Back to Journals » Journal of Inflammation Research » Volume 14
Multiple Cytokines Elevated in Patients with Keloids: Is It an Indication of Auto-Inflammatory Disease?
Authors Nangole FW, Ouyang K, Anzala O, Ogengo J, Agak GW
Received 21 March 2021
Accepted for publication 13 May 2021
Published 10 June 2021 Volume 2021:14 Pages 2465—2470
DOI https://doi.org/10.2147/JIR.S312091
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Ferdinand W Nangole,1 Kelsey Ouyang,2 Omu Anzala,3 Julius Ogengo,4 George W Agak2
1Department of Surgery, University of Nairobi, Nairobi, Kenya; 2Division of Dermatology, David Geffen School of Medicine at UCLA, Los Angeles, California, USA; 3Institute of Aids Vaccine Initiative, University of Nairobi, Nairobi, Kenya; 4Department of Human Anatomy, University of Nairobi, Nairobi, Kenya
Correspondence: Ferdinand W Nangole P.O. Box 2212-00202, Nairobi, Kenya
Email [email protected]
Background: Inflammation seems to play a major role in the pathophysiology of keloids. However, the role of cytokines in keloid pathophysiology has not been fully evaluated with only a few cytokines studied. We undertook this study to compare various cytokines in patients with keloids and a control group of patients without keloids nor family history of keloids so as to determine which cytokines are elevated and could thus be critical in keloid formation.
Methods: This was a cross-sectional study of patients with keloids and a control group of those without. Patients in both groups were matched for age, sex and body mass index. Their plasma was analyzed for both inflammatory and anti-inflammatory cytokines using the Bio-flex ElisaTM method. Comparisons of cytokines means in both groups were done using Student’s t-test.
Results: A total of 84 participants with 42 participants in each group were followed during the study. Male to female ratio was 1:2. Age ranges were similar with a mean of 29.6 years. A total of 28 cytokines were assayed. Statistically significant differences were noted in 15 of the 28 cytokines assayed with 11 being elevated more in keloid patients with only four in the non-keloid forming group. Among elevated cytokines in keloid patients were granulocyte colony-stimulating factors, granulocyte-monocyte-colony-stimulating factors, interleukins 4, 6 and 13.
Conclusion: Patients with keloids have significantly higher cytokines compared with non-keloid forming patients. This finding suggests that keloid formation could be influenced by multiple inflammatory cytokines, an indication that the patient’s immune system could play a role in keloid formation akin to auto-inflammatory disease.
Keywords: keloids, cytokines, auto-inflammatory, disease
Introduction
There is still insufficient evidence as to the probable cause of keloids.1,2 Among theories fronted is the Inflammatory Theory with the suggestion that keloids are a result of an exaggerated inflammatory response.1–3 Inflammation as a possible cause of keloids emanates from the fact that there are abundant inflammatory cells in keloid specimens.4,5 Further, keloid surveillance studies have demonstrated that keloids are more common in allergic and/or auto-immune diseases such as asthma or atopic dermatitis compared with the normal population.6,7
Cytokines and growth factors implicated in keloid formations include transforming growth factor beta (TGF-β), matrix metalloproteinases (MMPS), interleukin 6 and adiponectin.8–15 TGF-β is released from the platelets’ granules during the process of inflammation. TGF-β1 and TGF-β2 are thought to promote scar formation and fibrosis while TGF-β3 is thought to reduce scar tissue.8,9 TGF-β is thought to work by influencing MMPs activity.8–10 Interleukin 6 on the other hand has not only been shown to be greatly elevated in keloid tissues but also in serum of patients with keloids.11–13 Furthermore, keloid-like tissue was produced in immune-compromised rats by using keloid-derived stem cells and IL-6 strongly suggesting that it has a critical role in keloid formation13 A recent study by Luo et al. in 2021 on the other hand demonstrated that adiponectin could have a protective role in scarring and keloid formation.15
In spite of the above findings, the role of many cytokines in keloid formation has not been well documented. We therefore undertook this study to determine whether there was any difference in selected plasma cytokine levels in patients with keloids and those without.
Materials and Methods
The study was carried out at the Kenyatta National Hospital, a teaching hospital for the University of Nairobi between August 2018 and July 2020. This study was approved by the local ethics and research committee. The objective of the study was to determine selected plasma cytokine levels in patients with keloids and a control group with no keloids. Patients with keloids attending a plastic surgery clinic at the Kenyatta National hospital were systematically and randomly sampled into the study. A control group was of patients seeking aesthetic services with no keloids nor family history of keloids adjusted for age, sex and BMI with the keloid group. Consent was sought from all study participants. All participants were informed about the purpose of the study, and it was conducted in accordance with the Declaration of Helsinki.
Excluded from the study were patients with known chronic illnesses such as diabetes mellitus, hypertension, asthma, connective tissue disorders and HIV/AIDS. All study participants had a thorough medical history taken and physical examination done to rule out any underlying medical conditions. Patient’s weight and height were then taken and used to determine BMI.
Ten millilitres of blood was taken in sodium heparin tubes from each participant. Density centrifugation was done and plasma was separated and stored at −80°C ready for cytokine assays. Cytokine assays were done using Bio-plex multiplex ElisaTM assay technique manufactured by Bio–Rad Company based at Berkeley, CA, USA. The technique involved use of fluorescent dyed microphores (beads) each with a distinct colour code to permit identification of the individual cytokines. Within a multiplex suspension coupled beads reacted with the sample containing the cytokines of interest. After a series of washes to remove unbound protein, a biotinylated detection antibody was added to create a sandwich complex. The final detection complex was formed with the addition of streptavidin-phycoerythrin (SA-PE) conjugate. Phycoerythrin served as a fluorescent indicator analysed by Bio-Plex Data Pro* with data presented as median fluorescence intensity (MFI) as well as concentration (pg/mL). The concentration of analyte bound to each bead was proportional to the MFI of reporter signal. Cytokines assayed included interleukins, chemokines and growth factors with a total of 28 (Table 1). Data captured were summarized and analyzed using Student’s t-test to compare means and ANOVA test for comparison of variance. Probability values significance were at 0.05.
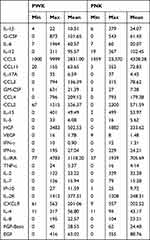 |
Table 1 Cytokines Mean and Range in Keloids and the Control Group |
Results
A total of 84 patients were recruited for the study with 42 patients in each group. The male to female ratio was 1:2 for both groups of patients. The mean age for the keloids group (PWK) was 28.5 years with an age range of 18−40.5 years while for the control (PNK) was 28.7 years with an age range of 18.2−40.7 years (p-value = 0.83).
A total of 52 keloids were seen in the PWK group while no keloids were encountered in the PNK group during the one year of follow up. Of the 52 keloids, trauma accounted for 63.4%, followed by infections (19.2%) and spontaneous keloids (17.4%) (Table 2). Auricular keloids accounted for 46% of the keloids followed by the cheek (15.2%) (Table 2). The mean surface areas of the keloids excised was 8.95 cm2 ± SD 2.254.
 |
Table 2 Keloids Etiology and Anatomical Location |
Out of 28 cytokines analyzed, 15 cytokines had a significance difference between the two groups (P < 0.05). Eleven of the 15 were markedly elevated in the PWK group with their means higher than those in the PNK group (Tables 1, 3 and 4). These were G-CSF, IL-10, IL-13, IL-6, CCL3, GM-CSF, CCL4, HGF, IL-IRA, IL −2R and IL-4. Cytokines significantly raised in PNK were CCL5, EGF, IL-1β and CCL2 (Table 4).
 |
Table 3 Cytokines Significantly Elevated in Patients with Keloids (PWK) in Comparison to Control (PNK) Group |
 |
Table 4 Cytokines Significantly Elevated in Non-Keloids (PNK) Patients |
Discussion
Keloid disease characterized by disfigurement, pain, pruritus and high recurrence rate has no well understood pathophysiology.1,2 Despite numerous studies and theories on keloid formation, inflammation seems to play a critical role with some researchers suggesting that inflammation could be the main cause.3,4 Whether keloids should be considered as an auto- inflammatory disease is not yet known. Auto-inflammatory diseases result from abnormal innate immune system, characterized by elevated inflammatory cytokines.16,17 Pharmacological interactions with cytokines either at the production or receptor levels have resulted in promising outcomes in some of these diseases.18 The majority of studies done to demonstrate cytokines levels in patients with keloids have largely been in vitro or limited to small sample sizes which makes it difficult to conclude whether keloids could fit into this group of disorders.11,12 Our study demonstrates marked elevation of various key inflammatory cytokines in patients with keloids compared with those without, implying that inflammation plays a greater role in keloid formation than previously thought. Manipulation of these cytokines by blocking them may thus provide an alternative treatment to patients with keloids.
Key cytokines elevated in the PWK group that play a critical role in inflammation include IL 4, IL6, IL10, IL 13, G-CSF and GM-CSF. Others were basic FGF and CCL3. Probably the most studied among these as far as keloid pathogenesis is concerned is IL-6.11,12,16 IL-6, considered as one of the most potent inflammatory cytokines, has also been noted to play a critical role in the pathogenesis of various disease processes including rheumatoid arthritis.10,12–1419,20 Qunzhou et al. also found IL-6 to be greatly increased in keloids compared with normal skin.12 They further demonstrated the ability to reproduce keloid-like tissue in immune-compromised rats by using keloid-derived stem cells and IL-6. This activity was halted by antibodies against IL-6 suggesting a possible role of IL-6 antibodies in the treatment of keloids. McCauley et al. in another study showed monocytes of patients who form keloids produced large amounts of IL-6 compared with normal patients.11 Hui Xue et al. demonstrated an elevation in the expression of IL-6 genes in keloid fibroblasts compared with normal fibroblasts.14 The significance of the above findings in the management of patients can be postulated from the fact that antibodies against the IL-6 cytokine receptor, tocilizumab (inhibits IL-6 binding to IL-6R) have successfully been used in the treatment of Castleman disease, an auto-inflammatory condition with high IL-6 levels.21
Other interleukins significantly elevated in PWK include IL-4 and IL-13. They do have synergistic effects and have been noted to play an important role in allergic conditions such as atopic dermatitis and asthma.22 Interestingly, population-based studies have demonstrated keloid prevalence to be higher in patients with this condition, suggesting a similar pathophysiology.6,7 IL-4 has been shown to stimulate B-lymphocytes to secrete immunoglobulin E (IgE) as well as up-regulation of IgE receptors on mast cells and basophils.23 IL-4 also promotes cellular inflammation through vascular cellular adhesion molecules (VCAM), promoting migration of T-lymphocytes, basophils and eosinophils from the intravascular compartments. In addition, they promote differentiation of naïve T-helper cells to T-helper 2 cells that secrete IL-4, IL-5, IL-9 and IL-13, all critical in the inflammatory response in wounds.22,23 Further, T-helper cells treated with IL-4 have fewer apoptotic activities, a fact that probably explains a higher TH2 ratio in keloids compared with normal skin and hypertrophic scars.22 Diaz et al. noted keloid lesions to have an increased signaling of IL-4 and IL-13 compared with normal skin.24 They further demonstrated resolution of keloid symptoms on patients who were given the IL-4 receptor antagonist dupilumab, prompting need for further research in this aspect of treatment.
Our study demonstrated cytokines that influence macrophage and neutrophil activities such as CCL3, CCL4, G-CSF and GM-CSF to be significantly elevated in the PWK compared with the PNK group. All these factors have been shown to be useful in the body’s innate inflammatory response. They work by activation of T lymphocytes, macrophages and dendritic cells to release various pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α. They also stimulate hematopoietic stem cells to proliferate and differentiate into macrophages and neutrophils that are responsible for both the non-specific and some aspects of the specific immune system. Their role in keloid pathogenesis has not been previously documented. This study therefore opens avenues for more studies to determine their significance in keloid formation and treatment.
There was no statistically significant difference between PWK and PNK in reference to TNF-α, IL −8, IL-12 and IFN-γ. Though TNF-α was higher in the PWK than the PNK group the difference was marginal. Similar findings were demonstrated by da Silva et al on in situ cytokines expression in keloids and normal tissue.25 However, McCauley et al. on the assay of blood monocytes in patients with or without keloids demonstrated elevated TNF-α in patients with keloids.11 Further, anti-TNF antibodies topically injected in keloids have shown reduction in keloid size and pruritus, suggesting a possible role in the management of the same.
Conclusion
There are multiple inflammatory cytokines that are elevated in patients’ plasma with keloids. There is a need to establish whether similar findings can be established in keloid tissue compared with normal skin tissues. This abnormal elevation of the inflammatory cytokines could be responsible for keloid formation akin to auto-inflammatory disorders. There is however need for more research to identify key cytokines in keloid formation, as these could be used as biomarkers as well as be manipulated to prevent and/or treat keloids in patients.
Data Sharing Statement
Data for this study are available and can be accessed through the corresponding author Dr. Ferdinand W. Nang’ole, Email: nang’[email protected].
Ethical Approval
The study was approved by the Kenyatta National Hospital/University of Nairobi ethics and research committee.
Consent to Publish
We give our full consent for the publication of this research in your Journal when accepted.
Author Contributions
All authors made a significant contribution to the work reported, whether in conception, study design, execution, acquisition of data, analysis and interpretation, drafting, revising or critically reviewing the article They also gave final approval of the version to be published and have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
George W AGAK is supported by NIHK01AR071479 and Burrows Wellcome CTRG1019954.
Disclosure
The authors declare that they had no conflict of interest in the study.
References
1. Jumper N, Raus R, Bayat A. Functional histopathology of Keloid disease. Histo-Pathol. 2015;1033–1057.
2. Chike-Obi CJ, Cole PD, Brissett AE. Keloids: pathogenesis, clinical features, and management. Semin Plast Surg. 2009;23(3):178–184. doi:10.1055/s-0029-1224797
3. Ogawa R. Keloid and Hypertrophic Scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci. 2017;18(3):606. doi:10.3390/ijms18030606
4. Nangole FW, Agak W. Keloid pathophysiology: fibroblast or inflammatory disorders? JPRAS Open. 2019;22:44–54. doi:10.1016/j.jpra.2019.09.004
5. Ali MM, Karanja FW, Nang’ole FW, Opot EON, Mulehane KO, Mbithi DZ. Determination of the prevalence, clinical characteristics and histo-pathological features of keloids in patients managed at the Kenyatta National Hospital. East Afri Med J. 2019;96(1):2220–2229.
6. Hajdarbegovic E, Bloem A, Balak D, Thio B, Nijsten T. The association between atopic disorders and keloids: a Case-control Study. Indian J Dermatol. 2015;60(6):635–639. doi:10.4103/0019-5154.169144
7. Ying-Yi L, Chun-Ching L, Yu W-W. Keloid risk in patients with atopic dermatitis: a nationwide retrospective cohort study in Taiwan. BMJ Open. 2018;89–97.
8. Jagajeevan J, Bayat A. Transforming growth factor B and keloid disease. Int J Surg. 2007;5:278–285. doi:10.1016/j.ijsu.2006.04.007
9. Liu Y, Yue L. Transforming Growth factor Beta 1, promote scar fibroblasts proliferation and trans differentiation via upregulating micro rna −21. Sci Rep. 2016. doi:10.1038/srep32231
10. Oliver B, Haiyan YU. Studies of TGFB1 –B3 and their receptors 1 and 2 in fibroblasts of keloids and hypertrophic scars. Arch Dermat Venereal. 2005;85:216–220.
11. Robert LM, Chopra V. Altered cytokines production in Black patients with keloids. J Clin Immunol. 2012;12:300–308.
12. Zhang Q, Yamaza T, Kelly AP. Tumor-like stem cells derived from human keloid are governed by the inflammatory niche driven by IL-17/IL-6 Axis. PLOS. 2009;4(11):e7798. doi:10.1371/journal.pone.0007798
13. Ghazizadeh M, Tosa M. Functional implications of IL-6 signalling pathways in keloid pathogenesis. J Invest Dermatol. 2007;127:98–105. doi:10.1038/sj.jid.5700564
14. Huixue. H, McCauley RL, Zhang W. Elevated interleukin −6 expression in keloid fibroblasts. J Surg Res. 2000;89(1):74–77.
15. Luo L, Li J, Wu Y, Qiao J, Fang H. Adiponectin, but Not TGF-β1, CTGF, IL-6 or TNF-α, May be a potential anti-inflammation and anti-fibrosis factor in keloid. J Inflamm Res. 2021;14:907–916. PMID: 33758530. PMCID:PMC7981148. doi:10.2147/JIR.S301971
16. Nézondet A-L, Poubelle E, Pelletier PM. The evaluation of cytokines to help establish diagnosis and guide treatment of auto-inflammatory and autoimmune diseases. J Leukoc Biol. 2020;108:647–657. doi:10.1002/JLB.5MR0120-218RRR
17. de Jesus AA, Canna SW, Liu Y, Goldbach-Mansky R. Molecular mechanisms in genetically defined auto-inflammatory diseases: disorders of amplified danger signaling. Annu Rev Immunol. 2015;33:823–874. doi:10.1146/annurev-immunol-032414-112227
18. Ciccarelli F, De Martinis M, Ginaldi*An L. Update on auto-inflammatory diseases. Curr Med Chem. 2013;21(3):261–269. doi:10.2174/09298673113206660303
19. Hirano T. Interleukin 6 in autoimmune and inflammatory diseases: a personal memoir. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(7):717–730. doi:10.2183/pjab.86.717
20. Xu H, Xiao X, He Y. Increased serum interleukin-6 levels in patients with hidradenitis suppurativa. Postepy Dermatol Alergol. 2017;34(1):82–84. doi:10.5114/ada.2017.65626
21. Galeotti C, Boucheron A, Guillaume S, Koné-Paut I. Sustained remission of multicentric castleman disease in children treated with tocilizumab. Pediatr Rheumatol Online J. 2011;9(Suppl 1):P6–9. doi:10.1186/1546-0096-9-S1-P6
22. Steinke JW, BorishTh L. cytokines and asthma — interleukin-4: its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir Res. 2001;2(2):66–70.
23. Brucato A, Emmi G, Cantarini L, et al. Management of idiopathic recurrent pericarditis in adults and in children: a role for IL-1 receptor antagonism. Intern Emerg Med. 2018;13:475–489. doi:10.1007/s11739-018-1842-x
24. Diaz A, Tan K, He H, Xu H, Cueto I. Keloid lesions show increased IL-4/IL-13 signaling and respond to Th2-targeting dupilumab therapy. J Eur Acad Dermatol Venereol. 2020;34(4):e161–e164. doi:10.1111/jdv.16097
25. da Silva IR, Tiveron LCRDC, da Silva MV, et al. In situ cytokine expression and morphometric evaluation of total collagen and collagens. Mediators Inflamm. 2017. doi:10.1155/2017/6573802
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
