Back to Journals » Journal of Pain Research » Volume 15
Multifidus Muscle Contractility Deficit Was Not Specific to the Painful Side in Patients with Chronic Low Back Pain During Remission: A Cross-Sectional Study
Authors Thu KW, Maharjan S, Sornkaew K, Kongoun S, Wattananon P
Received 24 February 2022
Accepted for publication 10 May 2022
Published 19 May 2022 Volume 2022:15 Pages 1457—1463
DOI https://doi.org/10.2147/JPR.S363591
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Khin Win Thu, Soniya Maharjan, Kanphajee Sornkaew, Sasithorn Kongoun, Peemongkon Wattananon
Spine Biomechanics Laboratory, Faculty of Physical Therapy, Mahidol University, Salaya, Nakhon Pathom, Thailand
Correspondence: Peemongkon Wattananon, Spine Biomechanics Laboratory, Faculty of Physical Therapy, Mahidol University, 999 Phuttamonthon 4 Road, Salaya, Nakhon Pathom, 73170, Thailand, Tel +66 2441 5450, Fax +66 2441 5454, Email [email protected]
Purpose: Morphology studies demonstrated that patients with chronic low back pain (CLBP) have bilateral multifidus muscle (LM) atrophy. This atrophy should result in LM contractility deficit bilaterally. Additionally, a recent study showed the effect of sex on LM thickness. Researchers proposed percentage LM contractility (LMCONT) as standardization to enable the comparison across participants. This study aimed to determine side-to-side difference in LMCONT and to determine the difference in LMCONT between males and females.
Patients and Methods: Twenty-five healthy individuals (NoLBP group; 10 males and 15 females) and 35 with CLBP (CLBP group; 16 males and 19 females; 23 unilateral pain and 12 bilateral pain) were recruited. Ultrasound imaging was used to measure LM thickness at rest, during maximum voluntary isometric contraction, and during combined maximum voluntary isometric contraction with electrical stimulation. These data were used to calculate LMCONT. For unilateral CLBP, right and left LMCON were renamed to painful and non-painful sides.
Results: Data demonstrated no significant difference (p > 0.05) between right (87.3 ± 13.7%) and left (87.2 ± 14.0%) in NoLBP, right (71.2 ± 15.7%) and left (76.5 ± 19.7%) in bilateral CLBP, and painful (70.3 ± 17.5%) and non-painful (77.7 ± 18.4%) in unilateral CLBP. No difference (p > 0.05) was found between males and females in both NoLBP (male 84.8 ± 6.5%, female 88.9 ± 15.4%) and CLBP groups (male 76.3 ± 15.5%, female 71.9 ± 14.0%).
Conclusion: The findings suggested that LM contractility deficit in CLBP is not specific to painful side. No effect was found of sex on LM contractility. Therefore, we can use averaged LM activation across painful and non-painful sides and across males and females to compare between NoLBP and CLBP groups.
Keywords: multifidus, muscle activation, low back pain, ultrasound imaging
Introduction
Low back pain is one of the leading causes of musculoskeletal problems with a great negative impact on society because of its global burden of disability.1 It affects nearly 60% to 80% of all populations throughout their lifetime.1 Once individuals suffer from low back pain, the recurrence rate throughout their lifetime will be approximately between 60% and 70% per year.2 Several studies have suggested lumbar multifidus muscle (LM) impairment might be a key factor contributing to low back pain.3–6 Studies also found that LM cannot be automatically recovered even after remission stage; therefore, it should be responsible for persistent/recurrent symptoms in patients with chronic low back pain (CLBP).7,8
LM is one of the deep trunk muscles responsible for static and dynamic stability of the lumbar spine.9 LM controls stability of the lumbar spine by stiffening the spine and takes part in more than two-thirds of the stiffness of the lumbar spine.10 Morphology studies using magnetic resonance image (MRI) and computerized tomography (CT) demonstrated reduction in LM cross-sectional area (CSA) bilaterally regardless of the side of pain.11–13 Functional studies using electromyography (EMG) demonstrated reduction in LM activation in both painful and non-painful sides in patients with CLBP.4,14,15 Recently, rehabilitative ultrasound imaging (RUSI) has been commonly used in clinical settings to evaluate LM contractility in patients with CLBP.8,16–18 Several studies demonstrated bilateral changes in LM morphology and activation regardless of side of pain.4,12–15 These changes are also related to muscle contractility. Therefore, questions remain whether any difference in LM contractility exists between left and right sides in healthy individuals, as well as painful and non-painful sides in patients with CLBP when using RUSI.
Although RUSI is widely used to measure LM thickness and CSA, one systematic review indicated the effect of sex on LM measurement in which males tended to have larger LM thickness and CSA than females.19 This can be a confounding factor when including both male and female data in analysis. According to EMG studies, researchers use maximum voluntary isometric contraction for standardization to enable the comparability across males and females.14,15 This standardization approach can be applicable for RUSI data to allow comparison across individuals.
Therefore, this study aimed to determine 1) side-to-side difference in LM contractility, 2) the effect of sex on LM thickness, and 3) ability of standardization to enable the comparison between males and females. Based on LM morphology studies, we hypothesized that patients with CLBP would demonstrate no differences in LM contractility between the painful and non-painful sides, 2) males would have larger LM thickness than female and 3) standardization would allow comparability between males and females.
Materials and Methods
Participants
Twenty-five healthy individuals (NoLBP group; 10 males and 15 females) and 35 with CLBP (CLBP group; 16 males and 19 females; 23 unilateral pain and 12 bilateral pain) aged between 20 and 40 years were recruited. The inclusion criteria for NoLBP group were no previous episodes of LBP, while inclusion criteria for CLBP were having LBP greater than three months, but currently pain-free, or a recurrent pattern of low back symptoms at least two episodes that interfered with activities of daily living or required treatment. The exclusion criteria were 1) clinical signs of systemic disease, 2) definitive neurological signs including pain, weakness, or numbness in the lower extremity, 3) previous spinal surgery, 4) diagnosed osteoporosis, severe spinal stenosis, or inflammatory joint disease, 5) body mass index greater than 30 kg/m2 and 6) active treatment of another medical illness that would preclude participation in any aspect of the study. This study was a part of an intervention study; therefore, we did not calculate sample size.
Instruments and Measures
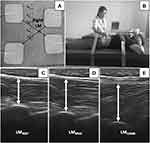
A B-mode rehabilitative ultrasound imaging device (RUSI; model CX50, Philips, NV, USA) with a broadband linear array (model L12-3) transducer was used to measure bilateral LM thickness at L4-5 facet joint (2 cm lateral to the lower half of the L4 spinous process) under three conditions including resting, maximum voluntary isometric contraction and combined MVIC with electrical stimulation.8 The tip of the L4-5 facet joint and thoracolumbar fascia in the RUSI image were used to measure LM thickness.17,18 Our previous work demonstrated that RUSI was valid and reliable to measure LM thickness.8
Procedure
This human research followed the principles of the Declaration of Helsinki. The study was approved by Mahidol University Central Institutional Review Board (MU-CIRB 2018/215.0712). The participants were asked to provide a written informed consent before data collection. Demographic data were collected for both the NoLBP and CLBP groups, while clinical data were collected for the CLBP group.
The participants were in a prone position with a towel roll between abdomen and pelvis to keep the lumbar spine in neutral position (lumbosacral angle less than 10 degrees). The thorax (T3) and pelvis (S2) were securely stabilized using the straps. The researcher captured 2 RUSI images of right and left LM thickness at rest (LMREST). Then, the participant was asked to perform two repetitions of maximum voluntary isometric contraction of back extension against the strap with 1-minute rest between repetitions, while the researcher simultaneously captured RUSI images for each side (LMMVIC).
Two electrical stimulation (ES) electrodes were attached at 3 cm lateral to the L3 and L5 spinous processes on the right side and another two electrodes were placed at 2 cm lateral to the spinous processes on the left side that created two diagonal lines intersecting at the right LM.8 The ES parameters were set as frequency at 6000 Hz and amplitude modulated at 20 to 50 Hz.8 The intensity was increased to maximum pain tolerance to activate LM motor unit, while the participant was asked to perform two repetitions of MVIC with 1-minute rest between repetitions. The researcher recorded RUSI images from the right LM during combined MVIC with ES (LMCOMB). Five-minute rest was provided to minimize muscle fatigue. Then, the researcher repeated the same protocol to measure the left LMCOMB. Figure 1 illustrates ES electrode placement, participant set up, and LM thickness at rest, during MVIC and during combined MVIC with ES. Person in Figure 1B provided informed consent for the image to be published.
Data Analysis
All RUSI images were exported to the MATLAB Program, Version R2014a (The MatWorks Inc, MA, USA) for LM thickness measurements in each condition. LMREST, LMMVIC, and LMCOMB were used to calculate percentage LM contractility (LMCONT) using the following equation.8
LMCONT = (LMMVIC-LMREST/LMCOMB-LMREST) X 100
The overall scheme for data analysis is presented in Figure 2. Data were classified to NoLBP or CLBP. For CLBP, data were sub-classified to bilateral CLBP and unilateral CLBP. Data from right and left sides in NoLBP and bilateral CLBP, as well as painful and non-painful sides in unilateral CLBP were used to determine side-to-side difference. In addition, data for each group were also classified to males and females. These data were further used to determine the effect of sex on LM thickness (LMREST, LMMVIC, and LMCOMB) and the ability of standardization by converting LM thickness to LM contractility (LMCONT) to enable the comparison between males and females.
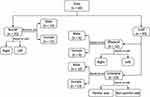 |
Figure 2 Overall scheme for data analysis. |
Statistical Analysis
Statistical analyses were performed using SPSS Software (Version 21, IBM Corp., NY, USA). Descriptive statistics was used to describe demographic and clinical data. The Shapiro–Wilk test demonstrated all data were normally distributed. Therefore, a dependent t-test was used to determine side-to-side difference, while an independent t-test was used to determine the difference in LM thickness and LM contractility between males and females. Significance level was held at 0.05.
Results
Table 1 demonstrates the demographic data for both NoLBP and CLBP groups and clinical data for the CLBP group. Results demonstrated no significant difference (p>0.05) between right and left in the NoLBP, right and left in the bilateral CLBP, and painful and non-painful in the unilateral CLBP groups (Table 2). Most LM thickness data demonstrated that males had significantly greater (p<0.05) LM thickness (LMREST, LMMVIC, and LMCOMB) than females (Table 3). However, no difference (p>0.05) was found in LMCONT between males and females in the NoLBP, bilateral CLBP, and unilateral CLBP groups.
 |
Table 1 Mean and Standard Deviation (SD) for Demographic Data |
 |
Table 2 Comparison of Percentage LM Activation Between Right and Left in NoLBP and Bilateral CLBP Groups, and Between Painful and Non-Painful Sides in Unilateral CLBP Group |
 |
Table 3 Comparison of LM Thickness at Rest, MVIC, COMB, and Percentage LM Activation Between Male and Female in NoLBP, Bilateral CLBP and Unilateral CLBP Groups |
Discussion
Our results based on RUSI approach supported the hypothesis in which patients with CLBP would demonstrate no difference in LM contractility between painful and non-painful sides, rather than specifically to the painful side. Our findings were consistent with morphology studies using MRI and CT scans.3,11–13 Those studies demonstrated LM atrophy and fatty infiltration bilaterally. These changes would compromise LM contractility and functions. This was also evident by the reduced LM activity in patients with CLBP in EMG studies.4,14,15
Hodges and Daneel4 suggested that structural and functional changes in patients with CLBP plausibly resulted from generally deconditioning, rather than reflex inhibition commonly found in acute low back pain or inflammatory mechanism found in subacute low back pain. In acute/subacute LBP, these neural and inflammatory mechanisms can cause changes in muscle activation patterns (decreased LM activation, delayed onset of LM, etc.).4,20–23 When left untreated, they can cause maladaptation over time.4 For instance, patients may use the bilateral lumbar erector spinae muscle, instead of LM, to achieve static and dynamic stability during daily activities.21 This may further cause disuse of the LM leading to deconditioning.4 Therefore, intervention should focus on bilateral LM, rather than specific to the side of pain.
No significant difference between sides can benefit future studies that researchers can use averaged LM contractility to obtain a statistically more stable data for further analysis. On the other hand, the researchers can select only one side for LM measurement (painful side or dominant side) to shorten study protocol and reduce the cost of the study.
Our findings partially supported our hypothesis that males would have larger LM thickness than females. We found evidence in only healthy individuals and patients with unilateral CLBP, but not patients with bilateral CLBP. Although patients with bilateral CLBP did not show significant difference between males and females, the overall mean LM thickness at rest and during contraction demonstrated that males had greater LM thickness than females. These findings suggested that LM thickness can be confounded by sex difference.18,19 This could lead to misinterpretation of difference in LM thickness between groups. Therefore, a direct comparison of LM thickness between groups without taking sex difference into account should be avoided.
We used RUSI in conjunction with ES as the standardization approach to allow comparability across males and females.8 This standardized LM thickness is used to represent LM contractility.8 Our findings indicated sex can influence LM thickness with males having greater thickness during resting and contraction than females. As a result, the interpretation of LM thickness might be problematic. Therefore, standardizing maximum contraction (LMMVIC) with reference values (LMREST and LMCOMB) can be used to control the effect of sex. Our findings indicated that the sex difference in LM thickness was no longer significant after standardization. Our findings were consistent with previous studies using percentage LM thickness change (relative LM thickness change) as standardization approach.18 Therefore, LM activation based on our approach can be used to standardize the effect of sex on LM thickness which in turn allowed us to compare across male and female data.
Limitations encountered in the study need to be considered. First, patients with CLBP in this study were in the remission phase. This would limit generalization to those patients with active pain. Our study did not take the dominant side into account. However, studies have reported dominant/non-dominant differences in LM thickness around 5% (<1% in our study) in healthy individuals, while no significant asymmetry in resting LM thickness was found in patients with unilateral CLBP.19 Therefore, any effect of dominant side in this study was unlikely. Our study aimed to explore whether LM contractility was specific to the painful side, however this was performed on a limited sample size. Therefore, replication of this study using a larger sample size and using a more robust statistical analysisfor example, linear regression, is warranted.
Conclusion
Findings demonstrated no difference between right and left sides in healthy individuals and patients with bilateral CLBP, as well as painful and non-painful side in patients with unilateral CLBP. These findings suggest that patients with CLBP have the same LM contractility level between painful and non-painful sides, rather than specifically to the painful side. Although we found differences in LM thickness between males and females, these differences were no longer present after using our approach as standardization. This allowed us to compare data across males and females.
Acknowledgments
We would like to thank the Spine Biomechanics Laboratory, Faculty of Physical Therapy, Mahidol University for providing the data collection space and equipment. We would also like to thank all participants in this study. This study was funded by Thailand Research Fund (MRG6280241) and Faculty of Physical Therapy, Mahidol University (RA fund).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Safiri S, Kolahi -A-A, Cross M, et al. Prevalence, Deaths, and Disability-Adjusted Life Years Due to Musculoskeletal Disorders for 195 Countries and Territories 1990–2017. Arthritis Rheumatol. 2021;73(4):702–714. doi:10.1002/art.41571
2. Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64:2028–2037.
3. Hides J, Gilmore C, Stanton W, et al. Multifidus size and symmetry among chronic LBP and healthy asymptomatic subjects. Man Ther. 2008;13:43–49.
4. Hodges PW, Danneels L. Changes in structure and function of the back muscles in low back pain: different time points, observations, and mechanisms. J Orthop Sports Phys Ther. 2019;49:464–476.
5. Russo M, Deckers K, Eldabe S, et al. Muscle control and non-specific chronic low back pain. Neuromodulation. 2018;21:1–9.
6. Sweeney N, O’Sullivan C, Kelly G. Multifidus muscle size and percentage thickness changes among patients with unilateral chronic low back pain (CLBP) and healthy controls in prone and standing. Man Ther. 2014;19:433–439.
7. Hides JA, Richardson CA, Jull GA. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine. 1996;21:2763–2769.
8. Wattananon P, Sungnak P, Songjaroen S, et al. Using neuromuscular electrical stimulation in conjunction with ultrasound imaging technique to investigate lumbar multifidus muscle activation deficit. Musculoskelet Sci Pract. 2020;50:102215.
9. Bogduk N, Macintosh JE, Pearcy MJ. A universal model of the lumbar back muscles in the upright position. Spine. 1992;17:897–913.
10. Macintosh JE, Valencia F, Bogduk N, et al. The morphology of the human lumbar multifidus. Clin Biomech. 1986;1:196–204.
11. Beneck GJ, Kulig K. Multifidus atrophy is localized and bilateral in active persons with chronic unilateral low back pain. Arch Phys Med Rehabil. 2012;93:300–306.
12. Danneels LA, Vanderstraeten GG, Cambier DC, et al. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J. 2000;9:266–272.
13. Fortin M, Macedo LG. Multifidus and paraspinal muscle group cross-sectional areas of patients with low back pain and control patients: a systematic review with a focus on blinding. Phys Ther. 2013;93:873–888.
14. Danneels LA, Coorevits PL, Cools AM, et al. Differences in electromyographic activity in the multifidus muscle and the iliocostalis lumborum between healthy subjects and patients with sub-acute and chronic low back pain. Eur Spine J. 2002;11:13–19.
15. MacDonald D, Moseley GL, Hodges PW. People with recurrent low back pain respond differently to trunk loading despite remission from symptoms. Spine. 2010;35:818–824.
16. Hebert JJ, Koppenhaver SL, Parent EC, et al. A systematic review of the reliability of rehabilitative ultrasound imaging for the quantitative assessment of the abdominal and lumbar trunk muscles. Spine. 2009;34:E848–56.
17. Kiesel KB, Uhl TL, Underwood FB, et al. Measurement of lumbar multifidus muscle contraction with rehabilitative ultrasound imaging. Man Ther. 2007;12:161–166.
18. Teyhen DS, Childs JD, Stokes MJ, et al. Abdominal and lumbar multifidus muscle size and symmetry at rest and during contracted States. Normative reference ranges. J Ultrasound Med. 2012;31:1099–1110.
19. Rummens S, Robben E, De Groef A, et al. Factors associated with the ultrasound characteristics of the lumbar multifidus: a systematic review. Pm r. 2020;12:82–100.
20. Kiesel KB, Butler RJ, Duckworth A, et al. Experimentally induced pain alters the EMG activity of the lumbar multifidus in asymptomatic subjects. Man Ther. 2012;17:236–240.
21. MacDonald D, Moseley LG, Hodges PW. Why do some patients keep hurting their back? Evidence of ongoing back muscle dysfunction during remission from recurrent back pain. Pain. 2009;142:183–188.
22. MacDonald DA, Moseley GL, Hodges PW. The lumbar multifidus: does the evidence support clinical beliefs? Man Ther. 2006;11:254–263.
23. Moseley GL, Nicholas MK, Hodges PW. Does anticipation of back pain predispose to back trouble? Brain. 2004;127:2339–2347.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.