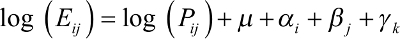Back to Journals » Cancer Management and Research » Volume 11
Mortality trends of bladder cancer in China from 1991 to 2015: an age-period-cohort analysis
Authors Yang Y, Cheng Z, Jia X, Shi N, Xia Z, Zhang W, Shi X
Received 30 September 2018
Accepted for publication 17 December 2018
Published 10 April 2019 Volume 2019:11 Pages 3043—3051
DOI https://doi.org/10.2147/CMAR.S189220
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Yongli Yang,1 Zhiwei Cheng,2 Xiaocan Jia,1 Nian Shi,3 Zhenhua Xia,1 Weiping Zhang,1 Xuezhong Shi1
1Department of Epidemiology and Biostatistics, College of Public Health, Zhengzhou University, Zhengzhou, Henan, China; 2Department of Case Management, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China; 3Department of Physical Diagnosis, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
Purpose: The effects of age, period, and cohort on mortality rates of bladder cancer in China remained vague. This study aimed to analyze the secular trends of bladder cancer mortality in China and estimate the independent effects of age, period, and cohort.
Methods: Data for bladder cancer mortality from 1991 to 2015 was obtained from the WHO Mortality Database and China Health Statistical Yearbook. The age-period-cohort model was used to estimate the effect of age, period, and cohort. The intrinsic estimator method was used to solve the nonidentification problem of collinearity among age, period, and cohort.
Results: The age-standardized mortality rates of total residents (2.33–1.87/100,000), male (3.45–2.89/100,000), and female (1.24–0.82/100,000) showed decreasing trends, which was more obvious in males than in females. Age effects increased consistently with age in all age groups (coefficients: –2.02 to 1.91 in the total population, –2.06 to 2.02 in males and –2.04 to 1.81 in females). Cohort effects decreased overall (coefficients: 0.96 to –1.62 in the total population, 1.11 to –1.66 in males and 0.78 to –1.46 in females). Period effects were not found in China.
Conclusion: Although a decreasing mortality was observed, the bladder cancer burden in China will likely increase in the next few years due to population aging, environmental pollution, and food safety. The findings suggested that preventive measures should be taken corresponding to the changes in age-and cohort-related factors in the population.
Keywords: bladder cancer, age effect, period effect, cohort effect, intrinsic estimator
Introduction
As one of the most common cancer of the urinary system, bladder cancer is the 9th most common cancer and 14th leading cause of cancer deaths worldwide, with an estimated 430,000 new cases and 165,100 deaths in 2012.1 Bladder cancer incidence and mortality rates have decreased slightly in most developed countries.2 Incidence and mortality rates in general decreased in most Western countries but increased in some Eastern European and developing countries, which leads to the burden of bladder cancer has been increasing.3 These changes could be caused by the exposure to risk factors for bladder cancer, including tobacco consumption, air pollution, urbanization and industrialization, and unhealthy lifestyles, which could be summarized as a cohort effect.4 In addition, the aging population, the development of death registration systems and changes in the ICD also contributed to changes in bladder cancer burden.5 We classified these factors as age effect and period effect. Pou et al6 used the age-period-cohort (APC) model to analyze the effects of age, period, and cohort on the mortality of bladder cancer in Córdoba, a province of Argentina, and suggested that mortality of bladder cancer is influenced by age and cohort.
China has experienced rapid economic growth and social change since the adoption of economic reforms from 1978 onward. As a result of this process, the epidemiological transition and the attendant rise in the chronic disease burden have taken place more rapidly than other countries.7 Bladder cancer is the sixth most common cancer in males and 15th in females according to the National Central Cancer Registry (NCCR) of China 2015 annual report.8 The incidence and mortality rates in China have increased gradually9 and shown different trends between males and females10 over the past few years. Increasing trends in incidence were observed for bladder cancer from 1973 to 2010 in urban Shanghai.5 Han et al11 and Wen et al 12 have examined the time trends of bladder cancer mortality using the data from the NCCR, but these studies did not reveal the effects of age, period, and cohort on bladder cancer mortality trends. Guo et al10 has estimated the bladder cancer cohort effect with generalized additive models using the national data, whereas the study ignored the effects of age and period due to the nonidentification problem of collinearity among age, period, and cohort.
Up until the present day, the effects of age, period, and cohort on bladder cancer mortality trends in China have remained vague. In this study, we examined the secular trends in bladder cancer mortality in China during the period 1991–2015 and analyzed the independent effects of chronological age, time period, and birth cohort simultaneously using the APC model combined with the intrinsic estimator (IE) algorithm, which could solve the nonidentification problem mentioned above.
Methods
Materials
The bladder cancer mortality data accessed is freely available from the WHO Cancer Mortality Database (International Agency for Research on Cancer, Lyon, France) for the period 1991–2000 and the China Public Health Statistical Yearbooks for the period 2002–2015. We replaced data in 2001 with the average of the four neighboring calendar years for 1999–2003 as it was unavailable.13 Data of 31 provinces was reported by the Center for Health Information and Statistics of China, which is an annual disease surveillance system set up in 1973 and based upon a 10% sample of the population.14
Bladder cancer patients aged 35–84 years were included in this study for the following reasons: the group aged ≥85 years did not meet the requirements of the APC models; the intervals of age and cohort need to be consistent; and the bladder cancer mortality of younger groups aged <35 years was approximately zero. Age, period, and cohort models were based on ten 5-year age intervals beginning at age 35 years (35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84) and five 5-year period groups (1991–1995, 1996–2000, 2001–2005, 2006–2010, 2011–2015). Age-standardized mortality rates (ASMRs) were calculated based on the data of the National Population Sampling Survey in 2015.
Methods
The APC model was used to estimate the effect of age, period, and cohort on bladder cancer mortality and the IE method was used to solve the nonidentification problem that the age, period, and cohort were collinear.15 The equation of the multiplicative APC model for mortalities rates is denoted as:
|
where Eij and Pij denote the death and exposure number in cell (i, j); m denotes the intercept death rate; ai denotes the coefficient for the ith age group; bj denotes the coefficient for the jth time period; gk denotes the coefficient for the kth cohort for cohorts. The cohort could be calculated according to the following equation: k = j - i + 1. The whole coefficients were parameterized using the IE method.15 Significant curvatures were assessed using chi-square test.16,17 The Akaike information criterion and the Bayesian information criterion were used to evaluate the goodness-of-fit of the model. STATA 12 was used to fit the model and graphics were manufactured by R 3.2.5 software. The significant level was 0.05.
Results
Overall trends of bladder cancer mortality rates
Figure 1 shows the ASMRs of bladder cancer from 1991 to 2015. Decreasing trends was observed in male, female, and total residents. The rate of decrease was more obvious in males than females.
  | Figure 1 Trends of ASMRs/100,000 population for bladder cancer in China. Abbreviation: ASMRs, age-standardized mortality rates. |
Age-specific bladder cancer mortality rates by time period and cohort
Age-specific bladder cancer mortality rates by period are shown in Figure 2. Overall bladder cancer mortality rates increased with age and this trend was more obvious in the >65 years age groups. The mortality rate for those aged 80–84 years was nearly 300-fold higher than for those aged 35–39 years in total residents. The multiples were 500 in male residents and 100 in female residents.
Period-specific bladder cancer mortality rates by age group
Figure 2 plots period-specific bladder cancer mortality rates by age group. The mortality rates showed stable trends in the ≤64 years age groups and a rapidly decreasing trend in the 65–79 years age groups. In the 80–84 years age group, the mortality rates during the period 2001–2005 were lower than the other periods.
Cohort-specific bladder cancer mortality rates by age group
As Figure 2 shows, the cohort-specific mortality rates of bladder cancer shared analogous trends with period-specific bladder cancer mortality rates. Mortality rates fluctuated slightly in the ≤64 years age groups and the >64 years age groups had a higher risk of mortality than the ≤64 years age groups. Bladder cancer mortality increased with age during the same cohort, especially in cohorts before 1946.
APC model
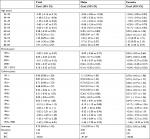
Table 1 shows the results of the full APC IE model. The net effects of age, period, and cohort on overall mortality trends can be seen in Figure 3. The separate effects of age, period, and cohort can be seen in Table 1.
Age effect
The coefficients of age effect increased consistently in all age groups. The age effect was found to be stronger in females than males aged ≤59 years and the age effect was more pronounced in males than females aged >59 years. Therefore, the effect of age on mortality increased faster in the male residents. Furthermore, the population aged ≤49 years showed lower risk among male residents. The high-risk groups are aged >65 years for males and >70 years for females.
Period effect
As Table 1 shows, the mortality rates of bladder cancer did not change during the observed periods. Therefore, there was no evidence to prove the period was a contributing factor in the trend of mortality rates.
Cohort effect
The cohort effects decreased in the male, female, and total population. In males, the cohort coefficients before 1931 were greater than zero and have statistical significance. This reveals that the population born before 1931 showed a greater risk of death from bladder cancer for male residents. As for females, none of the cohort coefficients had statistical significance. This finding indicates that the cohort is a factor that affects bladder cancer mortality in males only.
Discussion
In this study, bladder cancer mortality data from 1991 to 2015 was obtained to examine the secular trend of bladder cancer mortality in mainland China and APC analysis was used to explored age, period, and cohort effects. The results showed that the ASMRs presented a decreasing trend, which was more obvious in males than females. Furthermore, age effects increased consistently with age in all age groups, cohort effects decreased overall in the total population, and period effects were not observed in this study. These detections not only provide a comprehensive and up-to-date overview of temporal trends in bladder cancer mortality in China over the past 25 years but also produce some evidence for the prevention and control of bladder cancer.
The overall decreasing trends were observed in the total population and population by gender, which was clearer in males, consistent with the results of Guo et al10 and Antoni et al.2 Bladder cancer incidence was influenced by the environmental, industrial, and economic development of China, but the mortality is mainly determined by the treatment. Health care policy with regard to cancer, such as early detection and early diagnosis, is effective for improving treatment quality and reducing the mortality rate of bladder cancer in China. The disparity among genders may be interpreted by differential exposure to carcinogens and genetic and environmental factors, especially tobacco consumption.18 Nonetheless, prospects for bladder cancer burden in China are currently grim and will remain so during the next few years due to population aging, poor air quality, problematic food safety, and massive tobacco consumption.19 Smokers have about 50% increase in the incidence of bladder cancer mortality compared with nonsmokers.20 Therefore, tobacco control, environmental protection, early screening of bladder cancer and the potential impact of lifestyle, diet, inflammation status, or surgical treatment, such as transurethral resection, should be considered to control the mortality of bladder cancer.21,22
The age effect on bladder cancer mortality showed an uptrend in all populations, especially in males. The risk of mortality increased with age, which is analogous to the study of Olfert et al.4 The lengthy latent period may explicate the finding. There is often an interval of several decades between the first exposure to a carcinogen and the clinical appearance of the disease for most human cancers.23 Hence, many cases of bladder cancers are diagnosed and reported in the elderly. Furthermore, that the elderly may experience greater exposure to tobacco smoking and occupational risk factors is another interpretation for this finding. However, population aging in China has been rising owing to a sharp decline in fertility and prolongation of life expectancy. The percentage of people aged ≥65 years increased from 4.91% in 198224 to 10.47% in 2015,25 so we inferred bladder cancer burden in China will likely increase in the next few years.
Period effects usually indicate the impact of factors with immediate effects on cancer mortality and incidence, such as new diagnoses, improved medical interventions, or a change in ascertaining or coding the cause of mortality. The impact of the period for both males and females is not found in this study. One possible reason is that the study period (1991–2015) was relatively short, just as in the Argentinian study.6 An alternative reason may be that the effect of period on cancer mortality was small because of the highly developed of clinical treatment and screening program.26,27
The effects of the cohort showed a downward trend as a whole. This finding might be explained by the improved nutrition, living conditions, and health care services, which could reduce the risk factor exposure in younger cohorts. It has been confirmed that the status of medical insurance is closely related to chronic disease mortality, ignoring the impact of lifestyle.28 In China, three important medical security systems have been established since the 1990s, and the “Decision of the State Council on Setting up a Basic Medical Insurance System for Staff Members and Workers in Cities and Towns” was enacted in 1998. Furthermore, compared to the elderly cohorts, the younger cohorts had better nutrition and living conditions. The kindred trends were also found in other cancers’ mortality rates, eg, breast cancer, lung cancer, and cervical cancer.26,29,30 This phenomenon might highlight that the Chinese population has greatly benefited from improved nutrition, living conditions, and health care services due to rapid economic development and that this weakened the cohort effects.31
In this study, the APC model combined with the IE algorithm was used to solve the nonidentification problem of collinearity among age, period, and cohort, which produced a more reliable result. However, only 25 years of bladder cancer mortality rates were employed in this study because the disease surveillance system was perfected electronically in 1990s, which may affect the impact of the period on bladder cancer mortality rates. In particular, 2001 data was unavailable and an estimated value was input. Whether different imputation methods had an influence on the results is unclear.
Conclusion
This study clearly and simultaneously estimates the effects of age, period, and cohort on bladder cancer mortality. The findings indicated age and cohort effects exited in China population, which suggested that preventive measures should be taken corresponding to the changes in age-and cohort-related factors in the population.
Disclosure
The authors report no conflicts of interest in this work.
References
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. | ||
Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96–108. | ||
Bournele D, Beis D. Zebrafish models of cardiovascular disease. Heart Fail Rev. 2016;21(6):803–813. | ||
Olfert SM, Felknor SA, Delclos GL. An updated review of the literature: risk factors for bladder cancer with focus on occupational exposures. South Med J. 2006;99(11):1256–1263. | ||
Bao PP, Zheng Y, Wu CX, et al. Cancer incidence in urban Shanghai, 1973–2010: an updated trend and age-period-cohort effects. BMC Cancer. 2016;16(1):284. | ||
Pou SA, Osella AR, Diaz Mdel P. Bladder cancer mortality trends and patterns in Córdoba, Argentina (1986–2006). Cancer Causes Control. 2011;22(3):407–415. | ||
Yang G, Kong L, Zhao W, et al. Emergence of chronic non-communicable diseases in China. Lancet. 2008;372(9650):1697–1705. | ||
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. | ||
Pang C, Guan Y, Li H, Chen W, Zhu G. Urologic cancer in China. Jpn J Clin Oncol. 2016;46(6):497–501. | ||
Guo P, Huang ZL, Yu P, Li K. Trends in cancer mortality in China: an update. Ann Oncol. 2012;23(10):2755–2762. | ||
Han SJ, Zhang SW, Chen WQ, Li CL. Analysis of the status quo and trends: mortality in patients with bladder cancer in China. J Mod Urol. 2013;18:228–232. | ||
Wen DG, Shan BE, Zhang SW, et al. Analysis of incidence and mortality rates of bladder cancer in registration areas of China from 2003 to 2007. Tumor. 2012;32(4):256–262. | ||
Guo P, Li K. Trends in esophageal cancer mortality in China during 1987–2009: age, period and birth cohort analyzes. Cancer Epidemiol. 2012;36(2):99–105. | ||
Li M, Wan X, Wang Y, Sun Y, Yang G, Wang L. Time trends of esophageal and gastric cancer mortality in China, 1991–2009: an age-period-cohort analysis. Sci Rep. 2017;7(1):6797. | ||
Yang Y, Schulhofer-Wohl S, Fu WJ, Land KC. The intrinsic estimator for Age-Period-Cohort analysis: what it is and how to use it. Am J Sociol. 2008;113(6):1697–1736. | ||
Clayton D, Schifflers E. Models for temporal variation in cancer rates. I: Age-period and age-cohort models. Stat Med. 1987;6(4):449–467. | ||
Clayton D, Schifflers E. Models for temporal variation in cancer rates. II: Age-period-cohort models. Stat Med. 1987;6(4):469–481. | ||
Shariat SF, Sfakianos JP, Droller MJ, Karakiewicz PI, Meryn S, Bochner BH. The effect of age and gender on bladder cancer: a critical review of the literature. BJU Int. 2010;105(3):300–308. | ||
Chung RY, Schooling CM, Cowling BJ, Leung GM. How does socioeconomic development affect risk of mortality? An age-period-cohort analysis from a recently transitioned population in China. Am J Epidemiol. 2010;171(3):345–356. | ||
Liu W, Zhao X, Zhong Z. The role of tobacco smoke in bladder and kidney carcinogenesis: a comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol. 2016;70(4):e104–e105. | ||
Ferro M, Di Lorenzo G, Buonerba C, et al. Predictors of residual T1 high grade on re-transurethral resection in a large multi-institutional cohort of patients with primary T1 High-Grade/Grade 3 bladder cancer. J Cancer. 2018;9(22):4250–4254. | ||
Matteo F, Dorin M V, Francesco C, et al. High-grade T1 on re-transurethral resection after initial high-grade T1 confers worse oncological outcomes: results of a multi-Institutional Study. Urol Int. 2018;101(1):7–15. | ||
Levi F, La Vecchia C. Age, cohort and period effects on large bowel cancer incidence. Eur J Cancer Prev. 2002;11(6):515–517. | ||
Zhu J, Chen JG, Zhang YH, Chen YS, Ding LL. [Trend on mortality changes for lung cancer during 1972–2011 in Qidong, Jiangsu]. Zhonghua Liu Xing Bing Xue Za Zhi. 2012;33(9):933–936. Chinese. | ||
Wang YW, Chen HH, Wu MS, Chiu HM; Taiwanese Nationwide Colorectal Cancer Screening Program. Current status and future challenge of population-based organized colorectal cancer screening: lesson from the first decade of Taiwanese program. J Formos Med Assoc. 2018;117(5):358–364. | ||
Wang J, Bai Z, Wang Z, Yu C. Comparison of secular trends in cervical cancer mortality in China and the United States: an age-period-cohort analysis. Int J Environ Res Public Health. 2016;13(11):1148. | ||
Vartolomei MD, Ferro M, Cantiello F, et al. Validation of neutrophil-to-lymphocyte ratio in a multi-institutional cohort of patients with T1G3 non-muscle-invasive bladder cancer. Clin Genitourin Cancer. 2018;16(6):445–452. | ||
Bittoni MA, Wexler R, Spees CK, Clinton SK, Taylor CA. Lack of private health insurance is associated with higher mortality from cancer and other chronic diseases, poor diet quality, and inflammatory biomarkers in the United States. Prev Med. 2015;81:420–426. | ||
Li C, Yu C, Wang P. An age-period-cohort analysis of female breast cancer mortality from 1990–2009 in China. Int J Equity Health. 2015;14(1):76. | ||
Wang L, Yu C, Liu Y, et al. Lung cancer mortality trends in China from 1988 to 2013: new challenges and opportunities for the government. Int J Environ Res Public Health. 2016;13(11):1052. | ||
Ferro M, Vartolomei MD, Russo GI, et al. An increased body mass index is associated with a worse prognosis in patients administered BCG immunotherapy for T1 bladder cancer. World J Urol. 2018; 384(9945). |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.