitrophenol and methylene blue.69 Research on Cordyceps militaris showed the potential of this mushroom to synthesize spherical shaped, highly crystalline, and stable Ag-NPs with an average width of approximately 15 nm. The synthesized Ag-NPs showed considerable antibacterial activity against various pathogenic bacterial strains.70 A study on Ganoderma sp. reported the production of uniformly sized, highly stable Ag-NPs with a diameter of about 2 nm as observed under a Transmission Electron Microscope (TEM). Moreover, it was found that extracts of Ganoderma sp. contain reducing and capping agents that are responsible for the synthesis of these Ag-NPs.71 The list of different fungal species used to synthesize different nanoparticles have been comprehended in Table 1.
 |  |  |  |
Table 1 List of Fungal Species Used for Biosynthesis of Nanoparticles |
Benefits of Fungal Biosynthesis Over Other Biosynthesis Methods
It is well-established now that biological production processes are more efficacious than chemical-based processes regarding the costs, capacities, and procedures for metal NPs.33 Furthermore, there is the requirement for toxic solvents and additional treatment steps in chemical processes.132 There is high potential in biologically synthesized NPs by virtue of their chemical, optical and electronic features.133
The competent nature of fungi enables them to be used for the biosynthesis of NPs over other bacteria and plants. Cellular organization and metabolic activities are more developed in fungi than bacteria.134 Fungi have higher growth rates, fewer cultivation requirements, and high maximal yields with regards to the initial raw material in comparison to bacteria.107 The recovery of NPs produced by bacteria is complex compared to fungi that produce extracellular NPs that are much easier and cheaper to recover.135 Furthermore, the unwanted debris generated from production media and fungal biomass during the production of NPs from fungi can function as organic fertilizers and is easily biodegraded.136 The selection of fungi employed for the development of metal NPs on a large-scale is based upon some characteristics, including easy downstream process, rapid growth rate, easy biomass handling, and synthesis of surplus amount of enzymes and extracellular proteins.132,137–139 Contrarily, many mushrooms studied are either plant or human pathogens, besides studies being focused on the use of safe edible mushrooms. This fact makes them inappropriate to be used in productions on an industrial scale.136 A comparative schematic of bacterial and fungal biosynthesis of nanoparticles is shown in Figure 4
 |
Figure 4 Comparative schematic showing the bacterial and fungal mediated nanoparticle synthesis. |
Compared to plants, the fungal extracts perform better for metal nanoparticle synthesis. One of the critical reasons for this observation is the complex metabolome of plants. Because of high complexity, the plant extracts are highly heterogenous and variable in their biochemical composition. Therefore, the cost of standardization of the process increases. On the other hand, the maintenance cost of aseptic conditions for fungal cultures is saved in plant-mediated nanoparticle synthesis. In our opinion, the cost-benefit does not outweigh the efficacy of the fungal synthesis of nanoparticles. Due to the heterogeneity of plant extracts, the scale-up of the process is likely more tedious and, therefore, is a significant setback.
Applications of Fungal Nanoparticles in the Medical Field
The list of fungal nanoparticles having medical applications has been summarized in Table 2.
 |
Table 2 Enlist of Fungal Nanoparticles Showing Medical Applications |
Antibacterial Activity
Heavy use of antibiotics due to epidemics in the last few years, improper diagnosis, or self-medication has resulted in an increased resistance towards the microbes and their usual treatment drugs, i.e., antibiotics. NPs can be deemed as an effective measure to combat the resistance and devise new treatment methods.159,160 Studies depict that the biosynthesized Ag-NPs from Aspergillus niger exhibit inhibitory action against E. coli, a gram-negative bacteria, and S. aureus, a gram-positive bacterium. Ag-NPs bio-formulated by Sudhakar et al through Agaricus bisporus, an edible mushroom used as a bio-reductant, had antimicrobial action against harmful bacteria including Klebsiella spp., E. coli, and Proteus vulgaris.161 With reference to the study conducted by Durán and his associates (2007), the extracellular Ag-NPs produced by Fusarium sp. had the potential to be used to treat the textile fabrics as the cells of S. aureus were destroyed upon the exposure of fabric with Ag-NPs.162 It was concluded by Fayaz et al, based on their studies, that the growth of E. coli could be curbed with a MIC (Minimum Inhibitory Concentration) of 30 μg/mL by exposing the cells to biologically synthesized Ag-NPs produced through Trichoderma viride.163 Verma et al 2009 suggested that Aspergillus clavatus can be recognized as a biological factory to synthesize Ag-NPs. These NPs have an antimicrobial action against various human pathogens, including E. coli, P. fluorescens and C. albicans.75 Another researcher conducted a study in which he firstly screened around 20 strains of fungi and the results stated that two strains each of Trichoderma sp., Aspergillus sp., and Rhizopus sp. could be used for the biological synthesis of Ag-NPs that exhibited an antimicrobial action against bacteria including P. aeruginosa, E. coli and S. aureus.164 Govindappa et al, in their study, confirmed through SEM analysis the antibacterial action of Ag-NPs synthesized using the fungus Penicillium against P. aeruginosa and E. coli and also depicted that at higher concentrations, these Ag-NPs also exhibited anti-inflammatory, antioxidant, tyrokinase and anti-lipoxygenase nature.165 The microscopic analyses of atomic force and transmission electron microscopes revealed that the particle size of the biosynthesized Ag-NPs varied between the range of 4-30 nm. The composition and crystalline nature of Ag-NPs was established through EDS (energy dispersive spectroscopy), SAED (selective area electron diffraction), and XRD (X-ray diffraction) analyses. A schematic diagram of the mechanism of antibacterial activity of functionalized metallic nanoparticles is shown in Figure 5.166
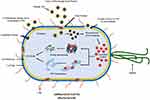 |
Figure 5 Schematic diagram showing the mechanism of antibacterial activity of functionalized metallic nanoparticles [Image Courtesy: 166. Under CC-BY license]. |
Antifungal Activity
Scientists worldwide have been trying to develop novel formulations to be used as effective treatments against the fatal diseases caused due to pathogenic microbes. Ag-NPs serve as potential candidates for the treatment of several diseases.167 Ag-NPs have been concluded to be used in formulations of products to be used in the treatment of nosocomial infections.167–171 An instance of biosynthesized Ag-NPs can potentially be used to treat nosocomial infections caused by the fungus Candida sp. The Ag-NPs possess antifungal action, synthesized through Bionectria ochroleuca and Aspergillus tubingensis. At 0.11–1.75μg/mL concentration, these Ag-NPs can destroy the fungus.172 A study by Ishida et al stated an approach of green chemistry (integrated microbial and nanotechnology) to synthesize NPs from the fungus Fusarium oxysporum. The NPs thus produced suppressed the outgrowth of pathogens, including Cryptococcus neoformans and Candida spp., as they exhibited a potentially high antifungal action. The fungal cells are killed due to the destruction of cells walls and damage to cytoplasmic membranes.173
Furthermore, the production of Ag-NPs by Schizophyllum commune, resulted in NPs with antifungal action against human fungal pathogens, essentially, dermatophytes, including Trichophyton rubrum, Trichophyton simii, and Trichophyton mentagrophytes.174 In a study conducted by Xue et al, the antifungal action of Ag-NPs biosynthesized from Arthroderma fulvum was studied.175 The study results depicted that Ag-NPs could destroy around ten human fungal pathogens when used at concentrations from 0.125 to 4.00 μg/mL, including Aspergillus spp., Fusarium spp., and Candida spp.
Antiviral Activity
Besides having antibacterial and antifungal properties, NPs also have antiviral properties. Antiviral action against HIV-1 has been recorded by Ag-NPs bio-manufactured from the fungus Aspergillus fumigatus. Research conducted by Narasimha et al stated that at a concentration of 30–180 ppm, the Ag-NPs produced by Aspergillus sp. exhibit antiviral action and decrease the no. of plaques and at a higher concentration ranging from 210–240 ppm, there is a complete suppression of virus particles in the bacterial cell host resulting in a total halt in the replication of virus.176 Studies conducted by Narasimha et al and Elechiguerra et al have concluded that the Ag-NPs ranging in size from 1–10 nm could prevent the HIV-1 virus from attaching to the host cell surface by binding to it.176,177
Anticancer Therapy
One of the major causes of fatalities worldwide is cancer. Scientists and researchers strive to establish less toxic and more effective treatments against cancer since chemotherapy has only been partially successful. Patients often acquire resistance to the chemicals or agents used in chemotherapy.178,179 According to various studies, free radicals are generated due to the induction of apoptotic pathways as a result of the administration of biogenic Ag-NPs.180 Hence, the use of Ag-NPs can serve as a diagnostic tool and for treatment of cancer in humans.180,181 Ag-NPs are reviewed to be successful angiogenic agents as they have anti-proliferative agents and anti-tumour properties. Cryptococcus laurentii (BNM0525), a yeast that biologically produces Ag-NPs has shown a potential anti-tumour behaviour in the cell lines of breast cancer, namely, T47D and MCF7.180 The cytotoxic effects caused by Ag-NPs breast cancer cells were examined by Gurunathan and his associates in 2013.182 They synthesized Ag-NPs biologically from Ganoderma neo-japonicum mycelia and concluded that the growth and activity of the breast cancer cells were restrained, accompanied by leakage in the cellular membrane after exposing the cells to solutions of Ag-NPs for 24 hrs at concentrations of 1 to 10 μg/mL. The anticancerous potential of Ag-NPs, formulated in broth cultures (shaken), with concentrations of 10-100 μg/mL, was also probed by Arun et al,174 who stated the results in terms of an MTT cytotoxicity assay that depicted around 27.2% - 64% cell death in human laryngeal carcinoma cells (HEP-2). Apart from the Ag-NPs, the Au-NPs are also reported to have anticancer properties. Basu et al reported that the green-synthesized Au-NPs using Tricholoma crassum showed dose-dependent induction of DNA damage and apoptosis in eukaryotic cells as revealed through comet assays.183 In an interesting development, Vahidi et al reported the synthesis of fungal-based tellurium nanoparticles. These NPs showed cytotoxicity against breast cancer cell line MCF-7 (IC50 39.83 μg/mL). Additionally, these tellurium nanoparticles showed significant antioxidant potential.184 Bhat and coworkers reported a unique photobiosynthetic mechanism of Au-NPs from Pleurotus florida. These functionalized Au-NPs showed in-vitro anticancer property against lung cancer cell line A549, chronic myelogenous leukaemia cell line K-562, cervix cancer cell line HeLa and mammary gland adenocarcinoma cell line MDA-MB.185
Drug Delivery
Drug delivery has been prospected to be a promising application for Nanoparticles where they can be used as carriers.186 Several mortal illnesses, including diabetes, cancer, and microbial infections, have been treated using novel drug delivery systems rooted in nanotechnology. This has also been applied to gene therapy.187 The application of this kind of drug delivery is advantageous due to its target specificity since it majorly affects the infected cells, thereby reducing the drug toxicity towards normal or uninfected cells. This improves the safety profile of the drug.188 Various approaches like quantum dots, nanotubes, nanopores, dendrimers, and liposomes release the bioactive compounds at the target-specific site(s) by coupling the drugs to NPs.187 For instance, regarding immunocompatibility and toxicity, Au-NPs are considerably safe and hence are used to formulate the scaffolds of drug delivery. A substitutive treatment for diabetes mellitus is the biologically synthesized NPs. An instance of the satisfactory therapeutic values of AU-NPs was in an experimental diabetic mouse model that showed low levels of liver enzymes like alkaline phosphatase and alanine transaminase due to the administration of Au-NPs, which further resulted in a low level of uric acid.189 Au-NPs, formulated through Trichoderma viride bound to the drug vancomycin, have also been used as an effective treatment to suppress the outgrowth or spread of vancomycin-resistant Staphylococcus aureus to a reduced concentration of 8 μg/mL by binding the Au-NPs to the surface of the microbial cell via ionic forces. Microscopic analysis through TEM reveals that the Au-NPs bound by vancomycin had permeated the membrane of S.aureus, resulting in cell death.190 Another study concluded that tumor growth could be reduced by a concentration of 86.8% by administering doxorubicin into magnetosomes of bacteria through covalent bonding.191 A study by Brown et al concluded that the delivery efficacy of oxaliplatin, an anticancer drug, could be improved when coupled with Au-NPs that had a monolayer of thiolated polyethylene glycol crested with a carboxylate group.171
Wound Healing
The first-ever product, including nano-silver particles to be commercialized for clinical use to treat ulcers, burns, epidermal necrolysis, and several other wounds, was developed by Robert Burrell.192–194 Huang et al also employed a similar approach to enhance wound healing in which he utilized a dressing laden with NPs. This approach provided the advantage of no adverse effects on patients along with the suppression of bacterial growth resulting in a decrease in the time for wound healing over the use of the standard treatment, silver sulfadiazine.195 Another study by Sundaramoorthi et al concluded that the use of Ag-NPs biologically formulated by Aspergillus niger could be detrimental for pathogenic bacteria as it could regulate the cytokines participating in wound healing and hence had sufficient potential for the recovery of wounds.196 Marcato et al in 2015, conducted an in vivo study on biosynthesized Ag-NPs by using Fusarium oxysporum. He formulated a biogenic silver formulation and depicted that this formulation coupled with enoxaparin could be an effective treatment for wound healing, free from any side effects, with the advantages of a reduced time for both the inflammatory action and demarcation of fibroblasts into myofibroblasts.197
Cosmetology Applications of Fungal Nanoparticles
The United States Federal Food, Drug, and Cosmetic Act defines cosmetics as
articles intended to be applied to the human body by being rubbed, poured, sprinkled, or sprayed for cleansing, beautifying, promoting attractiveness, or altering the appearance (U.S. Food and Drug Administration 2016).198
“Cosmeceutical” is defined as the cosmetic agents that assert peculiar curative or healing measures. The demand for cosmetic products has upsurged over the last few years, which has amplified its growth in the international market such that it went from $US31.84 billion in the year 2016 to $US42.4 billion worth in 2018 (GBI Research; RNCOS E-Services 2016).199 Nanotechnology remains one of the most potential and promising approaches in the cosmetic industry. The efficacy of cosmetics relies on their absorption into the skin, and the smaller particles are readily absorbed into the skin. Due to their small size, the nano-emulsions show potential applications in the cosmetic industry as they form more uniform layers (films) on the skin.200 Hence, NPs are frequently employed in the formulations of various cosmetic products in the cosmeceutical arena. There is a broad scope of nanotechnology in the cosmetic and dermatological industries, as the technology is used to manufacture multiple products, including toothpastes, soaps, perfumes, sunscreens, anti-wrinkle creams, moisturizers, skin cleansers, lipsticks, hair care products, and nail care products. Based on the size and functionality of NPs, they are classified into eight classes of product, namely, cubosomes, dendrimers, niosomes, nanogold, nanocrystals, nanosilver, nanocapsules, liposomes, and solid lipid nanoparticles.200 Novel eco-friendly approaches for manufacturing metal NPs of Au, Ag and Pt are highlighted.201 The approach is considered eco-friendly because they are produced through bio-factories like plants, fungi, bacteria, and yeasts cells.169
Silver Nanoparticles as Preservatives in Cosmetics
The use of preservatives in cosmetic products is necessary not only to restrain any sort of primary contamination from microbes after the formulation of the product but also to prevent the secondary contamination when the product is opened and closed by the consumer on a daily basis.202 Chemicals agents like parabens and phenoxyethanol, antibacterial in nature, are the generally used preservatives in cosmetic products. However, they subject the skin of the consumer to some harmful side effects like skin irritation and sensitivity to UV rays.203,204 Hence, it was the need of the hour for many years to substitute these harmful chemicals with some preservatives that have an indifferent activity towards the skin and antimicrobial nature towards microbes. The solution to this problem was metal nanoparticles like Ag-NPs. Based on their antimicrobial action, they are now employed to serve as preservatives in cosmetic products.205 There is extensive use of these nanoparticles in cosmetic products, including face packs, anti-aging creams, and deodorants.200 It was stated by Gajbhiye and Sakharwade (2016) that the Ag-NPs are also used as preservatives in shampoos and toothpastes owing to their antibacterial action. Ag-NPs are biologically synthesized through Penicillium, which is an endophytic fungal genus. Saponins, tannins, flavonoids, and terpenoids identified in the extracts of the fungus Penicillium serve as capping and reducing agents to convert Ag particles into Nanoparticles.165 Capping agents like carbonyl and amide groups discovered in Ag-NPs, synthesized from the fungus Fusarium semitectum, whose size ranges from 10–60 nm, are associated with a 6–8 weeks stability. Agglomeration is dodged in the metal NPs by using capping agents that also provide the product with stability.206 These properties affect the appearance of the product by providing it with an appearance homogenous in nature and enhancing its sensory functions by preventing the sedimentation of the product for over a year.202 Metal oxides in nanoparticles like nano-titanium oxide and zinc oxide improve the feel and the spreading ability of the cosmetic product. Besides imparting these properties to the cosmetic product, they also enhance the SPF (sun protection factor) and exhibit antimicrobial action contrary to their non-nano counterparts.207
Antimicrobial Agents in Cosmetics
PAg-NPs, the nanoparticles biologically synthesized from the Penicillium spp., can suppress the growth of P. aeruginosa and E. coli at a concentration of 100-μL culture filtrate/mL pathogen broth; they exhibit a sufficiently high antibacterial activity.165 The growth of other bacteria like V. cholera, S. aureus, B. subtilis, P. aeruginosa, and E. coli can also be suppressed by the antimicrobial activity of these Ag-NPs.208,209 Fungus, including various strains of the Candida spp., including C. glabrata, C. krusei, C. albicans, C. parapsilosis, and C. tropicalis are also subjected to damage by Ag-NPs due to a damaging effect to the cell envelope of fungal cells.210 Kokura et al demonstrated that at a low concentration of 1.0 ppm, Ag-NPs exhibited antimicrobial action against the mixed bacterial extracts containing P. aeruginosa, E. coli, S. aureus and against mixed fungal extracts including A. pullulans, A. niger, C. albicans and P. citrinum found in domestic wastewater like drainage and kitchen wastewater.202 Metal NPs like nano titanium oxide (TiO2) nanoparticles are used in cosmetic products like sunscreens and other cosmeceuticals like skin milk, whitening creams, morning and night creams (AzoNano2013). At a concentration of 40 μg mL−1, the titanium oxide nanoparticles synthesised by the fungus Aspergillus flavus suppress the growth of Gram-negative bacteria, E. coli.211
Antioxidants and Anti-Inflammatory Agents in Cosmetics
As mentioned above, nanoparticles enhance the sensory character and impart stability to the cosmetic product along with a better spreading ability and sun protection.205 FRAP (Ferric Reducing Antioxidant Power) and DPPH (1,1-diphenyl-2-picrylhydrazyl)-scavenging tests confirm the substantial amount of antioxidant action of PAg-NPs. The anti-inflammatory nature was also exhibited by these NPs, as they stabilize the membranes, thereby making them suitable preservatives in cosmetic products. Besides the advantages, Ag-NPs also promote wound healing when present in cosmetic products. However, there is not much evidence regarding the anti-inflammatory nature of the fungal Ag-NPs.206,212–215 But the fact to be considered is the risk of toxicity of NPs due to their effective penetration through the skin and the supposed nano-size of the particles. Recent studies show that Ag-NPs at a concentration of 0.002–0.02 ppm permeate the skin and are swept away from the bloodstream, not causing toxicity.205
Other Applications of Fungal Nanoparticles
Metal NPs developed through fungi can potentially function as sensors for electronic devices and optical devices.216 It was reported by Fayaz et al that metal NPs like Ag-NPs synthesized by Trichoderma viride were efficiently implemented in biosensing and bio-imaging operations.217 At 320–520 nm wavelengths, these silver nanoparticles were used for blue-orange light emission. Moreover, XRD analyses and EDX (Energy Dispersive X-ray) were employed for complete characterizations of these Ag-NPs. With reference to the studies of Zheng et al, NPs of Au-Ag alloy synthesized by using yeast cells were potentially used as a novel vanillin sensor, the sensitivity of which was five times greater than other approaches. This study concluded that Ag-NPs had high potential in being utilized in the form of sensors to determine vanillin production through vanilla tea and vanilla bean quantitatively.218 The studies of Thibault et al suggested that the activity of enzyme glucose oxidase (GOx) to function as an indicator was enhanced such that the glucose content could be easily resolved in the commercial injections of glucose. Au-NPs exhibit highly sensitive detection that forms the basis of activity of Au-NP-GOx-based biosensor.219 The diverse application of various fungal nanoparticles in biomedicine is illustrated in Figure 6.
 |
Figure 6 Diverse application of fungal based nanoparticles in the field of biomedicine. |
Future Prospect and Conclusion
Microorganisms (fungi, in particular) have been extensively used in the medical industry for various treatments and preventing diseases since the last century. With the intention of determining and producing medically important molecules, several primary and secondary metabolites (antibiotics, biosurfactants, enzymes, immune suppressor substances, and organic acids) have been produced at industrial scales. Only ~5% of the fungi available naturally have been studied. It is plausible that the interest in Science and technology related to fungal nanotechnology will heighten in the future. Chemically synthesized NPs are highly toxic and pose health hazards; hence, there is a need to conclude a non-toxic production method (production of NPs via biological pathways) that seems to have already gained interest in the scientific community. The green synthesized nanoparticles are more biocompatible than their chemically synthesized counterparts. There is a tremendous commercial potential for the green-synthesized nanoparticles in the industries such as diagnostics, textile, cosmetics, and medicine, among others, because billions of dollars’ worth of investments in nanotech-based products is expected. NPs of fungal origin seem to perform better due to a higher yield of biomass, higher enzyme levels, and easier downstream processing. The residual fungal biomass can also be used in biofertilizers. However, the cost of maintaining aseptic fungal cultures is a limiting factor, which can be overcome through meticulous process design and further research on low-cost substrates. Fungal nanoparticles can be synthesized through extracellular as well as intracellular mechanisms. In the extracellular mechanisms, cell surface molecules and enzymes may act as reducing agents. Intracellular mechanisms that utilize cytoplasmic oxidoreductases are typically involved in nanoparticle synthesis. Both intracellular and extracellular NPs show various biological activities, including, but not limited to, antiproliferative, antibacterial, antifungal, anti-parasitic, and antioxidant. These activities have been proven through research on a large number of fungal strains and a wide variety of metallic nanoparticles, including Ag, Au, ZnO, Pt, and TiO2. However, more studies report antimicrobial activity as compared to other activities. Besides medical applications, the fungal synthesized nanoparticles find applications in cosmetology as preservatives, antimicrobial, and anti-inflammatory agents. A noteworthy aspect is that the cosmetology applications of the NPs are seen at very low concentrations due to high skin penetration.
Currently, a plethora of applications of the fungal-synthesized nanoparticles are paving the way for several future technologies. The use of electrospinning to produce functional nanofibrous scaffolds can potentially play significant roles in nanobiotechnology in biomedical or nanomedicine industries, for instance, removing heavy metals during waste water treatment nanomembranes for environmental applications, enzyme immobilization, tissue engineering, and drug delivery for biomedical/nanomedicine applications. Thus, these nanomaterials are biocompatible, biodegradable, sustainable, antimicrobial, and non-toxic, all of which are of great relevance in the nanotechnology industry.
Acknowledgments
The authors are grateful to the Excellence project PrF UHK 2217/2022-2023 for the financial support.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lowry GV, Avellan A, Gilbertson LM. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat Nanotechnol. 2019;14(6):517–522. doi:10.1038/s41565-019-0461-7
2. Mittapally S, Aziz A, Student A, Afnan AA. A review on nanotechnology in cosmetics. Pharma Innov Int J. 2019;8(4):668–671.
3. Effiong DE, Uwah TO, Jumbo EU, et al. Nanotechnology in cosmetics: basics, current trends and safety concerns—A review. Adv Nanopart. 2019;9(1):1–22. doi:10.4236/ANP.2020.91001
4. Erkoc P, Ulucan-Karnak F. Nanotechnology-based antimicrobial and antiviral surface coating strategies. Prosthes. 2021;3(1):25–52. doi:10.3390/PROSTHESIS3010005
5. Tao C. Antimicrobial activity and toxicity of gold nanoparticles: research progress, challenges and prospects. Lett Appl Microbiol. 2018;67(6):537–543. doi:10.1111/LAM.13082
6. Marinescu L, Ficai D, Oprea O, et al. Optimized synthesis approaches of metal nanoparticles with antimicrobial applications. J Nanomater. 2020;2020:6651207. doi:10.1155/2020/6651207
7. Fouda A, El-din Hassan S, Salem SS, Shaheen TI. In-Vitro cytotoxicity, antibacterial, and UV protection properties of the biosynthesized Zinc oxide nanoparticles for medical textile applications. Microb Pathog. 2018;125:252–261. doi:10.1016/J.MICPATH.2018.09.030
8. Fouda A, Hassan SED, Saied E, Azab MS. An eco-friendly approach to textile and tannery wastewater treatment using maghemite nanoparticles (γ-Fe2O3-NPs) fabricated by Penicillium expansum strain (K-w). J Environ Chem Eng. 2021;9(1):104693. doi:10.1016/J.JECE.2020.104693
9. Badawy AA, Abdelfattah NAH, Salem SS, Awad MF, Fouda A. Efficacy assessment of biosynthesized Copper Oxide Nanoparticles (CuO-NPs) on stored grain insects and their impacts on morphological and physiological traits of wheat (Triticum aestivum L.) plant. Biology. 2021;10(3):233. doi:10.3390/BIOLOGY10030233
10. Syed A, Ahmad A. Extracellular biosynthesis of platinum nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B Biointerfaces. 2012;97:27–31. doi:10.1016/J.COLSURFB.2012.03.026
11. Canu IG, Schulte PA, Riediker M, Fatkhutdinova L, Bergamaschi E. Methodological, political and legal issues in the assessment of the effects of nanotechnology on human health. J Epidemiol Community Heal. 2018;72(2):148–153. doi:10.1136/JECH-2016-208668
12. Alavi M, Adulrahman NA, Haleem AA, et al. Nanoformulations of curcumin and quercetin with silver nanoparticles for inactivation of bacteria. Cell Mol Biol. 2021;67(5):151–156. doi:10.14715/CMB/2021.67.5.21
13. Nasrollahzadeh M, Sajjadi M, Sajadi SM, Issaabadi Z. Green Nanotechnology. Interface Sci Technol. 2019;28:145–198. doi:10.1016/B978-0-12-813586-0.00005-5
14. Oke AE, Aigbavboa CO, Semenya K. Energy savings and sustainable construction: examining the advantages of nanotechnology. Energy Procedia. 2017;142:3839–3843. doi:10.1016/J.EGYPRO.2017.12.285
15. Müller RH, Pyo SM. Why nanotechnology in dermal products?—Advantages, challenges, and market aspects. In: Cornier J, Keck CM, Voorde Van de M, editors. Nanocosmetics.
16. Gaikwad S, Ingle A, Gade A, et al. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int J Nanomedicine. 2013;8:4303–4314. doi:10.2147/IJN.S50070
17. Moghaddam AB, Namvar F, Moniri M, Tahir PM, Azizi S, Mohamad R. Nanoparticles biosynthesized by fungi and yeast: a review of their preparation, properties, and medical applications. Molecules. 2015;20(9):16540–16565. doi:10.3390/MOLECULES200916540
18. Singh T, Jyoti K, Patnaik A, Singh A, Chauhan R, Chandel SS. Biosynthesis, characterization and antibacterial activity of silver nanoparticles using an endophytic fungal supernatant of Raphanus sativus. J Genet Eng Biotechnol. 2017;15(1):31–39. doi:10.1016/J.JGEB.2017.04.005
19. Sharmin S, Rahaman MM, Sarkar C, Atolani O, Islam MT, Adeyemi OS. Nanoparticles as antimicrobial and antiviral agents: a literature-based perspective study. Heliyon. 2021;7(3):e06456. doi:10.1016/J.HELIYON.2021.E06456
20. Salvioni L, Morelli L, Ochoa E, et al. The emerging role of nanotechnology in skincare. Adv Colloid Interface Sci. 2021;293:102437. doi:10.1016/J.CIS.2021.102437
21. Kokura S, Handa O, Takagi T, Ishikawa T, Naito Y, Yoshikawa T. Silver nanoparticles as a safe preservative for use in cosmetics. Nanomedicine. 2010;6(4):570–574. doi:10.1016/J.NANO.2009.12.002
22. Wiesenthal A, Hunter L, Wang S, Wickliffe J, Wilkerson M. Nanoparticles: small and mighty. Int J Dermatol. 2011;50(3):247–254. doi:10.1111/J.1365-4632.2010.04815.X
23. Li Q, Liu F, Li M, Chen C, Gadd GM. Nanoparticle and nanomineral production by fungi. Fungal Biol Rev. 2021. doi:10.1016/J.FBR.2021.07.003
24. Alavi M, Rai M. Antisense RNA, the modified CRISPR-Cas9, and metal/metal oxide nanoparticles to inactivate pathogenic bacteria. Cell Mol Biomed Rep. 2021;1(2):52–59. doi:10.55705/CMBR.2021.142436.1014
25. Chinchilla-Rodríguez Z, Miguel S, Perianes-Rodríguez A, Sugimoto CR. Dependencies and autonomy in research performance: examining nanoscience and nanotechnology in emerging countries. Science. 2018;115(3):1485–1504. doi:10.1007/S11192-018-2652-7
26. Mitter N, Hussey K. Moving policy and regulation forward for nanotechnology applications in agriculture. Nat Nanotechnol. 2019;14(6):508–510. doi:10.1038/s41565-019-0464-4
27. Henchion M, McCarthy M, Dillon EJ, Greehy G, McCarthy SN. Big issues for a small technology: consumer trade-offs in acceptance of nanotechnology in food. Innov Food Sci Emerg Technol. 2019;58:102210. doi:10.1016/J.IFSET.2019.102210
28. Jain R, Sharma D. Applications and Ethical Issues of Nanotechnology in Real World. J Web Eng Technol. 2019;6(2):25–28.
29. Silva GA. A New Frontier: the convergence of nanotechnology, brain machine interfaces, and artificial intelligence. Front Neurosci. 2018;12:843. doi:10.3389/FNINS.2018.00843
30. Rana KL, Kour D, Yadav N, Yadav AN. Endophytic microbes in nanotechnology: current development, and potential biotechnology applications. In: Microb Endophytes Prospect Sustain Agric; 2020:231–262. doi:10.1016/B978-0-12-818734-0.00010-3
31. Kargozar S, Mozafari M. Nanotechnology and Nanomedicine: start small, think big. Mater Today Proc. 2018;5(7):15492–15500. doi:10.1016/J.MATPR.2018.04.155
32. Deshmukh R, Khardenavis AA, Purohit HJ. Diverse metabolic capacities of fungi for bioremediation. Indian J Microbiol. 2016;56(3):247. doi:10.1007/S12088-016-0584-6
33. Durán N, Marcato PD, Durán M, Yadav A, Gade A, Rai M. Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi, and plants. Appl Microbiol Biotechnol. 2011;90(5):1609–1624. doi:10.1007/S00253-011-3249-8
34. Hietzschold S, Walter A, Davis C, Taylor AA, Sepunaru L. Does nitrate reductase play a role in silver nanoparticle synthesis? Evidence for NADPH as the sole reducing agent. ACS Sustain Chem Eng. 2019;7(9):8070–8076. doi:10.1021/ACSSUSCHEMENG.9B00506
35. Ahmad Siddiqui E, Ahmad A, Julius A, et al. Biosynthesis of anti-proliferative gold nanoparticles using endophytic Fusarium oxysporum strain isolated from neem (A. indica) leaves. Curr Top Med Chem. 2016;16(18):2036–2042. doi:10.2174/1568026616666160215160644
36. Mukherjee P, Senapati S, Mandal D, et al. Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. ChemBioChem. 2002;3(5):461–463. doi:10.1002/1439-7633(20020503)3:5<461::AID-CBIC461>3.0.CO;2-X
37. Silva LP, Bonatto CC, Polez VLP. Green Synthesis of Metal Nanoparticles by Fungi: Current Trends and Challenges. 2016:71–89. doi:10.1007/978-3-319-42990-8_4
38. Khandel P, Shahi SK. Mycogenic nanoparticles and their bio-prospective applications: current status and future challenges. J Nanostruct Chem. 2018;8(4):369–391. doi:10.1007/s40097-018-0285-2
39. Kitching M, Ramani M, Marsili E. Fungal biosynthesis of gold nanoparticles: mechanism and scale up. Microb Biotechnol. 2015;8(6):904. doi:10.1111/1751-7915.12151
40. Gahlawat G, Choudhury AR. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019;9(23):12944–12967. doi:10.1039/C8RA10483B
41. Zhang X-F, Liu Z-G, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17(9):9. doi:10.3390/IJMS17091534
42. Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12(7):908–931. doi:10.1016/J.ARABJC.2017.05.011
43. Illath K, Wankhar S, Mohan L, Nagai M, Santra TS. Metallic nanoparticles for biomedical applications. Springer Ser Biomater Sci Eng. 2021;16:29–81. doi:10.1007/978-981-33-6252-9_2
44. Heuer-Jungemann A, Feliu N, Bakaimi I, et al. The role of ligands in the chemical synthesis and applications of inorganic nanoparticles. Chem Rev. 2019;119(8):4819–4880. doi:10.1021/ACS.CHEMREV.8B00733
45. Rauwel P, Küünal S, Ferdov S, Rauwel E. A review on the green synthesis of silver nanoparticles and their morphologies studied via TEM. Adv Mater Sci Eng. 2015;2015:1–9. doi:10.1155/2015/682749
46. Ojuederie O, Babalola O. Microbial and plant-assisted bioremediation of heavy metal polluted environments: a review. Int J Environ Res Public Health. 2017;14(12):1504. doi:10.3390/ijerph14121504
47. Azam Z, Ayaz A, Younas M, et al. Microbial synthesized cadmium oxide nanoparticles induce oxidative stress and protein leakage in bacterial cells. Microb Pathog. 2020:144. doi:10.1016/J.MICPATH.2020.104188
48. Salunke BK, Sawant SS, Lee SI, Kim BS. Microorganisms as efficient biosystem for the synthesis of metal nanoparticles: current scenario and future possibilities. World J Microbiol Biotechnol. 2016;32(5). doi:10.1007/S11274-016-2044-1
49. Yurtluk T, Akçay FA, Avcı A. Biosynthesis of silver nanoparticles using novel Bacillus sp. SBT8. Prep Biochem Biotechnol. 2018;48(2):151–159. doi:10.1080/10826068.2017.1421963
50. Abdo AM, Fouda A, Eid AM, et al. Green synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) by Pseudomonas aeruginosa and their activity against pathogenic microbes and common house mosquito, Culex pipiens. Materials. 2021;14(22):6983. doi:10.3390/MA14226983
51. Singh A, Gautam PK, Verma A, et al. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: a review. Biotechnol Rep. 2020;25:e00427. doi:10.1016/J.BTRE.2020.E00427
52. Guilger-Casagrande M, Lima de R. Synthesis of silver nanoparticles mediated by fungi: a review. Front Bioeng Biotechnol. 2019;7:287. doi:10.3389/FBIOE.2019.00287/BIBTEX
53. Guilger-Casagrande M, Lima de R. Synthesis of silver nanoparticles mediated by fungi: a review. Front Bioeng Biotechnol. 2019;7:287. doi:10.3389/fbioe.2019.00287
54. Menon S, Rajeshkumar S, Venkatkumar S. A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resour Technol. 2017;3(4):516–527. doi:10.1016/J.REFFIT.2017.08.002
55. Li X, Xu H, Chen ZS, Chen G. Biosynthesis of nanoparticles by microorganisms and their applications. J Nanomater. 2011;2011:1–16. doi:10.1155/2011/270974
56. Taha ZK, Hawar SN, Sulaiman GM. Extracellular biosynthesis of silver nanoparticles from Penicillium italicum and its antioxidant, antimicrobial and cytotoxicity activities. Biotechnol Lett. 2019;41(8–9):899–914. doi:10.1007/S10529-019-02699-X/FIGURES/12
57. Mohmed AA, Fouda A, Elgamal MS, EL-Din Hassan S, Shaheen TI, Salem SS. Enhancing of cotton fabric antibacterial properties by silver nanoparticles synthesized by new Egyptian strain Fusarium Keratoplasticum A1-3. Egypt J Chem. 2017;60:63–71. doi:10.21608/EJCHEM.2017.1626.1137
58. Fouda A, Hassan SED, Abdel-Rahman MA, et al. Catalytic degradation of wastewater from the textile and tannery industries by green synthesized hematite (α-Fe2O3) and magnesium oxide (MgO) nanoparticles. Curr Res Biotechnol. 2021;3:29–41. doi:10.1016/J.CRBIOT.2021.01.004
59. Fouda A, Awad MA, Eid AM, et al. An Eco-friendly approach to the control of pathogenic microbes and anopheles stephensi malarial vector using Magnesium Oxide Nanoparticles (Mg-NPs) fabricated by Penicillium chrysogenum. Int J Mol Sci. 2021;22(10):5096. doi:10.3390/IJMS22105096
60. Das RK, Pachapur VL, Lonappan L, et al. Biological synthesis of metallic nanoparticles: plants, animals and microbial aspects. Nanotechnol Environ Eng. 2017;2(1):1–21. doi:10.1007/S41204-017-0029-4
61. Ghosh S, Ahmad R, Zeyaullah M, Khare SK. Microbial nano-factories: synthesis and biomedical applications. Front Chem. 2021;194. doi:10.3389/FCHEM.2021.626834
62. Balakumaran MD, Ramachandran R, Kalaichelvan PT. Exploitation of endophytic fungus, Guignardia mangiferae for extracellular synthesis of silver nanoparticles and their in vitro biological activities. Microbiol Res. 2015;178:9–17. doi:10.1016/J.MICRES.2015.05.009
63. Chan YS, Don MM. Optimization of process variables for the synthesis of silver nanoparticles by Pycnoporus sanguineus using statistical experimental design. J Korean Soc Appl Biol Chem. 2013;56(1):11–20. doi:10.1007/S13765-012-2177-3
64. Siddiqi KS, Husen A. Fabrication of metal nanoparticles from fungi and metal salts: scope and application. Nanoscale Res Lett. 2016;11(1):1–15. doi:10.1186/S11671-016-1311-2
65. Owaid MN, Ibraheem IJ. Mycosynthesis of nanoparticles using edible and medicinal mushrooms. Eur J Nanomed. 2017;9(1):5–23. doi:10.1515/ejnm-2016-0016
66. Anthony KJP, Murugan M, Jeyaraj M, Rathinam NK, Sangiliyandi G. Synthesis of silver nanoparticles using pine mushroom extract: a potential antimicrobial agent against E. coli and B. subtilis. J Ind Eng Chem. 2014;20(4):2325–2331. doi:10.1016/J.JIEC.2013.10.008
67. Al-Bahrani R, Raman J, Lakshmanan H, Hassan AA, Sabaratnam V. Green synthesis of silver nanoparticles using tree oyster mushroom Pleurotus ostreatus and its inhibitory activity against pathogenic bacteria. Mater Lett. 2017;186:21–25. doi:10.1016/j.matlet.2016.09.069
68. Sen IK, Maity K, Islam SS. Green synthesis of gold nanoparticles using a glucan of an edible mushroom and study of catalytic activity. Carbohydr Polym. 2013;91(2):518–528. doi:10.1016/J.CARBPOL.2012.08.058
69. Narayanan KB, Park HH, Han SS. Synthesis and characterization of biomatrixed-gold nanoparticles by the mushroom Flammulina velutipes and its heterogeneous catalytic potential. Chemosphere. 2015;141:169–175. doi:10.1016/J.CHEMOSPHERE.2015.06.101
70. Wang L, Liu CC, Wang YY, Xu H, Su H, Cheng X. Antibacterial activities of the novel silver nanoparticles biosynthesized using Cordyceps militaris extract. Curr Appl Phys. 2016;16(9):969–973. doi:10.1016/J.CAP.2016.05.025
71. Nguyen VP, Le Trung H, Nguyen TH, Hoang D, Tran TH. Synthesis of biogenic silver nanoparticles with eco-friendly processes using Ganoderma lucidum Extract and evaluation of their theranostic applications. J Nanomater. 2021;2021:1–11. doi:10.1155/2021/6135920
72. Owaid MN, Naeem GA, Muslim RF, Oleiwi RS. Synthesis, characterization and antitumor efficacy of silver nanoparticle from Agaricus bisporus Pileus, Basidiomycota. Walailak J Sci Technol. 2018;17(2):75–87. doi:10.48048/wjst.2020.5840
73. Sarkar J, Ray S, Chattopadhyay D, Laskar A, Acharya K. Mycogenesis of gold nanoparticles using a phytopathogen Alternaria alternata. Bioprocess Biosyst Eng. 2011;35(4):637–643. doi:10.1007/S00449-011-0646-4
74. Saravanan M, Nanda A. Extracellular synthesis of silver bionanoparticles from Aspergillus clavatus and its antimicrobial activity against MRSA and MRSE. Colloids Surf B Biointerfaces. 2010;77(2):214–218. doi:10.1016/J.COLSURFB.2010.01.026
75. Verma VC, Kharwar RN, Gange AC. Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine. 2009;5(1):33–40. doi:10.2217/NNM.09.77
76. Abu-Tahon MA, Ghareib M, Abdallah WE. Environmentally benign rapid biosynthesis of extracellular gold nanoparticles using Aspergillus flavus and their cytotoxic and catalytic activities. Process Biochem. 2020;95:1–11. doi:10.1016/J.PROCBIO.2020.04.015
77. Ninganagouda S, Rathod V, Singh D; RATHOD Professor V. Extracellular biosynthesis of silver nanoparticles using Aspergillus Flavus and their antimicrobial activity against gram negative MDR strains. Int J Pharm Bio Sci. 2013;4(2):222–229.
78. Gupta S, Bector S. Biosynthesis of extracellular and intracellular gold nanoparticles by Aspergillus fumigatus and A. flavus. Antonie van Leeuwenhoek. 2013;103(5):1113–1123. doi:10.1007/S10482-013-9892-6
79. Shahzad A, Saeed H, Iqtedar M, et al. Size-controlled production of silver nanoparticles by Aspergillus fumigatus BTCB10: likely antibacterial and cytotoxic effects. J Nanomater. 2019;2019:1–14. doi:10.1155/2019/5168698
80. Magdi HM, Mourad MHE, El-Aziz MMA. Biosynthesis of silver nanoparticles using fungi and biological evaluation of mycosynthesized silver nanoparticles. Egypt J Exp Biol. 2014;10(1):1–12.
81. Binupriya AR, Sathishkumar M, Vijayaraghavan K, Yun SI. Bioreduction of trivalent aurum to nano-crystalline gold particles by active and inactive cells and cell-free extract of Aspergillus oryzae var. viridis. J Hazard Mater. 2010;177(1–3):539–545. doi:10.1016/J.JHAZMAT.2009.12.066
82. Binupriya AR, Sathishkumar M, Yun S-I. Myco-crystallization of silver ions to nanosized particles by live and dead cell filtrates of Aspergillus oryzae var. viridis and its bactericidal activity toward Staphylococcus aureus KCCM 12256. Ind Eng Chem Res. 2009;49(2):852–858. doi:10.1021/IE9014183
83. Vala AK. Exploration on green synthesis of gold nanoparticles by a marine-derived fungus Aspergillus sydowii. Environ Prog Sustain Energy. 2015;34(1):194–197. doi:10.1002/EP.11949
84. Ammar HAM, El-Desouky TA. Green synthesis of nanosilver particles by Aspergillus terreus HA1N and Penicillium expansum HA2N and its antifungal activity against mycotoxigenic fungi. J Appl Microbiol. 2016;121(1):89–100. doi:10.1111/JAM.13140
85. Priyadarshini E, Pradhan N, Sukla LB, Panda PK. Controlled synthesis of gold nanoparticles using Aspergillus terreus IF0 and its antibacterial potential against gram negative pathogenic bacteria. J Nanotechnol. 2014;2014:1–9. doi:10.1155/2014/653198
86. Nirwaan R, Sharma D, Chaturvedi M, Yadav JP. Green synthesis, characterization and antibacterial activity of silver nanoparticles of endophytic fungi Aspergillus terreus. Artic J Nanomed Nanotechnol. 2017. doi:10.4172/2157-7439.1000457
87. Laksee S, Puthong S, Teerawatananond T, Palaga T, Muangsin N. Highly efficient and facile fabrication of monodispersed Au nanoparticles using pullulan and their application as anticancer drug carriers. Carbohydr Polym. 2017;173:178–191. doi:10.1016/J.CARBPOL.2017.05.101
88. Rahi DK, Manhas L, Kaur M, Malik D, Rahi S. Extracellular synthesis of silver nanoparticles by an indigenous yeast aureobasidium pullulans RYLF 10: characterization and evaluation of antibacterial potential. Int J Pharm Biol Sci. 2018;8(3):312–321.
89. Castro ME, Cottet L, Castillo A. Biosynthesis of gold nanoparticles by extracellular molecules produced by the phytopathogenic fungus Botrytis cinerea. Mater Lett. 2014;115:42–44. doi:10.1016/J.MATLET.2013.10.020
90. Soni N, Prakash S. Efficacy of fungus mediated silver and gold nanoparticles against Aedes aegypti larvae. Parasitol Res. 2011;110(1):175–184. doi:10.1007/S00436-011-2467-4
91. Manjunath Hulikere M, Joshi CG. Characterization, antioxidant and antimicrobial activity of silver nanoparticles synthesized using marine endophytic fungus- Cladosporium cladosporioides. Process Biochem. 2019;82:199–204. doi:10.1016/J.PROCBIO.2019.04.011
92. Manjunath Hulikere M, Joshi CG, Danagoudar A, Poyya J, Kudva AK, Dhananjaya D. Biogenic synthesis of gold nanoparticles by marine endophytic fungus-Cladosporium cladosporioides isolated from seaweed and evaluation of their antioxidant and antimicrobial properties. Process Biochem. 2017;63:137–144. doi:10.1016/J.PROCBIO.2017.09.008
93. Salunkhe RB, Patil SV, Patil CD, Salunke BK. Larvicidal potential of silver nanoparticles synthesized using fungus Cochliobolus lunatus against Aedes aegypti (Linnaeus, 1762) and Anopheles stephensi Liston (Diptera; Culicidae). Parasitol Res. 2011;109(3):823–831. doi:10.1007/S00436-011-2328-1
94. Kaplan Ö, Gökşen Tosun N, Özgür A, et al. Microwave-assisted green synthesis of silver nanoparticles using crude extracts of Boletus edulis and Coriolus versicolor: characterization, anticancer, antimicrobial and wound healing activities. J Drug Deliv Sci Technol. 2021;64:102641. doi:10.1016/J.JDDST.2021.102641
95. Dar MA, Ingle A, Rai M. Enhanced antimicrobial activity of silver nanoparticles synthesized by Cryphonectria sp. evaluated singly and in combination with antibiotics. Nanomed Nanotechnol, Biol Med. 2013;9(1):105–110. doi:10.1016/J.NANO.2012.04.007
96. Zhang L, Wei Y, Wang H, et al. Green synthesis of silver nanoparticles using mushroom flammulina velutipes extract and their antibacterial activity against aquatic pathogens. Food Bioprocess Technol. 2020;13(11):1908–1917. doi:10.1007/S11947-020-02533-7
97. Birla SS, Gaikwad SC, Gade AK, Rai MK. Rapid synthesis of silver nanoparticles from Fusarium oxysporum by optimizing physicocultural conditions. Sci World J. 2013;2013:1–12. doi:10.1155/2013/796018
98. Korbekandi H, Ashari Z, Iravani S, Abbasi S. Optimization of biological synthesis of silver nanoparticles using Fusarium oxysporum. Iran J Pharm Res IJPR. 2013;12(3):289.
99. Naimi-Shamel N, Pourali P, Dolatabadi S. Green synthesis of gold nanoparticles using Fusarium oxysporum and antibacterial activity of its tetracycline conjugant. J Mycol Med. 2019;29(1):7–13. doi:10.1016/J.MYCMED.2019.01.005
100. Sawle BD, Salimath B, Deshpande R, Bedre MD, Prabhakar BK, Venkataraman A. Biosynthesis and stabilization of Au and Au–Ag alloy nanoparticles by fungus, Fusarium semitectum. Sci Technol Adv Mater. 2008;9(3). doi:10.1088/1468-6996/9/3/035012
101. Clarance P, Luvankar B, Sales J, et al. Green synthesis and characterization of gold nanoparticles using endophytic fungi Fusarium solani and its in-vitro anticancer and biomedical applications. Saudi J Biol Sci. 2020;27(2):706–712. doi:10.1016/J.SJBS.2019.12.026
102. Sogra Fathima B, Balakrishnan RM. Biosynthesis and optimization of silver nanoparticles by endophytic fungus Fusarium solani. Mater Lett. 2014;132:428–431. doi:10.1016/J.MATLET.2014.06.143
103. Gopinath K, Arumugam A. Extracellular mycosynthesis of gold nanoparticles using Fusarium solani. Appl Nanosci. 2013;4(6):657–662. doi:10.1007/S13204-013-0247-4
104. Mishra AN, Bhadauria S, Gaur MS, Pasricha R. Extracellular microbial synthesis of gold nanoparticles using fungus Hormoconis resinae. JOM. 2010;62(11):45–48. doi:10.1007/S11837-010-0168-6
105. Varshney R, Mishra AN, Bhadauria S, Gaur MS, Novel Microbial A. Route to synthesize silver nanoparticles using Fungus Hormoconis Resinae. Dig J Nanomater Biostruct. 2009;4(2):349–355.
106. Aziz N, Pandey R, Barman I, Prasad R. Leveraging the attributes of mucor hiemalis-derived silver nanoparticles for a synergistic broad-spectrum antimicrobial platform. Front Microbiol. 2016;7:1984. doi:10.3389/FMICB.2016.01984
107. Castro-Longoria E, Vilchis-Nestor AR, Avalos-Borja M. Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora crassa. Colloids Surf B Biointerfaces. 2011;83(1):42–48. doi:10.1016/J.COLSURFB.2010.10.035
108. Quester K, Avalos-Borja M, Vilchis-Nestor AR, Camacho-López MA, Castro-Longoria E. SERS properties of different sized and shaped gold nanoparticles biosynthesized under different environmental conditions by Neurospora Crassa extract. PLoS One. 2013;8(10):e77486. doi:10.1371/JOURNAL.PONE.0077486
109. Hamedi S, Shojaosadati SA, Shokrollahzadeh S, Hashemi-Najafabadi S. Extracellular biosynthesis of silver nanoparticles using a novel and non-pathogenic fungus, Neurospora intermedia: controlled synthesis and antibacterial activity. World J Microbiol Biotechnol. 2013;30(2):693–704. doi:10.1007/S11274-013-1417-Y
110. Kathiresan K, Manivannan S, Nabeel MA, Dhivya B. Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids Surf B Biointerfaces. 2009;71(1):133–137. doi:10.1016/J.COLSURFB.2009.01.016
111. Mishra A, Tripathy SK, Wahab R, et al. Microbial synthesis of gold nanoparticles using the fungus Penicillium brevicompactum and their cytotoxic effects against mouse mayo blast cancer C2C12 cells. Appl Microbiol Biotechnol. 2011;92(3):617–630. doi:10.1007/S00253-011-3556-0
112. Hitesh R. Biosynthesis of silver nanoparticles using fungus Penicillium brevicompactum and evaluation of their anti-bacterial activity against some human pathogens. Res J Biotechnol. 2016;11(8):44.
113. Majeed S, Abdullah Bin MS, Nanda A, Ansari MT. In vitro study of the antibacterial and anticancer activities of silver nanoparticles synthesized from Penicillium brevicompactum (MTCC-1999). J Taibah Univ Sci. 2018;10(4):614–620. doi:10.1016/J.JTUSCI.2016.02.010
114. Magdi HM, Bhushan B. Extracellular biosynthesis and characterization of gold nanoparticles using the fungus Penicillium chrysogenum. Microsyst Technol. 2015;21(10):2279–2285. doi:10.1007/S00542-015-2666-5
115. Deniz F, Mazmanci MA. The biosynthesis of silver nanoparticles with fungal cytoplasmic fluid obtained from Phanerochaete chrysosporium ME446. Environ Res Technol. 2020;3(4):187–192. doi:10.35208/ERT.788891
116. Birla SSS, Tiwari VVV, Gade AKK, Ingle APP, Yadav APP, Rai MKK. Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett Appl Microbiol. 2009;48(2):173–179. doi:10.1111/J.1472-765X.2008.02510.X
117. Gade A, Rai M, Kulkarni S. Phoma sorghina, a phytopathogen mediated synthesis of unique silver rods. Int J Green Nanotechnol. 2011;3(3):153–159. doi:10.1080/19430892.2011.628573
118. Sarkar J, Kalyan Roy S, Laskar A, Chattopadhyay D, Acharya K. Bioreduction of chloroaurate ions to gold nanoparticles by culture filtrate of Pleurotus sapidus Quél. Mater Lett. 2013;92:313–316. doi:10.1016/J.MATLET.2012.10.130
119. Chaturvedi VK, Yadav N, Rai NK, et al. Pleurotus sajor-caju-mediated synthesis of silver and gold nanoparticles active against colon cancer cell lines: a new era of herbonanoceutics. Molecules. 2020;25(13):3091. doi:10.3390/molecules25133091
120. Vala AK. Intra- and extracellular biosynthesis of gold nanoparticles by a marine-derived Fungus Rhizopus oryzae. Synth React Inorganic, Met Nano-Metal Chem. 2014;44(9):1243–1246. doi:10.1080/15533174.2013.799492
121. AbdelRahim K, Mahmoud SY, Ali AM, Almaary KS, Mustafa AE, Husseiny SM. Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer. Saudi J Biol Sci. 2017;24(1):208–216. doi:10.1016/J.SJBS.2016.02.025
122. Cunha FA, Cunha M da CSO, da Frota SM, et al. Biogenic synthesis of multifunctional silver nanoparticles from Rhodotorula glutinis and Rhodotorula mucilaginosa: antifungal, catalytic and cytotoxicity activities. World J Microbiol Biotechnol. 2018;34(9):1–15. doi:10.1007/S11274-018-2514-8
123. Roy K, Sarkar CK, Ghosh CK. Photocatalytic activity of biogenic silver nanoparticles synthesized using yeast (Saccharomyces cerevisiae) extract. Appl Nanosci. 2014;5(8):953–959. doi:10.1007/S13204-014-0392-4
124. Olobayotan I, Akin-Osanaiye B. Biosynthesis of silver nanoparticles using baker’s yeast, Saccharomyces cerevisiae and its antibacterial activities. Access Microbiol. 2019;1(1A):526. doi:10.1099/ACMI.AC2019.PO0316
125. Yen San C, Mat don M. Biosynthesis of silver nanoparticles from Schizophyllum commune and in-vitro antibacterial and antifungal activity studies. J Phys Sci. 2013;24(2):83–96.
126. Tripathi RM, Shrivastav BR, Shrivastav A. Antibacterial and catalytic activity of biogenic gold nanoparticles synthesised by Trichoderma harzianum. IET nanobiotechnology. 2018;12(4):509–513. doi:10.1049/IET-NBT.2017.0105
127. Ahluwalia V, Kumar J, Sisodia R, Shakil NA, Walia S. Green synthesis of silver nanoparticles by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and Klebsiella pneumonia. Ind Crops Prod. 2014;55:202–206. doi:10.1016/J.INDCROP.2014.01.026
128. El-Wakil DA. Antifungal activity of silver nanoparticles by Trichoderma species: synthesis, characterization and biological evaluation. Egypt J Phytopathol. 2020;48(1):71–80. doi:10.21608/EJP.2020.49395.1015
129. Gemishev OT, Panayotova MI, Mintcheva NN, Djerahov LP, Tyuliev GT, Gicheva GD. A green approach for silver nanoparticles preparation by cell-free extract from Trichoderma reesei fungi and their characterization. Mater Res Express. 2019;6(9):095040. doi:10.1088/2053-1591/AB2E6A
130. Elgorban AM, Al-Rahmah AN, Sayed SR, Hirad A, Mostafa AA-F, Bahkali AH. Antimicrobial activity and green synthesis of silver nanoparticles using Trichoderma viride. Biotechnol Biotechnol Equip. 2016;30(2):299–304. doi:10.1080/13102818.2015.1133255
131. Mukherjee P, Roy M, Mandal BP, et al. Green synthesis of highly stabilized nanocrystalline silver particles by a non-pathogenic and agriculturally important fungus T. asperellum. Nanotechnology. 2008;19(7):075103. doi:10.1088/0957-4484/19/7/075103
132. Vahabi K, Dorcheh SK, Monajembashi S, et al. Stress promotes Arabidopsis - Piriformospora indica interaction. Plant Signaling & Behavior. 2016;11(5). doi:10.1080/15592324.2015.1136763
133. Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res. 2007;10(3):507–517. doi:10.1007/S11051-007-9275-X
134. Jha AK, Prasad K. Understanding mechanism of fungus mediated nanosynthesis: a molecular approach. Adv Appl Through Fungal Nanobiotechnol. 2016;1–23. doi:10.1007/978-3-319-42990-8_1
135. Arnoldi M, Fritz M, Bäuerlein E, Radmacher M, Sackmann E, Boulbitch A. Bacterial turgor pressure can be measured by atomic force microscopy. Phys Rev E. 2000;62(1):1034. doi:10.1103/PhysRevE.62.1034
136. Vahabi K, Mansoori GA, Karimi S. Biosynthesis of Silver nanoparticles by Fungus Trichoderma Reesei (A Route for Large-Scale Production of AgNPs). Insci J. 2011;1(1):65–79. doi:10.5640/insc.010165
137. Narayanan KB, Sakthivel N. Mycocrystallization of gold ions by the fungus Cylindrocladium floridanum. World J Microbiol Biotechnol. 2013;29(11):2207–2211. doi:10.1007/S11274-013-1379-0
138. Prasad R, editor. Advances and applications through fungal nanobiotechnology. In: Fungal Nanobiotechnology; 2016. doi:10.1007/978-3-319-42990-8
139. Prasad R. Fungal nanotechnology. In: Prasad R, editor. Fungal Biology; 2017. doi:10.1007/978-3-319-68424-6
140. Musarrat J, Dwivedi S, Singh BR, Al-Khedhairy AA, Azam A, Naqvi A. Production of antimicrobial silver nanoparticles in water extracts of the fungus Amylomyces rouxii strain KSU-09. Bioresour Technol. 2010;101(22):8772–8776. doi:10.1016/J.BIORTECH.2010.06.065
141. Rajakumar G, Rahuman AA, Roopan SM, et al. Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim Acta Part A Mol Biomol Spectrosc. 2012;91:23–29. doi:10.1016/J.SAA.2012.01.011
142. Alani F, Moo-Young M, Anderson W. Biosynthesis of silver nanoparticles by a new strain of Streptomyces sp. compared with Aspergillus fumigatus. World J Microbiol Biotechnol. 2012;28(3):1081–1086. doi:10.1007/S11274-011-0906-0/FIGURES/5
143. Jaidev LR, Narasimha G. Fungal mediated biosynthesis of silver nanoparticles, characterization and antimicrobial activity. Colloids Surf B Biointerfaces. 2010;81(2):430–433. doi:10.1016/J.COLSURFB.2010.07.033
144. Pareek V, Bhargava A, Panwar J. Biomimetic approach for multifarious synthesis of nanoparticles using metal tolerant fungi: a mechanistic perspective. Mater Sci Eng B. 2020;262:114771. doi:10.1016/J.MSEB.2020.114771
145. Kalpana VN, Kataru BAS, Sravani N, Vigneshwari T, Panneerselvam A, Devi Rajeswari V. Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus Niger: antimicrobial textiles and dye degradation studies. OpenNano. 2018;3:48–55. doi:10.1016/J.ONANO.2018.06.001
146. Chauhan A, Zubair S, Tufail S, et al. Fungus-mediated biological synthesis of gold nanoparticles: potential in detection of liver cancer. Int J Nanomedicine. 2011;6:2305–2319. doi:10.2147/IJN.S23195
147. Govindappa M, Lavanya M, Aishwarya P, et al. Synthesis and characterization of endophytic fungi, Cladosporium perangustum mediated silver nanoparticles and their antioxidant, anticancer and nano-toxicological study. Bionanoscience. 2020;10(4):928–941. doi:10.1007/S12668-020-00719-Z/FIGURES/14
148. Munawer U, Raghavendra VB, Ningaraju S, et al. Biofabrication of gold nanoparticles mediated by the endophytic Cladosporium species: photodegradation, in vitro anticancer activity and in vivo antitumor studies. Int J Pharm. 2020;588:119729. doi:10.1016/J.IJPHARM.2020.119729
149. Durán N, Marcato PD, Alves OL, De Souza GIH, Esposito E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Nanobiotechnol. 2005;3(1). doi:10.1186/1477-3155-3-8/FIGURES/9
150. Chowdhury S, Basu A, Kundu S. Green synthesis of protein capped silver nanoparticles from phytopathogenic fungus Macrophomina phaseolina (Tassi) Goid with antimicrobial properties against multidrug-resistant bacteria. Nanoscale Res Lett. 2014;9(1):365. doi:10.1186/1556-276X-9-365/FIGURES/8
151. Vigneshwaran N, Kathe AA, Varadarajan PV, Nachane RP, Balasubramanya RH. Silver-protein (core-shell) nanoparticle production using spent mushroom substrate. Langmuir. 2007;23(13):7113–7117. doi:10.1021/LA063627P
152. Mishra A, Tripathy SK, Wahab R, et al. Microbial synthesis of gold nanoparticles using the fungus Penicillium brevicompactum and their cytotoxic effects against mouse mayo blast cancer C 2C 12 cells. Appl Microbiol Biotechnol. 2011;92(3):617–630. doi:10.1007/S00253-011-3556-0/FIGURES/15
153. Subramaniyan SA, Sheet S, Vinothkannan M, et al. One-pot facile synthesis of pt nanoparticles using cultural filtrate of microgravity simulated grown P. chrysogenum and their activity on bacteria and cancer cells. J Nanosci Nanotechnol. 2017;18(5):3110–3125. doi:10.1166/JNN.2018.14661
154. Feroze N, Arshad B, Younas M, Afridi MI, Saqib S, Ayaz A. Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity. Microsc Res Tech. 2020;83(1):72–80. doi:10.1002/JEMT.23390
155. Singh D, Rathod V, Ninganagouda S, Hiremath J, Singh AK, Mathew J. Optimization and characterization of silver nanoparticle by endophytic fungi penicillium sp. isolated from curcuma longa (Turmeric) and application studies against MDR E. coli and S. aureus. Bioinorg Chem Appl. 2014;2014:408021. doi:10.1155/2014/408021
156. Elamawi RM, Al-Harbi RE, Hendi AA. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt J Biol Pest Control. 2018;28(1):28. doi:10.1186/S41938-018-0028-1/FIGURES/10
157. Fayaz AM, Balaji K, Girilal M, Kalaichelvan PT, Venkatesan R. Mycobased synthesis of silver nanoparticles and their incorporation into sodium alginate films for vegetable and fruit preservation. J Agric Food Chem. 2009;57(14):6246–6252. doi:10.1021/JF900337H
158. Mukherjee P, Ahmad A, Mandal D, et al. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett. 2001;1(10):515–519. doi:10.1021/NL0155274
159. Das S, Sudhagar P, Kang YS, Choi W. Graphene synthesis and application for solar cells. J Mater Res. 2014;29(3):299–319. doi:10.1557/JMR.2013.297
160. Sharma A, Verma N, Sharma A, Deva D, Sankararamakrishnan N. Iron doped phenolic resin based activated carbon micro and nanoparticles by milling: synthesis, characterization and application in arsenic removal. Chem Eng Sci. 2010;65(11):3591–3601. doi:10.1016/J.CES.2010.02.052
161. Sudhakar T, Nanda A, Babu SG, Janani S, Evans MD, Markose TK. Synthesis of silver nanoparticles from edible mushroom and its antimicrobial activity against human pathogens. Int J PharmTech Res. 2014;6(5):1718–1723.
162. Durán N, Marcato PD, De Souza GIH, Alves OL, Esposito E. Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J Biomed Nanotechnol. 2007;3(2):203–208. doi:10.1166/JBN.2007.022
163. Mohammed fayaz A, Balaji K, Kalaichelvan PT, Venkatesan R. Fungal based synthesis of silver nanoparticles—An effect of temperature on the size of particles. Colloids Surf B Biointerfaces. 2009;74(1):123–126. doi:10.1016/J.COLSURFB.2009.07.002
164. Ottoni CA, Simões MF, Fernandes S, et al. Screening of filamentous fungi for antimicrobial silver nanoparticles synthesis. AMB Express. 2017;7(1):1–10. doi:10.1186/S13568-017-0332-2
165. Govindappa M, Farheen H, Chandrappa CP, Channabasava R, Rai RV, Raghavendra VB. Mycosynthesis of silver nanoparticles using extract of endophytic fungi, Penicillium species of Glycosmis mauritiana, and its antioxidant, antimicrobial, anti-inflammatory and tyrokinase inhibitory activity. Adv Nat Sci Nanosci Nanotechnol. 2016;7(3):035014. doi:10.1088/2043-6262/7/3/035014
166. Kumar H, Bhardwaj K, Cruz-Martins N, et al. Applications of fruit polyphenols and their functionalized nanoparticles against foodborne bacteria: a mini review. Molecules. 2021;26(11):3447. doi:10.3390/MOLECULES26113447
167. de Francisco L, Pinto D, Rosseto H, et al. Evaluation of radical scavenging activity, intestinal cell viability and antifungal activity of Brazilian propolis by-product. Food Res Int. 2018;105:537–547. doi:10.1016/J.FOODRES.2017.11.046
168. Correa-Royero J, Tangarife V, Durán C, Stashenko E, Mesa-Arango A. In vitro antifungal activity and cytotoxic effect of essential oils and extracts of medicinal and aromatic plants against Candida krusei and Aspergillus fumigatus. Rev Bras Farmacogn. 2010;20(5):734–741. doi:10.1590/S0102-695X2010005000021
169. Ingle A, Gade A, Pierrat S, Sonnichsen C, Rai M. Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Curr Nanosci. 2008;4(2):141–144. doi:10.2174/157341308784340804
170. Li GJ, Hyde KD, Zhao RL, et al. Fungal diversity notes 253–366: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016;78(1):1–237. doi:10.1007/S13225-016-0366-9
171. Brown SD, Nativo P, Smith J-A, et al. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J Am Chem Soc. 2010;132(13):4678–4684. doi:10.1021/JA908117A
172. Rodrigues AG, Ping LY, Marcato PD, et al. Biogenic antimicrobial silver nanoparticles produced by fungi. Appl Microbiol Biotechnol. 2012;97(2):775–782. doi:10.1007/S00253-012-4209-7
173. Ishida K, Cipriano TF, Rocha GM, et al. Silver nanoparticle production by the fungus Fusarium oxysporum: nanoparticle characterisation and analysis of antifungal activity against pathogenic yeasts. Mem Inst Oswaldo Cruz. 2013;109(2):220–228. doi:10.1590/0074-0276130269
174. Arun G, Eyini M, Gunasekaran P. Green synthesis of silver nanoparticles using the mushroom fungus Schizophyllum commune and its biomedical applications. Biotechnol Bioprocess Eng. 2014;19(6):1083–1090. doi:10.1007/s12257-014-0071-z
175. Xue B, He D, Gao S, Wang D, Yokoyama K, Wang L. Biosynthesis of silver nanoparticles by the fungus Arthroderma fulvum and its antifungal activity against genera of Candida, Aspergillus and Fusarium. Int J Nanomedicine. 2016;11:1899. doi:10.2147/IJN.S98339
176. Narasimha G. Antiviral activity of silver nanoparticles synthesized by fungal strain Aspergillus Niger. Nano Sci Nano Technol. 2012;6(1):18–20.
177. Elechiguerra JL, Larios-Lopez L, Liu C, Garcia-Gutierrez D, Camacho-Bragado A, Yacaman MJ. Corrosion at the nanoscale: the case of silver nanowires and nanoparticles. Chem Mater. 2005;17(24):6042–6052. doi:10.1021/CM051532N
178. Sharma P, Mehta M, Dhanjal DS, et al. Emerging trends in the novel drug delivery approaches for the treatment of lung cancer. Chem Biol Interact. 2019;309:108720. doi:10.1016/j.cbi.2019.06.033
179. Dhanjal DS, Mehta M, Chopra C, et al. Novel controlled release pulmonary drug delivery systems: current updates and challenges. In: Modeling and Control of Drug Delivery Systems. Academic Press; 2021:253–272. doi:10.1016/B978-0-12-821185-4.00001-4
180. Ortega FG, Fernández-Baldo MA, Fernández JG, et al. Study of antitumor activity in breast cell lines using silver nanoparticles produced by yeast. Int J Nanomedicine. 2015;10:2021. doi:10.2147/IJN.S75835
181. Xia ZK, Ma QH, Li SY, et al. The antifungal effect of silver nanoparticles on Trichosporon asahii. J Microbiol Immunol Infect. 2016;49(2):182–188. doi:10.1016/J.JMII.2014.04.013
182. Singh P, Kim YJ, Zhang D, Yang DC. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016;34(7):588–599. doi:10.1016/J.TIBTECH.2016.02.006
183. Basu A, Ray S, Chowdhury S, et al. Evaluating the antimicrobial, apoptotic, and cancer cell gene delivery properties of protein-capped gold nanoparticles synthesized from the edible mycorrhizal fungus Tricholoma crassum. Nanoscale Res Lett. 2018;13(1):154. doi:10.1186/S11671-018-2561-Y/FIGURES/10
184. Vahidi H, Kobarfard F, Alizadeh A, Saravanan M, Barabadi H. Green nanotechnology-based tellurium nanoparticles: exploration of their antioxidant, antibacterial, antifungal and cytotoxic potentials against cancerous and normal cells compared to potassium tellurite. Inorg Chem Commun. 2021;124:108385. doi:10.1016/J.INOCHE.2020.108385
185. Bhat R, Sharanabasava VG, Deshpande R, Shetti U, Sanjeev G, Venkataraman A. Photo-bio-synthesis of irregular shaped functionalized gold nanoparticles using edible mushroom Pleurotus Florida and its anticancer evaluation. J Photochem Photobiol B Biol. 2013;125:63–69. doi:10.1016/J.JPHOTOBIOL.2013.05.002
186. Prasad C, Krishna Murthy P, Hari Krishna RH, Sreenivasa Rao R, Suneetha V, Venkateswarlu P. Bio-inspired green synthesis of RGO/Fe3O4 magnetic nanoparticles using Murrayakoenigii leaves extract and its application for removal of Pb(II) from aqueous solution. J Environ Chem Eng. 2017;5(5):4374–4380. doi:10.1016/J.JECE.2017.07.026
187. Surendiran A, Sandhiya S, Pradhan SC, Adithan C. Novel applications of nanotechnology in medicine: eBSCOhost. Indian J Med Res. 2009;130(6):689–701.
188. Janith W, Chamindri W. Applications of nanotechnology in drug delivery and design-an insight-Indian journals. Curr Trends Biotechnol Pharm. 2016;10(1):78–91.
189. Daisy P, Saipriya K. Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabetes mellitus. Int J Nanomedicine. 2012;7:1189. doi:10.2147/IJN.S26650
190. Mohammed fayaz A, Girilal M, Mahdy SA, Somsundar SS, Venkatesan R, Kalaichelvan PT. Vancomycin bound biogenic gold nanoparticles: a different perspective for development of anti VRSA agents. Process Biochem. 2011;46(3):636–641. doi:10.1016/J.PROCBIO.2010.11.001
191. You C, Han C, Wang X, et al. The progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicity. Mol Biol Rep. 2012;39(9):9193–9201. doi:10.1007/S11033-012-1792-8
192. Chaloupka K, Malam Y, Seifalian AM. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28(11):580–588. doi:10.1016/J.TIBTECH.2010.07.006
193. Wang P, Li Y, Huang X, Wang L. Fabrication of layer-by-layer modified multilayer films containing choline and gold nanoparticles and its sensing application for electrochemical determination of dopamine and uric acid. Talanta. 2007;73(3):431–437. doi:10.1016/J.TALANTA.2007.04.022
194. Yun Y, Dong Z, Lee N, et al. Revolutionizing biodegradable metals. Mater Today. 2009;12(10):22–32. doi:10.1016/S1369-7021(09
195. Huang Y, Li X, Liao Z, et al. A randomized comparative trial between Acticoat and SD-Ag in the treatment of residual burn wounds, including safety analysis. Burns. 2007;33(2):161–166. doi:10.1016/J.BURNS.2006.06.020
196. Sundaramoorthi C, Kalaivani M, Mathews DM, Palanisamy S, Kalaiselvan V, Rajasekaran A. Biosynthesis of silver nanoparticles from Aspergillus Niger and evaluation of its wound healing activity in experimental rat model. Int J PharmTech Res. 2009;1(4):1523–1529.
197. Marcato PD, Paula De LB, Melo PS, et al. In vivo evaluation of complex biogenic silver nanoparticle and enoxaparin in wound healing. J Nanomater. 2015;2015:439820. doi:10.1155/2015/439820
198. Singh S, Dhanjal DS, Thotapalli S, Sharma P, Singh J. Importance and recent aspects of fungi-based food ingredients. In: New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier; 2020:245–254.
199. Singh S, Dhanjal DS, Thotapalli S, Sharma P, Singh J. Fungal enzyme inhibitors: repository of novel cancer therapeutics. In: New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier; 2020:121–133.
200. Lohani A, Verma A, Joshi H, Yadav N, Karki N. Nanotechnology-based cosmeceuticals. ISRN Dermatol. 2014;2(1):1–15. doi:10.1155/2014/843687
201. Maczey N, Dhendup K, Cannon P. Thitarodes namnai sp. nov. and T. caligophilus sp. nov. (Lepidoptera: hepialidae), hosts of the economically important entomopathogenic fungus Ophiocordyceps sinensis in Bhutan. Zootaxa. 2010;24(12):42–52. doi:10.11646/zootaxa.2412.1.3
202. Kokura S, Handa O, Takagi T, Ishikawa T, Naito Y, Yoshikawa T. Silver nanoparticles as a safe preservative for use in cosmetics. Nanomed Nanotechnol, Biol Med. 2010;6(4):570–574. doi:10.1016/J.NANO.2009.12.002
203. Handa O, Kokura S, Adachi S, et al. Methylparaben potentiates UV-induced damage of skin keratinocytes. Toxicology. 2006;227(1–2):62–72. doi:10.1016/J.TOX.2006.07.018
204. Ishiwatari S, Suzuki T, Hitomi T, Yoshino T, Matsukuma S, Tsuji T. Effects of methyl paraben on skin keratinocytes. J Appl Toxicol. 2007;27(1):1–9. doi:10.1002/JAT.1176
205. Gajbhiye S, Sakharwade S, Gajbhiye S, Sakharwade S. Silver Nanoparticles in Cosmetics. J Cosmet Dermatol Sci Appl. 2016;6(1):48–53. doi:10.4236/JCDSA.2016.61007
206. Gajbhiye M, Kesharwani J, Ingle A, Gade A, Rai M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed Nanotechnol, Biol Med. 2009;5(4):382–386. doi:10.1016/J.NANO.2009.06.005
207. Baskar K, Raj GA, Mohan PM, Lingathura S, Ambrose T, Muthu C. Larvicidal and growth inhibitory activities of entomopathogenic fungus, Beauveria bassiana against Asian army worm, Spodoptera litura Fab. (Lepidoptera: Noctuidae). J Entomol. 2012;9(3):155–162. doi:10.3923/je.2012.155.162
208. Park J-H, Choi G-J, Lee S-W, Kim K-M, Jung H-S. Griseofulvin from Xylaria sp. Strain F0010, an endophytic fungus of Abies holophylla and its antifungal activity against plant pathogenic fungi. J Microbiol Biotechnol. 2005;15(1):112–117.
209. Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346. doi:10.1088/0957-4484/16/10/059
210. Kim J, Pitts B, Stewart PS, Camper A, Yoon J. Comparison of the antimicrobial effects of chlorine, silver ion, and tobramycin on biofilm. Antimicrob Agents Chemother. 2008;52(4):1446–1453. doi:10.1128/AAC.00054-07
211. Rajakumar G, Rahuman AA, Roopan SM, et al. Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim Acta Part A Mol Biomol Spectrosc. 2012;91:23–29. doi:10.1016/J.SAA.2012.01.011
212. Ruma K, Sunil K, Prakash HS. Antioxidant, anti-inflammatory, antimicrobial and cytotoxic properties of fungal endophytes from Garcinia species. Int J Pharm Pharm Sci. 2013;5(3):889–897.
213. Bonderman D, Pretsch I, Steringer-Mascherbauer R, et al. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with Diastolic Heart Failure (DILATE-1): a randomized, double-blind, placebo-controlled, single-dose study. Chest. 2014;146(5):1274–1285. doi:10.1378/CHEST.14-0106
214. Naz S, Vallejo M, García A, Barbas C. Method validation strategies involved in non-targeted metabolomics. J Chromatogr A. 2014;1353:99–105. doi:10.1016/J.CHROMA.2014.04.071
215. Bhimba BV, Franco DAAD, Mathew JM, Jose GM, Joel EL, Thangaraj M. Anticancer and antimicrobial activity of mangrove derived fungi Hypocrea lixii VB1. Chin J Nat Med. 2012;10(1):77–80. doi:10.1016/S1875-5364(12
216. Lin J, Zhang H, Chen Z, Zheng Y. Penetration of lipid membranes by gold nanoparticles: insights into cellular uptake, cytotoxicity, and their relationship. ACS Nano. 2010;4(9):5421–5429. doi:10.1021/NN1010792
217. Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, Venketesan R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomed Nanotechnol, Biol Med. 2010;6(1):103–109. doi:10.1016/J.NANO.2009.04.006
218. Zheng D, Hu C, Gan T, Dang X, Hu S. Preparation and application of a novel vanillin sensor based on biosynthesis of Au–Ag alloy nanoparticles. Sens Actuators B Chem. 2010;148(1):247–252. doi:10.1016/J.SNB.2010.04.031
219. Thibault S, Aubriet H, Arnoult C, Ruch D. Gold nanoparticles and a glucose oxidase based biosensor: an attempt to follow-up aging by XPS. Microchim Acta. 2008;163(3):211–217. doi:10.1007/S00604-008-0028-Z
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
