Back to Journals » OncoTargets and Therapy » Volume 14
Multimodal Treatment Combining Salvage Surgery-Assisted Chemotherapy and Checkpoints Blockade Immunotherapy Achieves Complete Remission on a Recurrent Penile Cancer Patient: A Case Report
Authors Hu L , Shan X, Han D, Guo Z, Wang H, Xiao Z
Received 15 July 2021
Accepted for publication 3 September 2021
Published 22 September 2021 Volume 2021:14 Pages 4891—4896
DOI https://doi.org/10.2147/OTT.S319932
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Linjun Hu,1,* Xingli Shan,1,* Dongdong Han,1 Zhaoxia Guo,2 Huina Wang,2 Zejun Xiao3
1Department of Urology, Cancer Hospital of HuanXing Chaoyang District Beijing, Beijing, People’s Republic of China; 2Acornmed Biotechnology Co., Ltd, Beijing, People’s Republic of China; 3Department of Urology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zejun Xiao
Department of Urology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Email [email protected]
Abstract: Penile squamous cell carcinoma (pSCC) is a rare disease in developed countries. pSCC causes a severe health problem and social burden in developing countries. We reported a 49-year-old male recurrent pSCC patient with medium PD-L1 expression and low TMB. The patient obtained complete response after multimodal therapy (MMT). The clinical manifestation is a recurrence in the right groin with nearly ruptured pSCC. He had partial resection of penile cancer plus bilateral groin lymph node dissection and pelvic lymph node dissection during the first operation. Pathology of the recurrent tumor showed fibrous tissue with cancer infiltration and necrosis. We used MMT, including resection of palliative right inguinal metastases, four cycles of paclitaxel+bleomycin+cisplatin, and continuous sintilimab to treat the patient. The patient had a complete response (CR) after four cycles of therapy and sustained CR for 18 months with continuous sintilimab, showing a good tolerance and acceptable toxicity. This is the first case presenting a complete response in a relapsed pSCC patient. These results suggest that MMT is worth exploring.
Keywords: pSCC, immunotherapy, chemotherapy, multimodal treatment, case report
Introduction
Penile cancer is rare and has a high mortality1 and the recurrence rate is high after local excision. Penile cancer was predicted to account for 0.27% of new cancer cases in the US.2 Globally, penile cancer causes 34,000 new cancer cases a year, at the rate of 3.09% in 2018.3 About 95% of penile cancer is penile squamous cell carcinoma (pSCC).4 Prepuce elongation with a prevalence of 32% and HPV infection with a prevalence of 52% were the two major etiologies of penile squamous cell carcinoma in 2017, in China.5 It is generally accepted that the decisive treatment for early-stage penile cancer is extensive surgical resection or penile resection (partial or complete), usually with bilateral inguinal lymph node dissection with or without pelvic lymph node dissection.6 The European Association of Urology Guidelines approved that patients with advanced or recurrent pSCC or large-volume (> 4 cm) inguinal lymph nodes should be considered for multiple treatment modes including surgery, chemotherapy and/or radiotherapy.7 Cisplatin in combination with or without taxanes is still the main treatment for unresectable and relapsed penile cancer.8 The effect of chemotherapy and/or radiotherapy is not effective enough, with a median overall survival (OS) of recurrent patients of less than 6 months.9 To date, immunotherapy using immune checkpoint inhibitors (CPI) has become a preferred treatment option for metastatic and advanced cancers.10 PD-L1 was expressed in 48% of penile carcinomas and mainly in high-risk HPV negative tumors.11 However, there is a lack of clinical evidence showing the outcomes of CPI therapy in advanced or recurrent pSCC. A study shows that pSCC patients with high PD-L1 expression represent a promising prospect for anti-PD-L1/PD-1 therapy, while pSCC patients with medium or low PD-L1 expression may not respond well to anti-PD-L1/PD-1 therapy as a “immune exclusion” immunophenotype.12 However, the efficacy of utilizing CPI therapy to different PD-L1 expression levels remain elusive in pSCC.
Here we report a male patient with local recurrence of the right groin and nearly ruptured pSCC. He was treated with multimodal therapy (MMT) which included more aggressive salvage-assisted chemotherapy combined with CPI. The patient achieved complete response (CR) after three months of MMT. And he achieved progress complete response (pCR) with continuous CPI therapy.
Case Description
A 49-year-old male patient presented because of pain in the lymph nodes, with long prepuce and a 30-year history of gunshot wounds. The patient was diagnosed with pSCC in Jan 2019. The tumor was about 5.0 cm by 5.0 cm in size with bilateral inguinal lymph node metastases, T2N3M0 and phase IV poor differentiated. HPV status was negative. Under general anesthesia, partial penile resection and bilateral inguinal lymphadenectomy were performed. The pathology was poor differentiated pSCC ((Figure 1A), involving the cavernous body of the penis and the cavernous body of the urethra, with nerve invasion and lymphocyte infiltration, and no cancer seen at the cutting edge. Cancer metastasis could be seen in lymph nodes (2/10, left 1/6; right 1/4). In Mar 2019, under general anesthesia he subsequently underwent 3D laparoscopic pelvic lymph node dissection. The pathology suggested that no metastatic carcinoma was seen in the lymph nodes after operation (0/18). The patient refused adjuvant therapy.
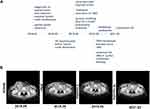
A right groin mass began to appear, and gradually increased in May. We found that the groin mass on the right side was unclear borders, red and swollen skin on the surface, a feeling of undulation, and near to rupture. The tumor showed as an irregular soft tissue shadow, about 10.3 cm by 7.0 cm, with some cystic changes, and no clear boundary with the local muscle (Figure 2B). Palliative resection of the right inguinal metastasis was performed. During the operation, the tumor was closely related to muscles and blood vessels, and the tumor was most likely to be removed. Pathology showed that fibrous tissue had cancer infiltration with necrosis (Figure 1E), consistent with recurrence or metastasis of penile squamous cell carcinoma.
According to our experiences and the reports, we determined to use combined adjuvant therapy as one of the therapeutic options. At the same time, we performed genetic profiling and PD-L1/CD8 protein-expressing analyses on the tumor tissue.
The results of genetic profiling and PD-L1/CD8 expressing analyses were as follows:
- Next-generation high-throughput gene-targeted sequencing, including 808 tumor diagnosis and treatment-related genes was performed. The tumor mutation burden (TMB, 2.25 Muts/Mb) was low, as the median TMB in pSCC was 4.5 Muts/Mb.13 The microsatellite stability is stable (MSS). And mutations positively or negatively related to immune efficacy were not detected.
- The tumor content accounts for 50% of the sample with active tumor-infiltrating lymphocytes (TIL). PD-L1 22C3 IHC analysis with Tumor Proportion Score (TPS) Interpretation was about 20–30%, while Combined Positive Score (CPS) was about 50–60% (Figure 1B–D). The CD8+ cytotoxic T-lymphocytes (CTLs) was about 30–40% (Figure 1F–H).
About 2 weeks after surgery, a postoperative combined adjuvant therapy was carried out. The patient’s treatment plans are presented in a timeline (Figure 2A). The therapeutic regimen (TBP, body surface area: 2.09 m2) is paclitaxel 270 mg intravenous drip on day 1; bleomycin 15 mg intravenous drip on days 1/8/15; cisplatin intravenous drip 50 mg on days 2/3/4 and sintilimab injection 200 mg on day 1. Every three weeks is a cycle. The patient reached complete imaging remission (CR) after 4 cycles of combined therapy (Figure 2B). Until Mar 2021, the enhanced CT after 28 cycles (21 months) of sintilimab still presented a sustainable CR (Figure 2B). The patient was still alive and there is no sign of recurrence or any adverse events.
Discussion
The 5-year overall mortality rate of locally advanced or lymph node infiltrating pSCC undergoing perioperative chemotherapy is approximately 40%.14 Due to the low incidence of penile cancer, there is no standard treatment plan thus far.15 Previous clinical studies showed that bilateral pelvic lymph node dissection (PLND) could improve the survival rate of patients with pN2, but patients with pN3 may not benefit from bilateral PLND.16 Our patient was diagnosed with penile cancer with bilateral inguinal lymph node metastases. He received partial penile resection and bilateral PLND, with no cancer seen at the cutting edge. Patients who have bilateral lymph node metastases or extranodal extension have 80–90% rate of recurrence17 and a poor 5-year OS.18 The recent systematic review by the European Urological Association’s Penile Cancer Guidelines Group indicated that these pN3 pSSC patients who received adjuvant radiotherapy after PLND could not benefit from either a reduced recurrence or an increased survival rate.19 And, according to contemporary North American and European guidelines, it is necessary to administer pN2-3 M0 pSCC neoadjuvant or adjuvant chemotherapy.1 Recent clinical reports confirmed that multimodal treatment could prolong survival, and was associated with longer survival of patients with stage IIIB/IV compared with surgery alone.20 However, our patient refused the adjuvant therapy, and this may have caused the earlier recurrence.
The prognosis of patients with recurrence of pSCC is dismal. Further prospective clinical studies are needed to improve these patients’ treatments. The guidelines recommend combined chemotherapy regimens including taxane and cisplatin as new adjuvant regimens in patients with locally advanced cancer.1,21 A combination of paclitaxel-ifosfamide-cisplatin (TIP) in penile squamous cell carcinoma patients with terminal lymph node metastasis could give a 63% (12/19) response rate and significantly higher overall survival (OS) and progression-free survival (PFS).22 A recent report of 21 patients with penile squamous cell carcinoma who received regional lymph node dissection after adjuvant taxane, cisplatin, and 5-Fluorouracil chemotherapy (TFP) had shown a median disease-free survival (DFS) of 8.9 months.23 Also a study (NCT00512096) shows that paclitaxel, ifosfamide, and cisplatin chemotherapy was effective as neoadjuvant treatment in patients with stage TX,N2-3,M0 penile cancer.24 However, chemotherapy may be efficient but is always accompanied by serious adverse reactions.
Recently, CPI has been approved in the treatment of many cancers including squamous cell carcinomas. The majority of pSCCs are PD-L1-positive,11 indicating that CPI therapies may benefit in pSCCs. A case reported that a penile cancer patient with strongly positive PD-L1 status showed PR since starting pembrolizumab 18 months ago.25 And another T2N2M0 penile cancer patient who used the PD-L1 inhibitor atezolizumab as second-line therapy reached near complete response after 2 years’ therapy.26 But the expression level of PD-L1 and/or TMB was lacking in these cases. While a recurrent metastatic penile cancer patient with positive PD-L1 ≥10% and a high TMB (8.87 Muts/Mb) treated with toripalimab got PR.27 Another stage IV patient with both high TMB and PD-L1 showing resistance to platinum-based chemotherapy and chemoradiation, got CR of lymph node metastasis after starting pembrolizumab.25 These cases shows that pSCC patients could benefit from CPI therapy, and a composite of high TMB plus high PD-L1 would further enrich the benefit to CPI.28 Meanwhile a recent study revealed that a relatively low TMB is commonly found in penile squamous cell carcinoma.29 Interestingly, sintilimab, a new anti-PD-1 antibody, with a combination with CapeOx shows no significant difference in the clinical responses between high TMB and low TMB patients in gastric cancer patients,30 which could be a possible option for low TMB patients. More exploration of CPI is needed in pSCC.
Conclusion
To our knowledge, this is the first case describing a reccurrent pSCC patient who achieved complete remission in China. The patient had a medium PD-L1 expression (20~30%), and low TMB (2.25 Muts/Mb). He was treated with MMT and achieved CR for 18 months, with a good tolerance and acceptable toxicity. This may provide MMT as a new option to maximize the benefit for advanced or recurrent penile cancer patients, even if these patients have a medium PD-L1 and low TMB. However, evidence of a single case report is limited. More large clinical trials are needed to explore the application of MMT in pSCC.
Abbreviations
pSCC, penile squamous cell carcinoma; MMT, multimodal therapy; CR, complete response; OS, overall survival; CPI, immune checkpoint inhibitors; pCR, progress complete response; TMB, tumor mutation burden; MSS, microsatellite stability stable; TIL, tumor-infiltrating lymphocytes; TPS, Tumor Proportion Score; CPS, Combined Positive Score; CTLs, CD8+ cytotoxic T-lymphocytes; PLND, pelvic lymph node dissection; PFS, progression-free survival; DFS, disease-free survival.
Consents
The authors have obtained informed consent from the patient for publication of this case report, and the ethics committee of Cancer Hospital of HuanXing approved this consent process and the publication of case details.
Disclosure
Linjun Hu and Xingli Shan are co-first authors for this study. The authors declared no conflicts of interest.
References
1. Hakenberg OW, Compérat EM, Minhas S, Necchi A, Protzel C, Watkin N. EAU guidelines on penile cancer: 2014 update. Eur Urol. 2015;67(1):142–150. doi:10.1016/j.eururo.2014.10.017
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
3. de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Global Health. 2020;8(2):e180–e190. doi:10.1016/S2214-109X(19)30488-7
4. Burgers JK, Badalament RA, Drago JR. Penile cancer: clinical presentation, diagnosis, and staging. Urol Clin North Am. 1992;19(2):247–256. doi:10.1016/S0094-0143(21)00387-6
5. Gu W, Zhang P, Zhang G, et al. Importance of HPV in Chinese penile cancer: a contemporary multicenter study. Front Oncol. 2020;10:1521. doi:10.3389/fonc.2020.01521
6. Protzel C, Alcaraz A, Horenblas S, Pizzocaro G, Zlotta A, Hakenberg OW. Lymphadenectomy in the surgical management of penile cancer. Eur Urol. 2009;55(5):1075–1088. doi:10.1016/j.eururo.2009.02.021
7. Gakis G, Witjes JA, Compérat E, et al. EAU guidelines on primary urethral carcinoma. Eur Urol. 2013;64(5):823–830. doi:10.1016/j.eururo.2013.03.044
8. Pizzocaro G, Nicolai N, Milani A. Taxanes in combination with cisplatin and fluorouracil for advanced penile cancer: preliminary results. Eur Urol. 2009;55(3):546–551. doi:10.1016/j.eururo.2008.07.014
9. Mistretta FA, Palumbo C, Knipper S, et al. Conditional survival of patients with stage I-III squamous cell carcinoma of the penis: temporal changes in cancer-specific mortality. World J Urol. 2020;38(3):725–732. doi:10.1007/s00345-019-02869-6
10. Sun L, Zhang L, Yu J, et al. Clinical efficacy and safety of anti-PD-1/PD-L1 inhibitors for the treatment of advanced or metastatic cancer: a systematic review and meta-analysis. Sci Rep. 2020;10(1):2083. doi:10.1038/s41598-020-58674-4
11. Ottenhof SR, Djajadiningrat RS, de Jong J, Thygesen HH, Horenblas S, Jordanova ES. Expression of programmed death ligand 1 in penile cancer is of prognostic value and associated with HPV status. J Urol. 2017;197(3 Pt 1):690–697. doi:10.1016/j.juro.2016.09.088
12. Chu C, Yao K, Lu J, et al. Immunophenotypes based on the tumor immune microenvironment allow for unsupervised penile cancer patient stratification. Cancers. 2020;12(7):1796.
13. Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. doi:10.1186/s13073-017-0424-2
14. Joshi SS, Handorf E, Strauss D, et al. Treatment trends and outcomes for patients with lymph node-positive cancer of the penis. JAMA Oncol. 2018;4(5):643–649. doi:10.1001/jamaoncol.2017.5608
15. Resch I, Abufaraj M, Hübner NA, Shariat SF. An update on systemic therapy for penile cancer. Curr Opin Urol. 2020;30(2):229–233. doi:10.1097/MOU.0000000000000733
16. Robinson R, Marconi L, MacPepple E, et al. Risks and benefits of adjuvant radiotherapy after inguinal lymphadenectomy in node-positive penile cancer: a systematic review by the European association of urology penile cancer guidelines panel. Eur Urol. 2018;74(1):76–83. doi:10.1016/j.eururo.2018.04.003
17. Pandey D, Mahajan V, Kannan RR. Prognostic factors in node-positive carcinoma of the penis. J Surg Oncol. 2006;93(2):133–138. doi:10.1002/jso.20414
18. Rodney S, Muneer A. HPV and penile cancer: perspectives on the future management of HPV-positive disease. Oncology. 2016;30(3):250–252.
19. Li ZS, Deng CZ, Yao K, et al. Bilateral pelvic lymph node dissection for Chinese patients with penile cancer: a multicenter collaboration study. J Cancer Res Clin Oncol. 2017;143(2):329–335. doi:10.1007/s00432-016-2292-3
20. Sirithanaphol W, Sookprasert A, Rompsaithong U, Kiatsopit P, Wirasorn K, Chindaprasirt J. Prognostic factors for penile cancer and survival in response to multimodality therapy. Res Rep Urol. 2020;12:29–34.
21. Dorff TB, Ballas LK, Schuckman AK. Current management strategy for penile cancer and future directions. Curr Oncol Rep. 2017;19(8):54. doi:10.1007/s11912-017-0615-4
22. Xu J, Li G, Zhu SM, et al. Neoadjuvant docetaxel, cisplatin and ifosfamide (ITP) combination chemotherapy for treating penile squamous cell carcinoma patients with terminal lymph node metastasis. BMC Cancer. 2019;19(1):625. doi:10.1186/s12885-019-5847-2
23. Necchi A, Lo Vullo S, Nicolai N, et al. Prognostic factors of adjuvant taxane, cisplatin, and 5-fluorouracil chemotherapy for patients with penile squamous cell carcinoma after regional lymphadenectomy. Clin Genitourin Cancer. 2016;14(6):518–523. doi:10.1016/j.clgc.2016.03.005
24. Pagliaro LC, Williams DL, Daliani D, et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: a phase II study. J Clin Oncol. 2010;28(24):3851–3857.
25. Chahoud J, Skelton WP, Spiess PE, et al. Case report: two cases of chemotherapy refractory metastatic penile squamous cell carcinoma with extreme durable response to pembrolizumab. Front Oncol. 2020;10:615298. doi:10.3389/fonc.2020.615298
26. Hui G, Ghafouri SN, Shen J, Liu S, Drakaki A. Treating penile cancer in the immunotherapy and targeted therapy era. Case Rep Oncol Med. 2019;2019:8349793. doi:10.1155/2019/8349793
27. Su X, Zhang J, Fu C, Xiao M, Wang C. Recurrent metastatic penile cancer patient with positive PD-L1 expression obtained significant benefit from immunotherapy: a case report and literature review. Onco Targets Ther. 2020;13:3319–3324. doi:10.2147/OTT.S231258
28. Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633–641. doi:10.1200/JCO.2017.75.3384
29. Stoehr R, Wendler O, Giedl J, et al. No evidence of microsatellite instability and loss of mismatch-repair-protein expression in squamous cell carcinoma of the penis. Pathobiology. 2019;86(2–3):145–151. doi:10.1159/000495251
30. Jiang H, Zheng Y, Qian J, et al. Safety and efficacy of sintilimab combined with oxaliplatin/capecitabine as first-line treatment in patients with locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma in a phase Ib clinical trial. BMC Cancer. 2020;20(1):760. doi:10.1186/s12885-020-07251-z
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.