Back to Journals » International Journal of General Medicine » Volume 16
Monocyte-to-High Density Lipoprotein Cholesterol Ratio Positively Predicts Coronary Artery Disease and Multi-Vessel Lesions in Acute Coronary Syndrome
Authors Du GL, Liu F, Liu H, Meng Q , Tang R , Li XM, Yang YN , Gao XM
Received 2 May 2023
Accepted for publication 23 August 2023
Published 28 August 2023 Volume 2023:16 Pages 3857—3868
DOI https://doi.org/10.2147/IJGM.S419579
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Yuriy Sirenko
Guo-Li Du,1,2,* Fen Liu,1,3,4,* Hua Liu,1,2,* Qi Meng,1,2 Ran Tang,1,2 Xiao-Mei Li,1,3 Yi-Ning Yang,1,5 Xiao-Ming Gao1,3,4
1State Key Laboratory of Pathogenesis, Prevention, and Treatment of High Incidence Diseases in Central Asia, Urumqi, People’s Republic of China; 2Department of Endocrinology, First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, People’s Republic of China; 3Department of Cardiology, First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, People’s Republic of China; 4Xinjiang Key Laboratory of Medical Animal Model Research, Clinical Medical Research Institute of First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, People’s Republic of China; 5People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, Xinjiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yi-Ning Yang; Xiao-Ming Gao, Email [email protected]; [email protected]
Purpose: We investigated the hypothesis that MHR (monocyte-to-high density lipoprotein cholesterol ratio) is related to the severity of coronary artery in ACS (acute coronary syndrome).
Methods: In this case–control study, we recruited 15,853 participants undergoing the first time percutaneous coronary intervention (PCI) including 4093 normal controls, 10,518 chronic coronary artery disease (CAD), and 1242 ACS cases. Examination of demographic clinical data and biochemical profiles, as well as MHR values, were performed before PCI. The relationship between MHR and severity of coronary artery lesion in ACS was analyzed. We also used a flow cytometric assay to distinguish CD14+/CD16- classical monocyte subsets in peripheral blood mononucleated cells from CAD patients.
Results: MHR was higher in patients with ACS compared with MHR in normal control and chronic CAD (normal control vs chronic CAD vs ACS: 0.46 ± 0.27 × 109/mmol vs 0.53 ± 0.29 × 109/mmol vs 0.73 ± 0.47 × 109/mmol, P < 0.001). MHR showed a significantly progressive increase as the angiographic severity of coronary lesions increased (single vessel lesion vs multi-vessel lesions in ACS: 0.54 ± 0.31 × 109/mmol vs 0.58 ± 0.35 × 109/mmol, P < 0.001), and classical monocyte subset to HDL-C ratio (CMHR) was increased in with CAD patients compared with control [4.69 (IQR, 1.06, 2.97) × 103/mmol vs 1.92 (IQR, 0.92, 3.04) × 103/mmol, P = 0.02]. Using a multivariate analysis, after adjusting for age, gender, body mass index (BMI), diabetes, and dyslipidemia, MHR was positively associated with multi-vessel lesions in ACS [OR (odds ratio): 1.28 (95% CI: 1.03– 1.59, P = 0.029)].
Conclusion: MHR level could be a potential predictor of coronary artery lesion severity in ACS.
Keywords: MHR, CAD, ACS, multi-vessel lesions, CMHR
Introduction
Coronary heart disease (CHD) remains the leading cause of death worldwide.1 Coronary atherosclerosis is considered to be a prerequisite for coronary events.2 Acute coronary syndrome (ACS) presents as unstable angina and/or acute myocardial infarction and for the reason of myocardial ischemia which may require immediate reperfusion therapy.2 The complexity of atherosclerotic cardiovascular disease pathogenesis poses significant challenges to making decisions about effective and targeted treatments. The use of multi-marker algorithms will improve predicting cardiovascular disease risk and decision-making.3
Inflammation has been shown to be an important factor in promoting the development of atherosclerosis.4 The atherosclerotic plaque develops as the monocytes infiltrate the intimal area of the coronary artery and mature into a macrophage and phagocytose oxidized low-density lipoprotein.5 Chronic low-grade inflammation causes the plaque’s destabilization and promotes plaque lesion progression and further plaque rupture results in obstructed blood flow. As a consequence, ACS leads to myocardial necrosis. Inflammation responds immediately at the site of necrosis and affects cardiac remodeling and recovery from myocardial infarction.6,7
Circulating monocytes, monocytes from the splenic reservoir, and newly formed monocytes in the bone marrow are mobilized and recruited to the site of necrosis and are involved in healing after AMI.8,9 TAPP et al showed that blood concentration of classical monocytes (CD14++CD16-) and intermediate monocytes (CD14++CD16+) in peripheral blood peaked on day one and three following an MI onset.10 Monocytes significantly correlated with biomarkers, cardiac markers, and clinical outcomes such as left-ventricle ejection fraction (LVEF) regarding ACS and may be a prognostic factor in MI recovery.11
Monocyte-to-lymphocyte ratio (MLR) and other hematological indices had been testified to their prognostic and diagnostic value in severity of coronary artery disease and metabolic diseases.12,13 Monocyte count to HDL cholesterol ratio (MHR), as an inflammatory prognostic maker, had been proved to play roles in prognosticating in-hospital major adverse cardiac events and mortality in patients with ST-segment elevation myocardial infarction (STEMI) undergoing the first time percutaneous coronary intervention (PCI) and angiographic no-reflow after PCI.14–19 However, the role of MHR in the prediction of ACS severity is still unknown. Therefore, in this study, we hypothesized MHR is an independent risk factor of multi-vessel lesions in ACS and could predict the severity of ACS and be valuable in the patient risk-stratify.
Materials and Methods
Study Population
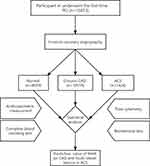
We designed this one-center, case-controlled trial and recruited 15,853 participants who underwent the first time PCI between January 2015 and January 2018 in the First Affiliated Hospital of Xinjiang Medical University. All participants provided written informed consent. The participants included patients presenting with ACS, patients presenting with chronic CAD (patients who had coronary sclerosis but did not present acutely), and participants with normal angiography, Figure 1. Patients were excluded if they had regional wall motion abnormalities, relevant valvular abnormalities in echocardiograms, carotid atherogenesis, acute inflammatory diseases, tumors, autoimmune disease, and severe hepatic and renal dysfunction. The study was following the Declaration of Helsinki guidelines and was approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University.
Anthropometric Measurements
An interview-based questionnaire was used to collect the following information: general health status, demographic status, cardiovascular risk factors (including hypertension, diabetes, smoking, drinking et al), medical history [including history of medication, stroke and CVD (CVD: cerebrovascular disease)] and clinical presentation. Evidence of CAD in a parent or sibling was considered as a positive family history of CAD. We also performed a physical examination in which height, body weight (BW), systolic blood pressure (SBP) and diastolic blood pressure (DBP), etc. The formula of weight BW (kg)/height (m2) was used to calculate the BMI. Hypertension was defined as systolic pressure (SBP) ≥140 mmHg and/or diastolic pressure (DBP) ≥90 mmHg or self-reported use of antihypertensive medication regardless of measured blood pressure.
Complete Blood Counting Test and Biochemical Assays
Complete blood counts and biochemical tests were performed at the Clinical Laboratory Center of the First Affiliated Hospital of Xinjiang Medical University. Complete blood counts including the concentration of white blood cells, neutrophils, lymphocytes, monocyte, hemoglobin, and platelet were performed by a Sysmex XN 2000 hematology analyzer (Tokyo, Japan). Venous blood was collected in a 5-mL tube with EDTA and processed to obtain plasma within 4 hours in the cardiology department of our hospital. The serum concentration of total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride, creatinine (Cr), and blood glucose were measured using equipment for chemical analysis (Dimension AR/AVL Clinical Chemistry System, Newark, NJ, USA).
Flow Cytometry
The patient’s whole blood was separated at 3500 × g for 15 min at room temperature. Ficoll-Paque PLUS was added to the tubes with the plasma, and the tubes were centrifuged for 30 minutes at 400 × g at room temperature. The supernatants were then centrifuged at 400 × g at room temperature for 10 min. Peripheral blood mononuclear cells (PBMCs) were gathered following centrifugation. Peripheral blood mononuclear cell phenotypic identification with flow cytometry. PBMCs were washed twice with cold PBS and incubated with FITC-labeled CD14 and PE-labeled CD16 antibodies for 30 min at 4°C in the dark. After washing with cold PBS, cell preparations were immediately analyzed on an LSRFortessa flow cytometer (BD Immunocytometry Systems, San Jose, CA, USA). Data were analyzed using FlowJo software (version V10; Tree Star, Inc., Ashland, OR, USA). Information on the antibodies used in this assay is given in Supplemental Table S1.
Invasive Coronary Angiography
Using the Seldinger technique, invasive coronary angiography was performed using the radial and femoral routes. All PCI procedures were performed by experienced interventional cardiologists. At least three experienced interventional cardiologists reviewed the PCI tomography and decided on the vessel lesion numbers according to angiographic measurements. It was defined as multi-vessel lesions in ACS if the number of vessel lesions was two or more.
Statistical Analysis
Data analysis was performed using the SPSS version 26.0 statistical software package (Chicago, IL, USA). Normally distributed data were expressed as the mean ± SD and analyzed using a t-test. Non-normally distributed data were expressed using the median with interquartile range (IQR) and analyzed by the rank-sum test. Categorical variables were compared using the Chi-square or Fisher’s test. Following univariate analysis, multivariate logistic regression models were built to analyze the possible predictors of CAD, ACS, and coronary artery lesion severity in ACS. We analyzed associated factors of coronary artery disease by comparing them to normal control, analyzed associated factors of ACS by comparing them to CAD, and analyzed associated factors of coronary artery lesion severity by comparing them to single vessel lesions in ACS. The receiver operating characteristic (ROC) curves were drawn using the GraphPad Prism 8 software. It was considered statistically significant as P < 0.05.
Results
Clinical and Laboratory Properties of ACS, Chronic CAD, and Control Subjects
The basal demographic properties, cardiovascular risk factors, medication history, comorbidities, lipid profiles, creatinine levels, coronary vessel lesions and hematological variables of the study groups and subgroups are shown in Table 1 and Supplemental Table S2. In this study, a total of 15,853 participants had percutaneous coronary intervention (Figure 2); 4093 patients (25.8%) had normal coronary arteries; 10,518 patients (66.3%) had chronic CAD, and 1242 patients (7.8%) were diagnosed as ACS. Compared with the normal group, patients of chronic CAD and ACS tended to be older, obese, more often male, and had higher blood pressure, blood glucose, and more cardiovascular risk factors, such as hypertension, family history of CAD, dyslipidemia, and lower LVEF. ACS patients tended to take more medications such as aspirin, angiotensin-converting enzyme inhibitors (ACEI) ⁄ angiotensin receptor blockers (ARB), and statins compared with normal control, but less than chronic CAD patients.
 |
Table 1 Clinical and Laboratory Properties of Study Groups |
Compared with ACS patients with single vessel lesions, those with multi-vessel lesions groups had more family history of CAD, higher creatinine levels, more hypertension, higher blood glucose, higher neutrophil, lower lymphocyte, and lower hemoglobin (P < 0.05 respectively). Also, ACS patients with multi-vessel lesion groups took more medicine including aspirins, ACEI ⁄ ARB, and statins (P < 0.05, respectively, Table 1).
Clinical and Laboratory Properties of the Study Groups According to the Median of MHR
We also categorized participants into two groups according to a median of MHR on admission (MHR median: 0.46; lower half: ≤ 0.46, n = 7938; upper half: > 0.46, n = 7916). Table 2 presents the clinical, and laboratory properties of the study groups according to a median of MHR on admission. Patients with MHR higher than 0.46 showed young age, more males, higher BMI, blood glucose and hypertension, lower LVEF, higher rates of diabetes, smoking, and dyslipidemia (P < 0.001 respectively); lower level of total cholesterol, LDL cholesterol, HDL cholesterol and higher level of triglycerides and creatinine (P < 0.001 respectively); higher WBC count, neutrophil count, hemoglobin and platelet count and lower level of lymphocyte count (P < 0.001 respectively).
 |
Table 2 Clinical and Laboratory Characteristics of Participants Based on Median MHR |
Angiographic Severity of Coronary Artery Disease and MHR Levels
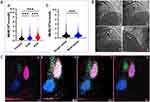
Figure 2A presents MHR levels according to the angiographic severity of coronary artery disease in ACS. Compared with normal control, the MHR level in both chronic CAD, and total ACS groups increased significantly (0.46 ± 0.27 × 109/mmol vs 0.53 ± 0.29 × 109/mmol vs 0.73 ± 0.47 × 109/mmol, P < 0.001). In the subgroup of ACS, MHR showed a significantly progressive increase in ACS patients with multi-vessel compared with those with single vessel lesions (0.54 ± 0.31 × 109/mmol vs 0.58 ± 0.35 × 109/mmol, P < 0.001, Figure 2B). Figure 2C also presents representative monocytes count in control, chronic CAD, and ACS patients (single vessel and multi-vessel lesions).
Flow Cytometry Identification of Classical Monocytes
The monocyte subsets in PBMCs from healthy and CAD were analyzed by flow cytometry. We used an exclusion gating strategy to identify monocytes and to separate the CD14+/CD16- classical monocytes (CM) and the CD14low/CD16+ nonclassical monocytes (NCM) (Figure 3A). Compared with normal control, the CM concentration in the chronic CAD group increased significantly [1.64 (IQR, 0.57, 2.52) ×103/L vs 4.12 (IQR, 2.50, 7.22) ×103/L, P = 0.002, Figure 3B]. And the CMHR level of CAD group was significantly higher than the control group [1.92 (IQR, 0.92, 3.04) ×103 /L vs 4.69 (IQR, 1.06, 2.97) × 103/L, P = 0.02, Figure 3C].
Predictors Analysis of ACS
Firstly, a univariate logistic regression model was built to analyze the possible predictors of total CAD (including chronic CAD and ACS, P < 0.05), and a significant association was found between MHR and total CAD (Supplemental Table S3). We found that age, male, body mass index, blood glucose, triglycerides level, WBC count, neutrophil count, platelet count, and MHR were significantly associated with CAD.
Multivariate logistic regression analysis showed that male (OR: 1.68; 95% CI: 1.37–2.08, P < 0.001), BMI (OR: 1.50; 95% CI: 1.26–1.80, P<0.001), LVEF (OR: 0.98; 95% CI: 0.97–0.99, P < 0.001), neutrophil count (OR: 1.03; 95% CI: 1.03–1.04, P < 0.001), platelet count (OR: 1.00; 95% CI: 1.00–1.01, P < 0.001) and MHR (OR: 2.85; 95% CI: 2.31–3.53, P < 0.001) were independent predictors of ACS after adjustment of all other variables (Supplemental Table S4).
Predictors Analysis of ACS with Multi-Vessel Lesions
According to univariate analysis, compared with single vessel lesions in ACS patients, we found that age, male, body mass index, blood glucose, WBC count, neutrophil count, lymphocyte count, platelet count, and MHR were significantly associated with multi-vessel lesions in ACS patients (P < 0.05). Multivariate logistic regression analysis showed that age (OR: 1.02; 95% CI: 1.02–1.03, P < 0.001), male (OR: 1.49; 95% CI: 1.32–1.67, P < 0.001), BMI (OR: 1.02; 95% CI: 1.01–1.03, P = 0.003), blood glucose (OR: 1.06; 95% CI: 1.04–1.07, P < 0.001), platelet count (OR: 1.00; 95% CI: 1.00–1.00, P < 0.001) and MHR (OR: 1.28; 95% CI: 1.03–1.59, P = 0.029) were independent predictors of multi-vessel lesions in ACS after adjustment of all other variables (Table 3).
 |
Table 3 Univariate and Multivariate Logistic Regression Analyses for Predictors of ACS Patients with Multi-Vessel Lesions |
The ROC analysis and area under the curve (AUC) concerning the outcome are shown in Figure 4. The ROC curve constructed showed that MHR with AUC: 0.60 and cutoff value of 0.5, P < 0.001 was a good predictor of CAD (sensitivity of 48.93% and specificity of 65.48%, Figure 4). In addition, MHR could be used to discriminate ACS patients from chronic CAD patients with an AUC value of 0.65, P < 0.001. And the cutoff value was 0.57 with a sensitivity of 60.71% and a specificity of 64.19% (Figure 4).
Discussion
In this study, we found that MHR levels were higher in ACS patients compared with patients of chronic CAD and normal controls, and MHR showed a significantly progressive increase as the extent of angiographic lesions deteriorated. Moreover, MHR values were independently associated with angiographic severity of coronary arteries in ACS. CMHR was associated with chronic CAD.
In ACS, myocardial infarction results from sustained ischemia and oxygen deprivation. The innate immune system mediates both injury and repair. In the early phase, monocytes are mobilized and act as mediators of injury, whereas in the later phase, they become polarized and mediate remodeling. This complex biological process plays a critical role in cardiac remodeling and recovery.20 As the innate immune system initiates, monocytes are major players in inflammation and thrombosis, attending the process of phagocytosis, cytokine production, and tissue repair.7 In a steady state, patrolling monocytes account for 3–8% of blood leukocytes and defense the body from microorganisms and mediating inflammatory responses. In the 1970s, the accumulation of monocytes in atherosclerotic lesions in pigs was reported, and monocytes were involved in the formation of atherosclerotic plaques.21 Increased monocyte-platelet aggregates in ACS patients persist even one month after the acute event.22 In addition, particles from monocytes are present in large numbers in patients with ACS.23
Olivares et al showed a significantly higher percentage of monocytes in subjects who developed coronary artery disease.24 There are three major subsets for human monocyte and they differ in various states.25 The first subset referred to non-classic monocytes, which express low levels of CD14 and high levels of CD16 (CD14+CD16++), and they play important roles in monitoring healthy tissues and atherosclerotic plaque formation.26 A second human monocyte subset is called classical monocytes (CM) expressing CD14++CD16‐, and the third monocyte subset is called the intermediate monocyte (IM) expressing CD14 and CD16 (CD14++CD16+).27,28 CM and IM peak at one day after MI onset and act as players in inflammation,10 then, they can differentiate into macrophage and play roles in injury tissue repair. Based on these mechanisms, monocyte count has been evaluated as a potential marker of coronary events.24
The transition of stable CAD to ACS is associated with the escalation of inflammatory processes largely dependent on monocyte-related actions.29 Berg et al showed that evaluation of the classical CD16- monocyte subset allowed for independent predicted future risk of cardiovascular disease.30 The number of CD16-expressing monocytes is higher in patients with acute myocardial infarction.11 To the best of our knowledge, this is the first time that we evaluate the association between CMHR and CAD, and we found that CM concentration is increased in CAD, as well as CMHR in CAD compared to controls, making it an important role in predicting CAD.
HDL cholesterol shows its role in oxidation and inflammation. HDL prevents monocyte recruitment into the arterial wall by reducing the F-actin content of monocytes, thereby reducing the expression of CD11b on monocyte and endothelial adhesion molecules and thus inhibiting monocyte adhesion to the endothelium.31 HDL cholesterol plays an important role in inhibiting the oxidative modification of low-density lipoprotein cholesterol and expression of adhesion molecules in endothelial cells, preventing monocyte activation and spreading and also inhibiting activated monocytes thus reducing the recruitment of monocytes into the artery wall, suppressing monocyte differentiation to macrophages and protects against the development of atherosclerosis.32–35
Previous large epidemiological and prospective population studies have shown that serum HDL cholesterol levels are negatively associated with the risk of coronary heart disease.36 In 115 patients, the presence and extent of CAD were strongly associated with a decrease in HDL cholesterol.37 One study showed that HDL function was significantly impaired in 150 patients with ACS and that impaired HDL function was also associated with higher odds of developing ACS.38 These findings closely agree with the recent report where HDL was the best predictor of both the presence and extent of CAD.39
Given these two interactive parameters combined, could they effectively predict the inflammation even the myocardial infarction severity? In previous studies, the MHR was associated with cardiovascular diseases. Karatas found that elevated MHR levels were associated with in-hospital MACE.14 Previous studies have found that ACS patients with elevated MHR values showed a 1.4-fold higher incidence of hospitalization and long-term MACEs compared with ACS patients with lower MHR values.40 Acikgoz showed that increased MHR could predict in-hospital mortality (HR = 3.745, 95% CI: 1.308–5.950) and five-year mortality (HR = 2.048, 95% CI: 1.225–4.091, P = 0.014) in ST-segment elevation myocardial infarction.19 Cicek et al showed that higher admission MHR was associated independently with increased in-hospital, longer hospital stays, and increased short-term and long-term mortality in STEMI patients who undergo PCI successfully the first time.41 Canbolat et al showed that pre-procedural MHR levels are an independent predictor of atrial fibrillation recurrence after cryoballoon-based catheter ablation.15 Ucar et al showed that elevated MHR is a potential marker of bare metal stent restenosis.18
As mentioned above, elevated MHR proved to be associated with inflammation and a strong predictor for adverse cardiovascular events, bare metal stent restenosis, and even atrial fibrillation recurrence after ablation.14,42
In our study, we found that MHR on admission was an independent predictor of CAD, ACS, and even ACS with multi-vessel lesions. Since the monocytes play critical roles in the inflammation and recovery after MI and HDL cholesterol plays critical roles in anti-inflammation and oxidation, the new combined MHR parameter might be an “inflammatory and severity marker” in ACS patients. These findings are likely to help clinicians predict coronary artery lesion severity in ACS and aid in decision-making for the treatment.
MHR results are more readily available in clinical work. And one routine blood examination and blood lipids are enough to obtain valuable predictive marker of the MHR, which is faster and simpler.
Further more, according to our investigation, MHR can positively predict coronary artery disease and multi-vessel lesions in acute coronary syndrome, this indicator is highly variable predictor of patient outcomes. This can attract the attention of clinical experts early and can also help clinicians examination and treatment, providing a strong basis for patients to receive treatment early.43,44
Finally, MHR provides a new idea of whether there is a ratio association between monocytes and other cells in the blood vessel, and if so, whether it can improve our diagnostic accuracy of disease.
Limitations
There are some limitations in the study although the sample size of this study is relatively large, all participants are tested by PCI and statistics are well powered. First, it is a single-institute study design and these data need to be confirmed in multicenter studies in the future. Second, we are still investigating the association between MHR and ACS mortality, although we find positive results up to now (data not shown). Third, a large sample size will be designed to evaluate the roles of CMHR in predicting coronary artery lesion severity in ACS. Despite these, we think that our study may be of interest as we find MHR on admission is a valuable predictor of coronary artery lesion severity in ACS.
Conclusion
In conclusion, we consider that MHR on admission could be helpful to predict coronary artery lesion severity especially multi-vessel lesions in ACS.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; were accountable for all aspects of the work.
Funding
This study was supported by Xinjiang Science and Technology Support Project (XJTSP, 2016E02072). National Natural Science Foundation of China (No. 81960078, No. 81870272), Natural Science Foundation of Xinjiang Uygur Autonomous Region, Outstanding Youth Science Foundation Project (2021D01E28), opening project of the Xinjiang Key Laboratory (2021D04020) and State Key Laboratory of Pathogenesis, Prevention and Treatment of Central Asia High Incidence Diseases (SKL-HIDCA-2021-2, SKL-HIDCA-2021-XXG1 and SKL-HIDCA2022-XXG).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J. 2013;34(10):719–728. doi:10.1093/eurheartj/ehs411
2. Arbab-Zadeh A, Nakano M, Virmani R, Fuster V. Acute coronary events. Circulation. 2012;125(9):1147–1156. doi:10.1161/circulationaha.111.047431
3. Morrow DA. Cardiovascular risk prediction in patients with stable and unstable coronary heart disease. Circulation. 2010;121(24):2681–2691. doi:10.1161/circulationaha.109.852749
4. Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487(7407):325–329. doi:10.1038/nature11260
5. Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7(2):77–86. doi:10.1038/nrcardio.2009.228
6. Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res. 2013;112(12):1624–1633. doi:10.1161/circresaha.113.300890
7. Dutta P, Nahrendorf M. Monocytes in myocardial infarction. Arterioscler Thromb Vasc Biol. 2015;35(5):1066–1070. doi:10.1161/atvbaha.114.304652
8. Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325(5940):612–616. doi:10.1126/science.1175202
9. van der Laan AM, Ter Horst EN, Delewi R, et al. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur Heart J. 2014;35(6):376–385. doi:10.1093/eurheartj/eht331
10. Tapp LD, Shantsila E, Wrigley BJ, Pamukcu B, Lip GY. The CD14++CD16+ monocyte subset and monocyte-platelet interactions in patients with ST-elevation myocardial infarction. J Thromb Hemost. 2012;10(7):1231–1241. doi:10.1111/j.1538-7836.2011.04603.x
11. Tsujioka H, Imanishi T, Ikejima H, et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54(2):130–138. doi:10.1016/j.jacc.2009.04.021
12. Ji H, Li Y, Fan Z, et al. Monocyte/lymphocyte ratio predicts the severity of coronary artery disease: a syntax score assessment. BMC Cardiovasc Disord. 2017;17(1):90. doi:10.1186/s12872-017-0507-4
13. Li Y, Liu X, Luo Y. Monocyte to high-density lipoprotein cholesterol ratio and serum uric acid in Chinese adults: a cross-sectional study. BMC Endocr Disord. 2022;22(1):48. doi:10.1186/s12902-022-00966-z
14. Karatas MB, Canga Y, Ozcan KS, et al. Monocyte to high-density lipoprotein ratio as a new prognostic marker in patients with STEMI undergoing primary percutaneous coronary intervention. Am J Emerg Med. 2016;34(2):240–244. doi:10.1016/j.ajem.2015.10.049
15. Canpolat U, Aytemir K, Yorgun H, et al. The role of preprocedural monocyte-to-high-density lipoprotein ratio in prediction of atrial fibrillation recurrence after cryoballoon-based catheter ablation. Europace. 2015;17(12):1807–1815. doi:10.1093/europace/euu291
16. Wang Z, Ren L, Lei L, Ye H, Peng J. The relationship between neutrophil counts on admission and angiographic no-reflow after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Acta Cardiol. 2016;71(2):241–246. doi:10.2143/ac.71.2.3141856
17. Balta S, Celik T, Ozturk C, et al. The relation between monocyte to HDL ratio and no-reflow phenomenon in the patients with acute ST-segment elevation myocardial infarction. Am J Emerg Med. 2016;34(8):1542–1547. doi:10.1016/j.ajem.2016.05.031
18. Ucar FM. A potential marker of bare metal stent restenosis: monocyte counts - to- HDL cholesterol ratio. BMC Cardiovasc Disord. 2016;16(1):186. doi:10.1186/s12872-016-0367-3
19. Açıkgöz SK, Açıkgöz E, Şensoy B, Topal S, Aydoğdu S. Monocyte to high-density lipoprotein cholesterol ratio is predictive of in-hospital and five-year mortality in ST-segment elevation myocardial infarction. Cardiol J. 2016;23(5):505–512. doi:10.5603/CJ.a2016.0026
20. Ruparelia N, Godec J, Lee R, et al. Acute myocardial infarction activates distinct inflammation and proliferation pathways in circulating monocytes, prior to recruitment, and is identified through conserved transcriptional responses in mice and humans. Eur Heart J. 2015;36(29):1923–1934. doi:10.1093/eurheartj/ehv195
21. Badimon L, Storey RF, Vilahur G. Update on lipids, inflammation and atherothrombosis. Thromb Haemost. 2011;105(Suppl 1):S34–42. doi:10.1160/ths10-11-0717
22. Hoshi S, Goto M, Koyama N, Nomoto K, Tanaka H. Regulation of vascular smooth muscle cell proliferation by nuclear factor-kappaB and its inhibitor, I-kappaB. J Biol Chem. 2000;275(2):883–889. doi:10.1074/jbc.275.2.883
23. Selzman CH, Shames BD, McIntyre RC, Banerjee A, Harken AH. The NFkappaB inhibitory peptide, IkappaBalpha, prevents human vascular smooth muscle proliferation. Ann Thorac Surg. 1999;67(5):
24. Olivares R, Ducimetiere P, Claude JR. Monocyte count: a risk factor for coronary heart disease? Am J Epidemiol. 1993;137(1):49–53. doi:10.1093/oxfordjournals.aje.a116601
25. Arfvidsson J, Ahlin F, Vargas KG, Thaler B, Wojta J, Huber K. Monocyte subsets in myocardial infarction: a review. Int J Cardiol. 2017;231:47–53. doi:10.1016/j.ijcard.2016.12.182
26. Saederup N, Chan L, Lira SA, Charo IF. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2-/- mice: evidence for independent chemokine functions in atherogenesis. Circulation. 2008;117(13):1642–1648. doi:10.1161/circulationaha.107.743872
27. Weber C, Belge KU, von Hundelshausen P, et al. Differential chemokine receptor expression and function in human monocyte subpopulations. J Leukoc Biol. 2000;67(5):699–704. doi:10.1002/jlb.67.5.699
28. Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in the blood. Blood. 2010;116(16):e74–80. doi:10.1182/blood-2010-02-258558
29. Idzkowska E, Eljaszewicz A, Miklasz P, Musial WJ, Tycinska AM, Moniuszko M. The Role of Different Monocyte Subsets in the Pathogenesis of Atherosclerosis and Acute Coronary Syndromes. Scand J Immunol. 2015;82(3):163–173. doi:10.1111/sji.12314
30. Berg KE, Ljungcrantz I, Andersson L, et al. Elevated CD14++CD16- monocytes predict cardiovascular events. Circ Cardiovasc Genet. 2012;5(1):122–131. doi:10.1161/circgenetics.111.960385
31. Zambon A, Gervois P, Pauletto P, Fruchart JC, Staels B. Modulation of hepatic inflammatory risk markers of cardiovascular diseases by PPAR-alpha activators: clinical and experimental evidence. Arterioscler Thromb Vasc Biol. 2006;26(5):977–986. doi:10.1161/01.ATV.0000204327.96431.9a
32. Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95(8):764–772. doi:10.1161/01.res.0000146094.59640.13
33. Murphy AJ, Woollard KJ, Hoang A, et al. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol. 2008;28(11):2071–2077. doi:10.1161/atvbaha.108.168690
34. Parthasarathy S, Barnett J, Fong LG. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta. 1990;1044(2):275–283. doi:10.1016/0005-2760(90)90314-n
35. Susser LI, Rayner KJ. Through the layers: how macrophages drive atherosclerosis across the vessel wall. J Clin Invest. 2022;132(9). doi:10.1172/JCI157011
36. Toth PP, Barter PJ, Rosenson RS, et al. High-density lipoproteins: a consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7(5):484–525. doi:10.1016/j.jacl.2013.08.001
37. Drexel H, Amann FW, Rentsch K, et al. Relation of the level of high-density lipoprotein subfractions to the presence and extent of coronary artery disease. Am J Cardiol. 1992;70(4):436–440. doi:10.1016/0002-9149(92)91186-8
38. Thakkar H, Vincent V, Roy A, et al. HDL functions and their interaction in patients with ST-elevation myocardial infarction: a case-control study. Lipids Health Dis. 2020;19(1):75. doi:10.1186/s12944-020-01260-4
39. Romm PA, Green CE, Reagan K, Rackley CE. Relation of serum lipoprotein cholesterol levels to presence and severity of angiographic coronary artery disease. Am J Cardiol. 1991;67(6):479–483. doi:10.1016/0002-9149(91)90007-8
40. Cetin MS, Ozcan Cetin EH, Kalender E, et al. Monocyte to HDL cholesterol ratio predicts coronary artery disease severity and future major cardiovascular adverse events in acute coronary syndrome. Heart Lung Circ. 2016;25(11):1077–1086. doi:10.1016/j.hlc.2016.02.023
41. Çiçek G, Kundi H, Bozbay M, Yayla C, Uyarel H. The relationship between admission monocyte HDL-C ratio with short-term and long-term mortality among STEMI patients treated with successful primary PCI. Coron Artery Dis. 2016;27(3):176–184. doi:10.1097/MCA.0000000000000343
42. Ganjali S, Gotto AM, Ruscica M, et al. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J Cell Physiol. 2018;233(12):9237–9246. doi:10.1002/jcp.27028
43. Ma X, Han K, Yang L, et al. Adjustment of the GRACE risk score by monocyte to high-density lipoprotein ratio improves prediction of adverse cardiovascular outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Front Cardiovasc Med. 2021;8:755806. doi:10.3389/fcvm.2021.755806
44. Oylumlu M, Oylumlu M, Arik B, et al. Monocyte to high-density lipoprotein cholesterol and lymphocyte to monocyte ratios are predictors of in-hospital and long-term mortality in patients with acute coronary syndrome. Int J Clin Pract. 2021;75(5):e13973. doi:10.1111/ijcp.13973
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.