Back to Journals » Cancer Management and Research » Volume 13
Monoclonal Immunoglobulin-Associated Renal Lesions in Patients with Newly Diagnosed Multiple Myeloma: A Report from a Single Center
Authors Lin ZS , Yu XJ, Zhang X , Wang SX, Cen XN, Zhou FD, Zhao MH
Received 18 March 2021
Accepted for publication 29 April 2021
Published 13 May 2021 Volume 2021:13 Pages 3879—3888
DOI https://doi.org/10.2147/CMAR.S301818
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Zi-Shan Lin,1,* Xiao-Juan Yu,1,* Xu Zhang,1,2 Su-Xia Wang,1,2 Xi-Nan Cen,3 Fu-De Zhou,1 Ming-Hui Zhao1,4
1Renal Division, Department of Medicine, Peking University First Hospital; Institute of Nephrology, Peking University, Renal Pathology Center, Institute of Nephrology, Peking University, Key Laboratory of Renal Disease, Ministry of Health of China, Key Laboratory of CKD Prevention and Treatment, Ministry of Education of China, Beijing, People’s Republic of China; 2Laboratory of Electron Microscopy, Pathological Centre, Peking University First Hospital, Beijing, People’s Republic of China; 3Department of Hematology, Peking University First Hospital, Beijing, People’s Republic of China; 4Peking-Tsinghua Center for Life Sciences, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fu-De Zhou
Renal Division, Department of Medicine, Peking University First Hospital, Institute of Nephrology, Peking University; Renal Pathology Center, Institute of Nephrology, Peking University, Key Laboratory of Renal Disease, Ministry of Health of China, Key Laboratory of CKD Prevention and Treatment, Ministry of Education of China, No. 8 Xishiku Street, Xicheng District, Beijing, 100034, People’s Republic of China
Tel +86-13501162527
Fax +86-10-83575694
Email [email protected]
Background: Monoclonal immunoglobulin-associated renal lesions in patients with newly diagnosed myeloma vary. We aimed to determine the pathological spectrum and analyze associated prognostic factors.
Methods: Fifty-six patients with newly diagnosed multiple myeloma and biopsy-proven renal lesions were enrolled. Kidney biopsies were reanalyzed, and the baseline clinical characteristics, treatments and outcomes were recorded.
Results: Fifty-one patients had monoclonal immunoglobulin-associated renal lesions, with myeloma cast nephropathy (MCN) being the most common pattern. We divided our cohort into pure MCN, MCN+ other pathologies and non-MCN. Patients with MCN had more severe renal injury than those with non-MCN. In our cohort, none of the patients with pure MCN or MCN + other pathologies presented with nephrotic syndrome. Patients with non-MCN had better renal and overall survival than those with pure MCN but similar survivals to those with MCN + other pathologies. Number of myeloma casts (HR 1.08, p = 0.012) was the only independent prognostic factor for renal survival. Male sex (HR: 3.64; p = 0.015) and number of casts (HR: 1.17; p = 0.001) were independent prognostic factors for overall survival.
Conclusion: Patients with MCN had more severe renal injury than those with non-MCN. Patients with non-MCN had better renal and overall outcomes than those with pure MCN, but their outcomes were similar to those with MCN + other pathologies. The independent predictors of overall survival were male sex and number of myeloma casts.
Keywords: kidney biopsy, multiple myeloma, myeloma cast nephropathy, monoclonal immunoglobulin, prognosis
Introduction
Multiple myeloma (MM) is a hematological malignancy derived from the abnormal proliferation of clonal plasma cells, often resulting in organ dysfunction.1 Depending on the definition used, the incidence of renal involvement in MM, which is associated with poor outcomes, ranges from 20% to 50%.2,3
Renal failure in most patients with MM is caused by the nephrotoxicity of monoclonal immunoglobulin.3 The spectrum of renal lesions caused directly by monoclonal immunoglobulin in patients with MM includes myeloma cast nephropathy (MCN), monoclonal immunoglobulin deposition disease (MIDD), and amyloidosis.4,5 Other rarer patterns of renal lesions have also been reported.6 Patients with different patterns of renal lesions have different manifestations,7–10 and those with pure MCN present with more severe renal injury.7,9,10 The International Myeloma Working Group (IMWG) suggests that in patients with high serum free light chain levels and predominant light chain proteinuria, the most likely diagnosis is MCN and kidney biopsy is unnecessary.11 However, increasing evidence shows that kidney biopsy has significance for prognostic evaluation because the extent of cast formation, interstitial fibrosis and tubular atrophy (IFTA) are associated with renal survival in patients with MCN.12,13
Previous studies have shown that patients with kidney biopsy proven MCN or MCN combined with light chain deposition disease (LCDD) had worse outcomes than those with pure LCDD,9,10 but patients with monoclonal gammopathy of renal significance (MGRS) in those studies were not excluded. The manifestation and outcome of pure LCDD may be influenced by the absence of MM.
Here, we reviewed and analyzed 56 Chinese patients with newly diagnosed MM and renal involvement who had undergone kidney biopsy at Peking University First Hospital from 1999 to 2017. Our study aimed to determine the pathological spectrum, clinical characteristics and prognostic factors of monoclonal immunoglobulin-associated renal lesions in newly diagnosed myeloma patients.
Materials and Methods
Patients
The medical records of newly diagnosed MM patients between 1999 and 2017 were reviewed. An ultrasound-guided percutaneous native kidney biopsy was performed in 56 patients. Indications for kidney biopsy were acute kidney injury or acute kidney disease (64%), new-onset proteinuria or proteinuria accompanied by hematuria (22%), and nephrotic syndrome (14%). With the result of kidney biopsy, we recognized the underlying disease maybe MM or other hematological malignancy. Then we perform bone marrow smear and biopsy to confirm. The diagnosis of MM was strictly established depending on the guidelines of the IMWG.14
The study was approved by the Ethics Committee of our hospital and complied with the Declaration of Helsinki. Each patient provided informed consent.
Clinical Data
Demographic, clinical, laboratory, treatment and outcome data were collected. The following clinical manifestations were defined: hypoalbuminemia (serum albumin <30 g/L), nephrotic-range proteinuria (proteinuria >3.5 g/24 h), nephrotic syndrome (serum albumin <30 g/L with proteinuria >3.5 g/24 h), hypercalcemia (serum calcium > 2.5 mmol/L), acute kidney disease (changes in renal structure or function for less than 3 months), acute kidney injury (a ≥50% increase in the serum creatinine level occurring over 1–7 days or the presence of oliguria for more than 6 hours), anuria (urine output <100 mL/24h), oliguria (urine output 100–400 mL/24 h), and worsening renal function (a decrease of more than 30mL/min/1.73 m2 at admission in eGFR). The endpoints included end-stage renal disease (ESRD) and death.
Renal Histopathology
All biopsies were available for review. Kidney biopsy specimens were stained and analyzed by light microscopy, immunofluorescence and electron microscopy. Two pathologists evaluated the renal biopsy specimens separately.
According to a previous study, for patients with MCN, the mean number of myeloma casts per ×200 microscopic fields was recorded.12 The scores of tubular atrophy, interstitial fibrosis, interstitial inflammation and acute tubular injury were assessed as follows: <10% (0), 10–24% (1), 25–50% (2), >50% (3).13 The presence of interstitial edema was also assessed.13
Statistical Analysis
All statistical analyses were performed using SPSS version 24.0 (SPSS, Armonk, NY, USA). P<0.05 was considered statistically significant. For normally distributed data, the mean ± standard deviation was used to describe the quantitative variables and t test was used to compare the differences in quantitative parameters. For non-normally distributed data, the median with the range was used to describe the quantitative variables, and the Mann–Whitney U-test was used to compare the differences in quantitative parameters. Categorical data were described by absolute percentages, and differences between 2 groups were compared using χ2 test. The Kaplan-Meier method was used to plot the survival curves, which were statistically compared using the Log rank test. The associations between variables and outcomes were analyzed using a Cox regression model. The hazard ratios (HRs) with 95% confidence intervals (CIs) and p values were reported.
Results
Patient Characteristics
Three hundred ninety-seven MM patients with a mean age of 60.3 ± 11.4 years were newly diagnosed at our center (234 men and 163 women) between 1999 and 2017. Two hundred fifty-six patients (64%) had renal involvement (serum creatinine > 178 μmol/L or proteinuria). Kidney biopsy was only performed in 56/256 patients (22%). The baseline clinical characteristics of these patients are summarized in Table 1. The patients who had undergone kidney biopsy were younger and had significantly higher hemoglobin levels than those without kidney biopsy.
 |
Table 1 Baseline Clinical Characteristics of Patients with Renal Involvement |
Pathological Characteristics of Monoclonal Immunoglobulin-Associated Renal Lesions
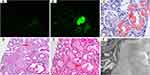
Fifty-one patients had monoclonal immunoglobulin-associated lesions and five patients had non-monoclonal immunoglobulin-associated lesions. Eighteen patients with monoclonal immunoglobulin-associated lesions (35%) were κ-restricted (Table 2). MCN was the most common monoclonal immunoglobulin-associated renal lesion in 31 patients, including 2 with the coexistence of amyloidosis (Figure 1) and 4 with the coexistence of LCDD. Two patients with pure MCN exhibited diffuse amyloid casts, which were λ-restricted. Other renal histopathologic diagnoses included pure amyloidosis (9 patients), pure LCDD (5 patients), light and heavy chain deposition disease (2 patients), light chain proximal tubulopathy (2 patients including one patient with coexisting C3 glomerulopathy), and fibrillary glomerulonephritis (2 patients). Non-monoclonal immunoglobulin-associated lesions were observed in 5 patients.
 |
Table 2 Kidney Biopsy Findings of Monoclonal Immunoglobulin-Associated Renal Lesions in 51 NDMM Patients |
Next, we divided our cohort into three groups: pure MCN (n=25), MCN+ other pathologies (n=6) and non-MCN (n=20). As shown in Table 3, patients with MCN+ other pathologies (median score, 2.5) had more severe acute tubular injury than those with non-MCN (median score, 1), but they showed no significant difference compared with those with pure MCN (median score, 2). Patients with pure MCN had significantly higher scores of tubular atrophy than those with MCN+ other pathologies and those non-MCN (p = 0.001). Patients with non-MCN had significantly lower scores of interstitial fibrosis than those with pure MCN or MCN+ other pathologies (p = 0.008).
 |
Table 3 Pathological Characteristics of Monoclonal Immunoglobulin-Associated Renal Lesions at Diagnosis |
Clinical Characteristics of Patients with Kidney Biopsy Proven Monoclonal Immunoglobulin-Associated Renal Lesions
The clinical characteristics at the time of kidney biopsy are listed in Table 4. The male/female ratio was 34/17, with a mean age of 55±11 years. The median hemoglobin level was 93 (range: 70–162) g/L, and it was significantly higher in the non-MCN group (114.5 g/L) than in the pure MCN group (83.0 g/L), but was not significantly different from that in the MCN + other pathologies group (90.5 g/L). The median serum creatinine level was 272 μmol/L (range: 31.0–1294.1) at the time of kidney biopsy, and it was significantly higher in patients with pure MCN (606.9 μmol/L) or with MCN + other pathologies (325.3 μmol/L) than in those with non-MCN (135.1 μmol/L). Patients with pure MCN (56%) had a higher incidence of dialysis requirements than those with non-MCN (10%), but there was no significant difference was found compared with those with MCN+ other pathologies (30%).
 |
Table 4 Clinical Characteristics of Patients with Kidney Biopsy-Proven Monoclonal Immunoglobulin-Associated Renal Lesions at Diagnosis |
The median proteinuria level was 3.8 g/24 h (range: 0–23.5), but no significant difference was found among the three groups. Nephrotic syndrome was observed in 8 patients (16%). Urine protein electrophoresis was studied in 30 patients, and the extent of albuminuria was higher in those with non-MCN (73.6%). The incidence of the albumin fraction (> 50% total urine protein) was significantly higher in the non-MCN group (92%), but no significant difference was found in the other albumin fractions (<10% and 10–50% total urine protein). The rate of hematuria was higher in the non-MCN group (48%) than in the pure MCN (12.5%) and MCN+ other pathologies (0%) groups.
Immunofixation electrophoresis of serum and urine was performed in 50 patients. Forty-six patients (92%) were positive by serum and/or urine immunofixation electrophoresis. Whole monoclonal immunoglobulin was identified in 17 patients (34%), and isolated monoclonal free light chains were detected in 33 patients (66%). No significant difference was found in the M-protein types among the three groups, but patients with MCN were more likely to have isolated serum-free light chains.
All the patients were assessed for extrarenal organ involvement. Eighteen patients exhibited extrarenal manifestations. Thirteen patients had bone lesions (7 patients with pure MCN, 3 patients with MCN + other pathologies and 3 patients with non-MCN), but no significant difference was found among the three groups (p = 0.208). Cardiac involvement was identified in 4 patients (2 patients with renal amyloidosis, one patient with MCN+ amyloidosis and one patient with LCDD). Liver involvement was identified in one patient with renal amyloidosis. Liver and cardiac involvement was diagnosed in one patient with amyloidosis. Patients with MCN had a significantly higher percentage of plasma cells in diagnostic aspirate smears than those with non-MCN (p = 0.013).
Treatment, Follow-Up and Outcome
The follow-up data were unavailable in 6 patients. The remaining 45 patients (88%) were followed up for a median time of 18 months (range: 2–109). All the patients received chemotherapy after kidney biopsy. For patients with MCN or anuria/oliguria, supportive therapy was administrated (urinary alkalization and free access to water). As shown in Table 5, the use of proteasome inhibitors or other chemotherapy was evenly distributed among the three groups. Among 37 patients who were treated with proteasome inhibitors, 6 received high-dose melphalan followed by autologous peripheral blood stem cell transplantation (HDM/ASCT). Eight patients were treated with other chemotherapy regimens, including the VAD regimen (vinorelbine, pirarubicin and dexamethasone) in 5 patients and MPT regimen (melphalan, prednisone and thalidomide) in 3 patients. Among the 15 patients who were on dialysis at diagnosis, 3 patients with λ-restricted pure-MCN discontinued dialysis for a period of 3, 9 and 12 months. Fifteen patients (33%) progressed to ESRD within a median duration of one month (range 1–60).
 |
Table 5 Treatment and Outcomes |
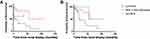
The rate of ESRD in the pure MCN group (50%) was significantly higher than that in the non-MCN group (11%), but it was not significantly different from that in the MCN+ other pathologies group (40%). The median renal survival was 6.9 months in the pure MCN group and was significantly shorter than that in the non-MCN group (not reached); however, it was not significantly different from that in the MCN + other pathologies groups (not reached; Figure 2A). Cox regression revealed that the predictors of progression to ESRD in the univariate analysis were the serum creatinine level at diagnosis and number of myeloma casts (Table 6). The independent predictor of progression to ESRD in the multivariate analysis was the number of myeloma casts (HR: 1.08, 95% CI: 1.08–1.15; p=0.012).
 |
Table 6 Prognostic Factors Associated with Renal Survival |
Of the 45 patients with available follow-up data, 17 (38%) died, 6 of whom died within 12 months after kidney biopsy. The causes of death in our patients included myeloma (6 patients, 35%), infection (4 patients, 23%), cardiovascular diseases (3 patients, 18%) and cerebrovascular disease (1 patient, 6%). The cause of death was unknown in 3 patients (18%). The median patient survival time was 54 months (95% CI: 39.8–68.2), and the mean time from diagnosis to death was 28.2 months (range: 3–84) in patients who died.
No significant difference was found in the rate of death among the three groups. The median survival was 8.7 months in the pure MCN group and was significantly shorter than that in the non-MCN group (not reached); however, it was not significantly different from that in the MCN + other pathologies group (not reached; Figure 2B). Univariate analysis revealed that the predictors of patient death were male sex, the serum creatinine level, the degree of tubular atrophy, and the number of myeloma casts (Table 7). In the multivariate analysis, male sex (HR: 3.64; 95% CI: 1.28–10.34; p=0.015) and the number of myeloma casts (HR: 1.17; 95% CI: 1.07–1.29; p=0.001) were correlated with survival.
 |
Table 7 Prognostic Factors Associated with Overall Survival |
Discussion
In our study, pure MCN was the most common monoclonal immunoglobulin-associated lesion (49%), followed by pure amyloidosis (17%) and pure LCDD (10%). The percentage of pure MCN in our cohort was higher than that in a previous report by Nasr et al7 (pure MCN, 28%; pure amyloidosis, 18%; pure MIDD, 17%) and by Ivanyi et al15 (pure MCN, 32%; pure amyloidosis, 11%; pure LCDD, 5%). However, more than two types of renal histopathologic patterns could be diagnosed with the same biopsy specimen. In our study, nearly 20% of patients with MCN had a second renal pathology pattern. Notably, 4 of 31 MCN patients (13%) had biopsy-proven renal LCDD. In a previous study by Mohan et al, 13 of 69 MM patients (13%) with LCCD had biopsy-proven MCN.16 In another study reported by Zand et al, 13 of 42 MM patients (31%) with MCN had biopsy-proven LCCD.9
We divided our cohort into 3 groups (pure MCN, MCN + other pathologies and non-MCN) and found many clinical differences. Patients with MCN were more likely to present with anemia, hypoproteinemia, and acute kidney disease and have isolated serum-free light chains than those with non-MCN. However, patients with non-MCN were more likely to present with nephrotic syndrome, albuminuria and microscopic hematuria. In summary, the clinical condition of renal damage in patients with pure MCN or MCN + other pathologies are more serious and more urgent. Notably, according to our study, patients with pure MCN or MCN + other pathologies can also present with nephrotic-range proteinuria but not nephrotic syndrome. Two factors may explain this finding: (1) MCN patients presented with acute kidney injury that was found very early, thus, serum albumin did not have sufficient time to decrease to the hypoalbuminemia level; (2) proteinuria of most patients with MCN contains very little albumin, a possible reason why they did not present with nephrotic syndrome. Some studies have shown that renal impairment caused by MCN can be reversed and survival can be improved if an early diagnosis and treatment are available.17 These clinical differences may be helpful for distinguishing the type of pathology and determining logical treatment, particularly when patients are ineligible for a kidney biopsy.
In our cohort, the median patient survival was 54 months, which was similar to that in previous studies7,12,13,16 but longer than that in a much earlier report.18 The better patient survival over the past decade may be due to the introduction of new therapeutic regimens, including bortezomib, and improved supportive care and hemodialysis technology. The introduction of novel agents has improved renal impairment reversal, and favorable outcomes can be achieved in most patients with renal impairment when the appropriate therapy is promptly applied.19 Similar to previous study, myeloma, cardiovascular diseases and infection were the main causes of death in our study.20
Recent studies have demonstrated that, in MCN patients, the extent of cast formation, tubular atrophy and interstitial fibrosis were prognostic factors for the renal outcome.12,13 In our study, we also enrolled patients with non-MCN and suggested that the number of myeloma casts was an independent predictor of patient survival. Several factors can explain this finding: (1) MCN usually occurs in patients with a heavy tumor load (serum free light chain>500 mg/L), and more myeloma casts correspond to a heavier tumor load;17 (2) patients with MCN are more likely to progress to ESRD with maintenance dialysis, resulting in poor survival,20–22 and a large number of casts and diffuse tubular atrophy lead to a poor renal prognosis.12,13 For MM patients, dialysis is a risk factor for death.20
We also found that male sex was an independent predictor of overall survival. A previous study showed that, in MM patients, male sex was an independent risk factor for infection, ranking as the second most important cause of death in our cohort.23 Furthermore, a recent study showed that CKD in males progressed more quickly.24 The relationship between CKD and cardiovascular diseases is well established. The precise reason why male sex is an independent predictor requires further investigation.
The limitations of our study include the short follow-up time, unstandardized treatments, lack of other parameters, and the small sample size, which cannot represent the entire MM population. In our study, 132 patients (52%) had a creatinine level greater than 178 µmol/L, which is more than double that in a previous study in China.25 The reason may be a selection bias because our hospital has a highly regarded nephrology department, and patients with renal impairment are more likely to choose our hospital for treatment. Therefore, a large-sample, multicenter study with a long-term follow-up is needed.
Conclusions
In patients with newly diagnosed MM, pure MCN is the most common monoclonal immunoglobulin-associated renal lesion. Patients with pure MCN or MCN+ other pathologies had similar presentations, both having more severe renal injury and possibly presenting without nephrotic syndrome. The independent predictors of overall survival were male sex and the number of myeloma casts.
Data Sharing Statement
All data generated in this study are included in the main manuscript.
Ethical Approval and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of the Peking University First Hospital (IRB approval number 2017[1280]) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. A signed informed consent was obtained from all patients for participation in this study. All data used in this manuscript were anonymized.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Number: 82070747), and CAMS Innovation Fund for Medical Sciences (2019-I2M-5-046). The funders had no role in study design, data collection, and analysis, interpretation of the data, the decision to publish, or preparation of the manuscript.
Disclosure
The authors declare that they have no conflicts of interests.
References
1. Rollig C, Knop S, Bornhauser M. Multiple myeloma. Lancet. 2015;385(9983):2197–2208. doi:10.1016/S0140-6736(14)60493-1
2. Eleutherakis-Papaiakovou V, Bamias A, Gika D, et al. Renal failure in multiple myeloma: incidence, correlations, and prognostic significance. Leuk Lymphoma. 2007;48(2):337–341. doi:10.1080/10428190601126602
3. Dimopoulos MA, Kastritis E, Rosinol L, Blade J, Ludwig H. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia. 2008;22(8):1485–1493. doi:10.1038/leu.2008.131
4. Motwani SS, Herlitz L, Monga D, Jhaveri KD, Lam AQ. Paraprotein-related kidney disease: glomerular diseases associated with paraproteinemias. Clin j Am Society Nephrol. 2016;11(12):2260–2272. doi:10.2215/CJN.02980316
5. Lin ZS, Qin AB, Wang SX, et al. A non-invasive differential diagnostic model for light chain cast nephropathy in newly diagnosed multiple myeloma patients with renal involvement: a multicenter study. J Nephrol. 2021. doi:10.1007/s40620-020-00926-7
6. Sethi S, Rajkumar SV, D’Agati VD. The complexity and heterogeneity of monoclonal immunoglobulin-associated renal diseases. J Am Society Nephrol. 2018;29(7):1810–1823. doi:10.1681/ASN.2017121319
7. Nasr SH, Valeri AM, Sethi S, et al. Clinicopathologic correlations in multiple myeloma: a case series of 190 patients with kidney biopsies. Am j Kidney Dis. 2012;59(6):786–794. doi:10.1053/j.ajkd.2011.12.028
8. Nasr SH, Valeri AM, Cornell LD, et al. Renal monoclonal immunoglobulin deposition disease: a report of 64 patients from a single institution. Clin j Am Society Nephrol. 2012;7(2):231–239. doi:10.2215/CJN.08640811
9. Zand L, Nasr SH, Gertz MA, et al. Clinical and prognostic differences among patients with light chain deposition disease, myeloma cast nephropathy and both. Leuk Lymphoma. 2015;56(12):3357–3364. doi:10.3109/10428194.2015.1040011
10. Lin J, Markowitz GS, Valeri AM, et al. Renal monoclonal immunoglobulin deposition disease: the disease spectrum. J Am Society Nephrol. 2001;12(7):1482–1492. doi:10.1681/ASN.V1271482
11. Dimopoulos MA, Sonneveld P, Leung N, et al. International myeloma working group recommendations for the diagnosis and management of myeloma-related renal impairment. J clin oncol. 2016;34(13):1544–1557. doi:10.1200/JCO.2015.65.0044
12. Ecotiere L, Thierry A, Debiais-Delpech C, et al. Prognostic value of kidney biopsy in myeloma cast nephropathy: a retrospective study of 70 patients. Nephrol Dial Transplant. 2016;31(1):64–72. doi:10.1093/ndt/gfv283
13. Royal V, Leung N, Troyanov S, et al. Clinicopathologic predictors of renal outcomes in light chain cast nephropathy: a multicenter retrospective study. Blood. 2020;135(21):1833–1846. doi:10.1182/blood.2019003807
14. Durie BG, Kyle RA, Belch A, et al. Myeloma management guidelines: a consensus report from the Scientific Advisors of the International Myeloma Foundation. Hematol J. 2003;4(6):379–398. doi:10.1038/sj.thj.6200312
15. Iványi B. Frequency of light chain deposition nephropathy relative to renal amyloidosis and Bence Jones cast nephropathy in a necropsy study of patients with myeloma. Arch Pathol Lab Med. 1990;114(9):986–987.
16. Mohan M, Buros A, Mathur P, et al. Clinical characteristics and prognostic factors in multiple myeloma patients with light chain deposition disease. Am J Hematol. 2017;92(8):739–745. doi:10.1002/ajh.24756
17. Hutchison CA, Cockwell P, Stringer S, et al. Early reduction of serum-free light chains associates with renal recovery in myeloma kidney. J Am Society Nephrol. 2011;22(6):1129–1136. doi:10.1681/ASN.2010080857
18. Montseny JJ, Kleinknecht D, Meyrier A, et al. Long-term outcome according to renal histological lesions in 118 patients with monoclonal gammopathies. Nephrol Dial Transplant. 1998;13(6):1438–1445. doi:10.1093/ndt/13.6.1438
19. Gavriatopoulou M, Terpos E, Kastritis E, Dimopoulos MA. Current treatments for renal failure due to multiple myeloma. Expert Opin Pharmacother. 2016;17(16):2165–2177. doi:10.1080/14656566.2016.1236915
20. Decourt A, Gondouin B, Delaroziere JC, et al. Trends in survival and renal recovery in patients with multiple myeloma or light-chain amyloidosis on chronic dialysis. Clin j Am Society Nephrol. 2016;11(3):431–441. doi:10.2215/CJN.06290615
21. Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Outcomes of newly diagnosed myeloma patients requiring dialysis: renal recovery, importance of rapid response and survival benefit. Blood Cancer J. 2017;7(6):e571. doi:10.1038/bcj.2017.49
22. Cobb J, Plantinga L, Luthi JC, et al. Pre-ESRD care and mortality in incident ESRD patients with multiple myeloma. Am J Clin Oncol. 2018;41(4):367–370. doi:10.1097/COC.0000000000000275
23. Sørrig R, Klausen TW, Salomo M, Vangsted A, Gimsing P. Risk factors for infections in newly diagnosed Multiple Myeloma patients: a Danish retrospective nationwide cohort study. Eur J Haematol. 2019;102(2):182–190. doi:10.1111/ejh.13190
24. Ricardo AC, Yang W, Sha D, et al. Sex-related disparities in CKD Progression. J Am Society Nephrol. 2019;30(1):137–146. doi:10.1681/ASN.2018030296
25. Lu J, Lu J, Chen W, et al. Clinical features and treatment outcome in newly diagnosed Chinese patients with multiple myeloma: results of a multicenter analysis. Blood Cancer J. 2014;4:e239. doi:10.1038/bcj.2014.55
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.