Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Multidimensional Outcomes of IV Thrombolysis in Minor Ischemic Stroke: Motor, Psychocognitive, and Dependence
Authors Li N , Zhang J, Li SJ, Du Y , Zhou Q, Gu HQ, Zhao XQ
Received 10 August 2023
Accepted for publication 24 October 2023
Published 1 November 2023 Volume 2023:19 Pages 2341—2351
DOI https://doi.org/10.2147/NDT.S434296
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Richard J Porter
Ning Li,1 Jia Zhang,1,2 Si-Jia Li,1 Yang Du,1,2 Qi Zhou,2 Hong-Qiu Gu,2 Xing-Quan Zhao1– 3
1Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, People’s Republic of China; 2China National Clinical Research Center for Neurological Diseases, Beijing, People’s Republic of China; 3Research Unit of Artificial Intelligence in Cerebrovascular Disease, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China
Correspondence: Xing-Quan Zhao, Beijing Tiantan Hospital, Capital Medical University, No. 119 South 4th Ring West Road, Fengtai District, Beijing, 100070, People’s Republic of China, Tel +86-13501031486, Email [email protected]
Background: The presence of mild deficit is the most common reason for nonuse of intravenous alteplase in ischemic stroke. We analyzed within a national prospective cohort on whether patients with minor stroke can benefit from intravenous alteplase.
Methods: This observational study included patients with acute ischemic stroke with a National Institutes of Health Stroke Scale (NIHSS) score 0 to 5 at admission. The short-term outcomes at discharge and 3-month were analyzed including the modified Rankin Scale score, gait speed, Montreal Cognitive Assessment, Patient Health Questionnaire-9, General Anxiety Disorder-7 and Stroke Impact Scale-16. Multivariate regression models were performed to evaluate the association between intravenous thrombolysis and clinical outcomes.
Results: A total of 1876 consecutive patients were included in the current analyses with 102 patients (5.4%) received alteplase and 1774 patients (94.5%) were in non-alteplase group. We found that 10.9% patients presented unfavorable functional outcome with a mRS ≥ 2 at 3-month. Patients with alteplase treatment had a more favorable outcome in SIS-16 at discharge (OR, 5.45; 95% CI, 2.22– 8.68) and 3-month after stroke (OR, 2.34; 95% CI, 0.17– 4.50). There was an association of alteplase with better gait speed in the restricted sample of age > 60 (OR,0.14; 95% CI, 0.02– 0.25), while an unfavorable effect was found in anxiety (OR, 2.23; 95% CI, 2.23, 0.91– 3.55) and depression (OR, 1.54; 95% CI, 0.17– 2.91) in female.
Conclusion: Alteplase showed a suggestive benefit in function and motor outcomes in patients with low NIHSS score of 0– 5. Meanwhile, female seemed more inclined to post-stroke emotional problems after alteplase treatment, which should be further explored in the future.
Keywords: alteplase, minor stroke, ischemic, outcomes
Background
Over half of patients with acute ischemic stroke have a low National Institutes of Health Stroke Scale (NIHSS) score at presentation.1,2 A number of these patients were untreated with intravenous thrombolysis (IVT) on account of the presence of mild symptoms in clinical practice.3 Yet prospective data suggested that 30% patients have residual disability at 3 months after minor stroke.4,5 A significant proportion of patients with mild and rapidly improving acute ischemic stroke not treated with thrombolytics have suboptimal short-term outcomes.6 Thus, it is of great significance to explore the benefits of IVT for minor stroke.
Current guidelines on intravenous alteplase in acute ischemic stroke are based on evidence in which alteplase treatment showed an overall benefit of reducing functional disability.7 However, clinical results of alteplase treatment were inconsistent in patients with mild neurologic deficits. Previous series of studies usually defined functional outcome by the modified Rankin Scale (mRS) to evaluate the efficacy of thrombolysis. Previous study from the Austrian Stroke Unit Registry (ASUR) involving 445 matched pairs, revealing that intravenous thrombolysis was beneficial for mild deficit patients with a NIHSS score of 0–5 after 3 months.8 Nevertheless, the mRS may be unmodified by IVT treatment at some situations. The randomized, double-blind clinical trial from Khatri et al reported that intravenous alteplase appears unlikely to increase the likelihood of favorable outcome compared to aspirin.9
Overall, global scales based on the subjective judgment of physicians may not be sufficiently sensitive to capture the changes of disability after minor stroke.10 Thus, Wendt et al suggested that individual neurologic deficits including language impairment, distal paresis, gait disorder and their impact on functional impairment should be considered in the judgment of a disabling stroke but not just based on the global score.11 Our study was designed to describe the association between intravenous alteplase and multidimensional outcomes of patients with minor stroke to provide more insights into the effectiveness of thrombolysis.
Methods
Study Design and Participants
We designed a retrospective analysis enrolled patients from the Impairment of CognitiON and Sleep after acute ischemic stroke or transient ischemic attack (ICONS) study, which is one subgroup study of The Third China National Stroke Registry (CNSR-III).12 The CNSR-III is a national prospective multicenter registry for patients with a diagnosis of AIS or TIA within seven days from 201 study sites in China that collected 15,166 stroke patients from August 2015 to March 2018 The detailed design, rationale, and basic description of the CNSR-III study have been described before.1
The inclusion criteria of ICONS were the same as CNSR-III, including: (1) age older than 18 years; (2) within 7 days from the onset of ischemic stroke symptoms to enrollment. The exclusion criteria were added to ICONS, including: (1) prior diagnosis of cognitive impairment, schizophrenia, or psychosis disease; (2) illiterate patients; (3) severe aphasia defined as National Institutes of Health Stroke Scale (NIHSS) item 9>2, visual impairment, hearing loss, dyslexia, severe unilateral neglect, or consciousness disorders (NIHSS item 1a >1 or 1b >1). We recruited patients with a diagnosis of minor stroke at admission in this study. The minor stroke was defined as the National Institutes of Health Stroke Scale (NIHSS) score ≤5. Patients were excluded if they met one of the following criteria: (1) premorbid mRS ≥2; (2) can not to walk for 10 m; (3) treated with intravenous urokinase.
Demographics and Clinical Profile
Data on demographics and clinical characteristics were obtained from medical recordings during hospitalization at baseline. All the clinical data were collected by unified trained investigators through the electronic data capture system (EDC). We collected demographic information including age, gender, body mass index (BMI) and education level, medicare, medical history (including hypertension, diabetes mellitus, hyperlipidemia, previous stroke, coronary artery disease, atrial fibrillation, current smoking, current drinking), baseline medication history (including antiplatelet agents, anticoagulant agents, antihypertensive, antilipidemic, antidiabetic), arrival and stroke severity (onset to arrival time, baseline NIHSS) and etiology classification of ischemic stroke (TOAST, Trial of Org 10172 in Acute Stroke Treatment).13 All the patients were divided into two groups: alteplase group and non-alteplase group according to the using of intravenous alteplase or not. The recommended dose of alteplase for Asian people was 0.9 mg/kg (maximum 90 mg).14
This study was conducted in accordance with the Declaration of Helsinki and the protocol of CNSR-III and ICONS was approved by the ethics committee of Beijing Tiantan Hospital (IRB approval number: KY2015-001-01).1 Written informed consents were obtained from all patients or their legal representatives.
Outcomes Assessment
The follow-up standardized tests were done by face-to-face interview at discharge and 3-month after stroke onset.
Motor Outcomes
Motor function was evaluated by gait speed through 10-meter-walking test.15 Participants were required to walk on a 10-meter walkway at their usual pace and the gait speed was calculated as distance in meters divided by time in seconds.16 Each test was conducted three times and the average value was kept for the analyses. Participants who used assistive devices were also included.
Psychocognitive Outcomes
The mood of patients was assessed with the Patient Health Questionnaire-9 (PHQ-9) and General Anxiety Disorder-7 (GAD-7).17,18 The PHQ-9 was used to evaluate depression during the past 2 weeks, with a total score of 27 points and the higher score means severe depression conditions. The GAD-7 was used to assess anxiety during the past 2 weeks, with a total score of 21 points and the higher score means severe anxiety conditions.
The cognitive function data was assessed with Montreal Cognitive Assessment (MoCA),19 with a total score of 30 points and the higher score means better cognitive conditions.
Functional Outcomes
The functional dependence status was assessed by the modified Rankin Scale (mRS)20 and Stroke Impact Scale (SIS-16).21 The mRS was used to measure global functional status with a score range from 0 to 5, which means no symptoms to serious functional impairment. The poor functional outcome was defined as a score of 2 or more on the mRS.22 The SIS-16 evaluated poststroke physical limitations with a broad score range from 0 to 100, which means fully dependent to fully independent.23
Statistical Analyses
All analyses were performed using the SAS, version 9.4, software (SAS Institute, Cary, NC, USA). Baseline characteristics between groups were compared using χ2 or Fisher exact test for categorical variables, t tests for normally distributed continuous variables and Mann–Whitney U-test for other continuous variables. Multivariate linear regression models were used to evaluate the association between intravenous alteplase and clinical outcomes including gait speed, MoCA, PHQ-9, GAD-7, and SIS-16 at 3-month after stroke, whereas multivariate logistic regression models were used to evaluate the association between intravenous alteplase and the dichotomous outcome (mRS 0–1 vs 2–5). First, unadjusted regression models were examined. The first model adjusted for age, gender, and baseline NIHSS. The second model additionally included education level, medicare, hypertension, diabetes mellitus, hyperlipidemia, previous stroke, current smoking, atrial fibrillation, coronary artery disease, medication history of anticoagulation, medication history of antiplatelet, onset-arrival time and TOAST classification. Prespecified stratified analyses were conducted by age (>60 and ≤60), gender (male and female) and baseline NIHSS (0–2 and 3–5) controlling for the variables in model 2. A two-tailed p value less than 0.05 was statistically significant.
Results
Baseline Characteristics
Among 2625 patients with AIS collected in the CNSR-III-ICONS database, 1925 of them had a final diagnosis of TIA or minor stroke (NIHSS<5), 49 patients were excluded for meeting the exclusion including with premorbid mRS≥2, failed to complete 10-meter-walk test or accepted intravenous urokinase. Thus, a total of 1876 consecutive patients were included in the current analyses (Figure 1). Overall, 102 patients (5.4%) received alteplase and 1774 patients (94.5%) were in non-alteplase group.
The baseline and clinical features of the cohort by alteplase treatment are demonstrated in Table 1. The mean age of the total population was 60.8 years and 26.5% of them was female. Of the study participants, 20.7% of patients had a prior history of stroke and 76.0% of them scored 0 on mRS before the index event (n = 1425). Most of the final diagnosis represented ischemic strokes (90.8%), with a mean NIHSS score at admission of 2. The stroke mechanisms in those with a final diagnosis of ischemic stroke according to the Toast classification included large artery atherosclerosis in 23.0%, cardioembolism in 5.0%, small artery occlusion in 26.3%, other determined cause in 0.9%, undetermined cause in 44.8%.
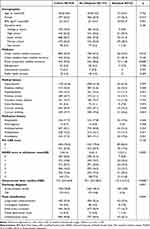 |
Table 1 Baseline Patients Characteristics by Alteplase Treatment |
As noted, the alteplase group had a higher proportion of female than the non-alteplase group (percentage, 36.3% vs 25.9%). They were more likely to have urban worker medical insurance (percentage, 54.9% vs 42.2%) and less likely to have rural cooperation medical insurance (percentage, 11.8% vs 30.0%). Patients treated with alteplase had higher NIHSS score at admission (mean±SD, 2.5±1.5) than non-alteplase (mean±SD, 2.0±1.5). The alteplase group possessed more patients who had a high NIHSS score of 3 to 5 than the non-alteplase group (percentage, 45.1% vs 36.9%). We found no significant differences at baseline in age, BMI, education level, medical history and medication history (p > 0.05).
Outcomes at Discharge and 3-Month
The clinical outcomes at discharge and 3-month are described in Table 2. In the overall cohort, the neurological functions of patients presented a trend of recovery or improvement in all evaluated dimensions at 3-month compared with discharge. Among patients treated with alteplase, the mean dosage of 56.2±10.7 mg, with a median onset-arrival time of 1.8 hours. None of the individuals was found symptomatic intracerebral hemorrhage (sICH) or life-threatening systemic bleeding within 36 hours. Only 1 of the alteplase group had severe complication other than hemorrhages, 7 (6.9%) of these had non-severe complications (Supplemental Table 1).
 |
Table 2 The Distribution of Outcomes at Discharge and 90 Days |
The Association Between Alteplase and Outcomes at Discharge
We analyzed the association between alteplase and discharge outcomes with 2 multivariable models and prespecified subgroups of age, gender and NIHSS score (Table 3). Significantly, we found an association of alteplase treatment on favorable functional outcome (higher SIS-16 score) at discharge in univariate analysis (β, 3.99; 95% CI, 0.84–7.13). The association still exist after adjusting for age, gender, and baseline NIHSS (Model 1; β, 5.30; 95% CI, 2.29–8.32) or additionally adjusting for education level, medicare, hypertension, diabetes mellitus, hyperlipidemia, previous stroke, current smoking, atrial fibrillation, coronary artery disease, medication history of anticoagulation, medication history of antiplatelet, onset-arrival time, TOAST classification (Model 2; β, 5.45; 95% CI, 2.22–8.68).
 |
Table 3 Association of Alteplase Treatment with Outcomes at Discharge (No Alteplase as Reference) |
The Association Between Alteplase and Outcomes at 3-Month
In the above-mentioned multivariable models, similar association between alteplase treatment and higher SIS-16 score was also observed at 3-month after stroke (β, 5.45; 95% CI, 2.22–8.68) in Table 4. The clinical outcomes including motor function, cognitive function and emotion were similar between groups in univariate and multivariable analysis at discharge and 3-month.
 |
Table 4 Association of Alteplase Treatment with Outcomes at 90 Days (No Alteplase as Reference) |
Subgroup Analysis
In subgroup analysis restricted to age >60, the associations between alteplase treatment and higher gait speed were observed at discharge (β, 0.13; 95% CI, 0.01–0.25) and 3-month (β, 0.14; 95% CI, 0.02–0.25). When restricting to male, alteplase treatment was associated with lower anxiety score (PHQ-9) at discharge (β, −1.40; 95% CI, −2.37- −0.42), while the association was not observed at 3-month. However, we found alteplase treatment was associated with higher anxiety score (β, 1.54; 95% CI, 0.17–2.91) and depression score (β, 2.23; 95% CI, 0.91–3.55) in female at 3-month. The relationship of alteplase treatment on higher SIS-16 score was consistent between different subgroups of age, gender or NIHSS score (all interaction p > 0.05 at discharge and 3 months). There was no significant difference in the relationship of alteplase treatment on mRS or MoCA outcomes between different subgroups.
Discussion
In this study, we described multidimensional outcomes after alteplase treatment in patients with minor stroke or TIA. We found that 10.9% of minor stroke patients with NIHSS score of 0–5 presented unfavorable functional outcome in mRS at 3-month. Patients with alteplase treatment had a more favorable functional outcome in SIS-16 at 3-month after stroke. In a prespecified analysis restricted to age >60, we identified a better outcome of alteplase treatment in gait speed, while an unfavorable effect was found in anxiety and depression in female.
Patients with minor stroke were generally defined as having a low NIHSS score. However, objectively mild deficits do not necessarily mean non-disabling, especially in posterior circulation stroke.24 Patients may suffer disproportionately impact from minor stroke based on different functional status, profession, or value system. The Potential of rtPA for Ischemic Strokes With Mild Symptoms (PRISMS) trial tested alteplase in minor acute ischemic minor stroke with a new operational definition of nondisabling, which aimed to capture patients who lack evidence of treatment.9 Although the study was terminated early, it enlightened researchers to focus on patients who might benefit from rtPA treatment and the imbalance of treatment benefits in communities.
We found 10.9% of minor stroke patients presented disability (mRS score ≥2) at 3-month, which was lower than the reported 20–30% in previous observational studies.4,25,26 But in accordance with the report from the CNSR-III study, 13.2% minor stroke patients experienced all-cause mortality or major disability in 1-year follow-up.27 Our study provided the association of alteplase with favorable motor function and quality of life at 3-month, which was in accordance with some observational studies. A meta-analysis including seven studies with a total of 1591 minor stroke patients showed that treatment with intravenous thrombolysis was associated with better functional outcome and the treatment-related risk of sICH does not contribute to disability or mortality.28 The Mild and Rapidly Improving Stroke Study (MaRISS) focused on multidimensional outcomes of minor stroke (NIHSS 0–5) and the effect of alteplase at 3-month, a better outcome was identified restricted to the NIHSS of 3–5 group in the SIS-16 but not in mRS.29 We reassured that alteplase treatment was associated with higher quality of life at discharge and 3-month, which was reflected in higher SIS-16 scores. Although the mRS scale was widely used to evaluate the recovery after stroke, the scale may not reflect the extent and the impact to daily life in disability among people with minor strokes.30
The efficacy of alteplase treatment in patients with minor stroke is difficult to interpretation cause the term of minor deficit was defined based on different cut-off points of NIHSS score.6,25,31 We defined the minor stroke in accordance with IST-3 and other recently published studies.9,26 Similar to previous studies, the alteplase treated group had higher baseline NIHSS scores compared with the non-alteplase group.10 Although possible covariates were adjusted in the following multivariable analysis, there may be residual confounding in the initial treatment decision.29 As above-mentioned, more than fifty percent patients included in our study had a baseline NIHSS score of 0–2, and approximal ninety percent achieved a nondisabled outcome (mRS 0–1) at 3-month. The eligibility criterion of “minor” might be essentially close to the definition of the favorable outcome of an mRS score of 0 −1.32 Therefore, it may create a ceiling effect for alteplase to show treatment benefit based on mRS outcome. Previous study has identified that the efficacy of reperfusion treatment depends on the disabling degree of the symptoms at admission.10 In addition, poor outcomes in minor stroke could be related to inadequate detection of functional impairment, such as cognitive or neuropsychological deficits.4 The positive outcome in the SIS-16 showed that this scale may better catch the severity of functional dependence than the scale based on subjective judgment of physicians.33
The Get With The Guidelines Stroke (GWTG-S) registry including 42,394 patients with a baseline NIHSS score of 0–5 untreated with alteplase reported that 27.2% could not ambulate independently at discharge.6 Similar study in Chinese population reported that 11.1% among 6752 minor stroke patients received alteplase had independent mobility difficulty at discharge.34 We observed that alteplase treatment was associated with favorable motor function in gait speed in minor stroke patients with age >60, with no significantly increased risk of sICH. As reported in a pooled analysis of 9 cohort studies, gait speed was associated with survival in community-dwelling older adults, with a pooled hazard ratio of 0.88 (per 0.1m/s).35 The potential cause of the observed benefit in older patients may be that the gait speed declines with age,36 therefore the improvements in gait speed from treatment may be easier to spot in older adults.
In our study, female seemed to have higher scores on the anxiety and depression evaluation after alteplase treatment. Female gender was a significant predictor of post-stroke depression in prospective cohort studies.37 In patients undergoing percutaneous transluminal coronary angioplasty, female was more susceptible to intrusive thought with feelings of anxiety and increased sensitivity.38 Some other factors such as social pressures and life stressors may have different effects on depression and anxiety in different genders.39 This finding implied that post-stroke depression and anxiety should not be underestimated, especially when invasive treatment was applied. And its management calls for a multifaceted approach including social, psychological, and biological dimensions.40
The treatment-related adverse events were not further analyzed in this study because none of the patients presented with symptomatic intracerebral hemorrhage or death during hospitalization. That may be speculated by the large proportion of non-alteplase treated patients in our study, though the proportion of minor stroke patients treated with thrombolysis has increased recently.41 However, the patients in our cohort were managed strictly according to the guidelines, patients accepted thrombolytic therapy would receive electrocardiograph monitoring for at least 24 hours and be reviewed with neuroimaging prior to antiplatelet or anticoagulant medication. Furthermore, previous study also reported a low rate of sICH in the same population.29,42
Our study provided a more nuanced view of multidimensional 3-month outcomes in minor stroke and innovatively analyzed the gait speed as an essential outcome related to life quality to discuss the efficient of thrombolysis. There were also some limitations. First, the potential selection bias cannot be excluded in nonrandomized data. However, CNSR-III multicenter registry enrolled patients prospectively from 201 hospitals that cover 22 provinces and 4 municipalities in China.1 The registry is part of a governmental quality assessment program and was essentially representative of Chinese clinical practice. Second, the neuroimaging markers were not analyzed in our study. Some abnormal parenchymal imaging and large-vessel occlusion were effective predictors of greater disability after minor stroke or TIA,43,44 their relationship to other functional outcomes may also be significant. Finally, since this was an observational registration study, further surveys including interventional trials are needed to test our preliminary results. In particular, randomized controlled trials should be performed to exclude the bias by indication.
Conclusion
Our study identified a suggestion of benefit from alteplase treatment in patients with low NIHSS score of 0–5. An expected efficacy of alteplase was also found in motor function in the older subgroup. Meanwhile, female gender seemed more inclined to post-stroke emotional problems after alteplase treatment, which should be further explored in the future. Thus, we suggest adopting multidimensional tools to correctly evaluate outcomes of patients with mild deficit. Further, randomized clinical trials are needed to confirm these preliminary findings.
Acknowledgments
The authors would like to express sincere gratitude to all participants involved in the study.
Funding
This study was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2019-I2M-5-029) and the National Key R&D Program of China (2022ZD0118003).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang Y, Jing J, Meng X, et al. The Third China National Stroke Registry (CNSR-III) for patients with acute ischaemic stroke or transient ischaemic attack: design, rationale and baseline patient characteristics. Stroke Vasc Neurol. 2019;4(3):158–164. doi:10.1136/svn-2019-000242
2. Dhamoon MS, Moon YP, Paik MC, et al. Long-term functional recovery after first ischemic stroke: the Northern Manhattan Study. Stroke. 2009;40(8):2805–2811. doi:10.1161/STROKEAHA.109.549576
3. Messé S, Khatri P, Reeves MJ, et al. Why are acute ischemic stroke patients not receiving IV tPA? Results from a national registry. Neurology. 2016;87(15):1565–1574. doi:10.1212/WNL.0000000000003198
4. Khatri P, Conaway MR, Johnston KC, et al. Ninety-day outcome rates of a prospective cohort of consecutive patients with mild ischemic stroke. Stroke. 2012;43(2):560–562. doi:10.1161/STROKEAHA.110.593897
5. Smith EE, Abdullah AR, Petkovska I, et al. Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke. Stroke. 2005;36(11):2497–2499. doi:10.1161/01.STR.0000185798.78817.f3
6. Romano JG, Smith EE, Liang L, et al. Distinct short-term outcomes in patients with mild versus rapidly improving stroke not treated with thrombolytics. Stroke. 2016;47(5):1278–1285. doi:10.1161/STROKEAHA.115.011528
7. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals From the American heart association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi:10.1161/STR.0000000000000211
8. Greisenegger S, Seyfang L, Kiechl S, et al. Thrombolysis in patients with mild stroke: results from the Austrian stroke unit registry. Stroke. 2014;45(3):765–769. doi:10.1161/STROKEAHA.113.003827
9. Yeatts SD, Broderick JP, Chatterjee A, et al. Alteplase for the treatment of acute ischemic stroke in patients with low National Institutes of Health Stroke Scale and not clearly disabling deficits (Potential of rtPA for Ischemic Strokes with Mild Symptoms PRISMS): rationale and design. Int J Stroke. 2018;13(6):654–661. doi:10.1177/1747493018765269
10. Merlino G, Smeralda C, Lorenzut S, et al. To Treat or Not to Treat: importance of Functional Dependence in Deciding Intravenous Thrombolysis of “Mild Stroke”. Patients J Clin Med. 2020;9(3):1.
11. Wendt M, Tütüncü S, Fiebach JB, et al. Preclusion of ischemic stroke patients from intravenous tissue plasminogen activator treatment for mild symptoms should not be based on low national institutes of health stroke scale scores. J Stroke Cerebrovasc Dis. 2013;22(4):550–553. doi:10.1016/j.jstrokecerebrovasdis.2013.01.021
12. Liao X, Zuo L, Dong Y, et al. Persisting cognitive impairment predicts functional dependence at 1 year after stroke and transient ischemic attack: a longitudinal, cohort study. BMC Geriatr. 2022;22(1):1009. doi:10.1186/s12877-022-03609-z
13. Adams HP, Biller J. Classification of subtypes of ischemic stroke: history of the trial of org 10172 in acute stroke treatment classification. Stroke. 2015;46(5):e114–e117. doi:10.1161/STROKEAHA.114.007773
14. Liao X, Wang Y, Pan Y, et al. Standard-dose intravenous tissue-type plasminogen activator for stroke is better than low doses. Stroke. 2014;45(8):2354–2358. doi:10.1161/STROKEAHA.114.005989
15. Kollen B, Kwakkel G, Lindeman E. Hemiplegic gait after stroke: is measurement of maximum speed required? Arch Phys Med Rehabil. 2006;87(3):358–363. doi:10.1016/j.apmr.2005.11.007
16. Graham JE, Ostir GV, Fisher SR, et al. Assessing walking speed in clinical research: a systematic review. J Eval Clin Pract. 2008;14(4):552–562. doi:10.1111/j.1365-2753.2007.00917.x
17. Kroenke K, Spitzer R, Williams J. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi:10.1046/j.1525-1497.2001.016009606.x
18. Shen Y, Yuan S, Liu J, et al. The reliability, validity and screening effect of the happiness index scale among inpatients in a general hospital. BMC Psychiatry. 2022;22(1):601. doi:10.1186/s12888-022-04219-0
19. Zietemann V, Georgakis MK, Dondaine T, et al. Early MoCA predicts long-term cognitive and functional outcome and mortality after stroke. Neurology. 2018;91(20):e1838–e1850. doi:10.1212/WNL.0000000000006506
20. van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. doi:10.1161/01.STR.19.5.604
21. Duncan P, Lai SM, Bode RK, et al. Stroke Impact Scale-16: a brief assessment of physical function. Neurology. 2003;60(2):291–296. doi:10.1212/01.WNL.0000041493.65665.D6
22. Gardener H, Romano LA, Smith EE, et al. Functional status at 30 and 90 days after mild ischaemic stroke. Stroke Vasc Neurol. 2022;7(5):375–380. doi:10.1136/svn-2021-001333
23. Beninato M, Portney L, Sullivan P. Using the international classification of functioning, disability and health as a framework to examine the association between falls and clinical assessment tools in people with stroke. Phys Ther. 2009;89(8):816–825. doi:10.2522/ptj.20080160
24. Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5(7):603–612. doi:10.1016/S1474-4422(06)70495-1
25. Ng FC, Coote S, Frost T, et al. Utility of computed tomographic perfusion in thrombolysis for minor stroke. Stroke. 2016;47(7):1914–1916. doi:10.1161/STROKEAHA.116.013021
26. Khatri P, Kleindorfer DO, Yeatts SD, et al. Strokes with minor symptoms: an exploratory analysis of the national institute of neurological disorders and stroke recombinant tissue plasminogen activator trials. Stroke. 2010;41(11):2581–2586. doi:10.1161/STROKEAHA.110.593632
27. Ding Y, Liu L, Chen Z, et al. Serum cystatin c predicts stroke clinical outcomes at 1 year independent of renal function. Front Neurol. 2021;12:676872. doi:10.3389/fneur.2021.676872
28. You S, Saxena A, Wang X, et al. Efficacy and safety of intravenous recombinant tissue plasminogen activator in mild ischaemic stroke: a meta-analysis. Stroke Vasc Neurol. 2018;3(1):22–27. doi:10.1136/svn-2017-000106
29. Romano JG, Gardener H, Campo-Bustillo I, et al. Predictors of outcomes in patients with mild ischemic stroke symptoms: maRISS. Stroke. 2021;52(6):1995–2004. doi:10.1161/STROKEAHA.120.032809
30. Dromerick AW, Edwards DF, Diringer MN. Sensitivity to changes in disability after stroke: a comparison of four scales useful in clinical trials. J Rehabil Res Dev. 2003;40(1):1–8. doi:10.1682/JRRD.2003.01.0001
31. Fischer U, Baumgartner A, Arnold M, et al. What is a minor stroke? Stroke. 2010;41(4):661–666. doi:10.1161/STROKEAHA.109.572883
32. Powers W. Intravenous alteplase for mild nondisabling acute ischemic stroke: a bridge too far? JAMA. 2018;320(2):141–143. doi:10.1001/jama.2018.8511
33. Katzan I, Fan Y, Uchino K, et al. The PROMIS physical function scale: a promising scale for use in patients with ischemic stroke. Neurology. 2016;86(19):1801–1807. doi:10.1212/WNL.0000000000002652
34. Xiong Y, Yan R, Gu H, et al. Intravenous thrombolysis in Chinese patients with mild acute ischemic stroke. Ann Transl Med. 2021;9(9):767. doi:10.21037/atm-21-40
35. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi:10.1001/jama.2010.1923
36. Cai Y, Tian Q, Gross AL, et al. Motor and physical function impairments as contributors to slow gait speed and mobility difficulty in middle-aged and older adults. J Gerontol a Biol Sci Med Sci. 2022;77(8):1620–1628. doi:10.1093/gerona/glac001
37. Sharpe M, Hawton K, Seagroatt V, et al. Depressive disorders in long-term survivors of stroke. Associations with demographic and social factors, functional status, and brain lesion volume. Br J Psychiatry. 1994;164(3):380–386. doi:10.1192/bjp.164.3.380
38. Edéll-Gustafsson U, Hetta J. Fragmented sleep and tiredness in males and females one year after percutaneous transluminal coronary angioplasty (PTCA). J Adv Nurs. 2001;34(2):203–211. doi:10.1046/j.1365-2648.2001.01746.x
39. Pase MP. The association between sleep duration and stroke differs by race and sex. Neurology. 2018;91(18):e1728–e1731. doi:10.1212/WNL.0000000000006420
40. Hachinski V. Post-stroke depression, not to be underestimated. Lancet. 1999;353:9166.
41. Asdaghi N, Wang K, Ciliberti-Vargas MA, et al. Predictors of thrombolysis administration in mild stroke: Florida-Puerto Rico collaboration to reduce stroke disparities. Stroke. 2018;49(3):638–645. doi:10.1161/STROKEAHA.117.019341
42. Romano JG, Smith EE, Liang L, et al. Outcomes in mild acute ischemic stroke treated with intravenous thrombolysis: a retrospective analysis of the get with the guidelines-stroke registry. JAMA Neurol. 2015;72(4):423–431. doi:10.1001/jamaneurol.2014.4354
43. Coutts S, Modi J, Patel SK, et al. What causes disability after transient ischemic attack and minor stroke?: results from the CT and MRI in the Triage of TIA and minor Cerebrovascular Events to Identify High Risk Patients (CATCH) Study. Stroke. 2012;43(11):3018–3022. doi:10.1161/STROKEAHA.112.665141
44. Xie X, Jing J, Meng X, et al. Predictive Value of the ABCD3-I for Short- and Long-Term Stroke after TIA with or without sICAS. J Atheroscler Thromb. 2021;29(9):1372–1382. doi:10.5551/jat.63050
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

