Back to Journals » Infection and Drug Resistance » Volume 16
Molecular Epidemiology of Staphylococcus aureus in a Tertiary Hospital in Anhui, China: ST59 Remains a Serious Threat
Authors Zhang H , Cao J , He Z , Zong X, Sun B
Received 7 November 2022
Accepted for publication 21 January 2023
Published 16 February 2023 Volume 2023:16 Pages 961—976
DOI https://doi.org/10.2147/IDR.S395220
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Video abstract of "Molecular epidemiology of Staphylococcus aureus" [ID 395220].
Views: 153
Huimin Zhang,1 Jiaxin Cao,1 Zhien He,2,3 Xianchun Zong,1 Baolin Sun2,3
1College of Life Science and Technology, Mudanjiang Normal University, Mudanjiang, People’s Republic of China; 2Department of Oncology, The First Affiliated Hospital, University of Science and Technology of China, Hefei, People’s Republic of China; 3School of Life Science and Medicine, University of Science and Technology of China, Hefei, People’s Republic of China
Correspondence: Baolin Sun, Email [email protected]
Purpose: This study aimed to investigate the molecular characteristics, antimicrobial resistance and hemolytic phenotype of Staphylococcus aureus isolated from Anhui, China.
Results: From August 2021 to January 2022, 214 S. aureus isolates were collected from the Anhui Provincial Hospital. This study identified 117 methicillin-resistant S. aureus and 97 methicillin-sensitive S. aureus isolates, and the detection rate of methicillin-resistant isolates was 1.8-fold higher than the average isolates reported in China (53.9% vs 30.5%). S. aureus isolates share identity at five or more of the seven MLST-based housekeeping loci, referred to as the clonal complex (CC). Forty ST types were found in 214 clinical S. aureus isolates, with the most extensive distribution of ST59 and ST6697 typing numbers and higher CC5 detection rates than any other clonal group. (The ST typing is the result of the MLST typing website query.) To detect the virulence of ST types of S. aureus, hemolysis experiments were performed on 214 clinical isolates, and it was concluded that ST59 had a relatively robust hemolytic capacity.
Conclusion: Anhui S. aureus isolates have unique molecular and antibiotic resistance profiles. The antibiotic resistance profile may be related to the random use of antibiotics.
Keywords: Staphylococcus aureus, epidemiological analysis, multilocus sequence typing, phenotype, hemolytic
Introduction
Staphylococcus aureus is a Gram-positive globular bacterium of the phylum Firmicutes.1 S. aureus is a leading pathogenic bacterium responsible for hospital- and community-acquired infections, ranking third after Escherichia coli and Klebsiella pneumonia. When the patient’s skin or mucosal barrier is broken, S. aureus can enter the underlying tissues or blood and cause infection; when invasive medical equipment, is used, complications like invasive infections might occur.2,3
The extensive use of antibiotics in clinical settings has led to a gradual increase in resistance to S. aureus, triggering widespread concern worldwide. Humans have been battling S. aureus for many years, starting with penicillin and ending with the advent of more antibiotics, but S. aureus quickly developed resistance to newer antibacterial drugs and spread rapidly.4 MRSA is the most common multidrug-resistant S. aureus and is one of the leading causes of infections associated with healthcare facilities.5 In addition, resistance to other antibiotics is present in S. aureus. For example, S. aureus, which is resistant to β-lactamase antibiotics, is almost ubiquitous.6 Clinical statistics indicate that S. aureus is an infectious pathogen,7 and the severity of its infection is associated with genotype and virulence factors.8 For example, S. aureus ST121 is an isolate with high virulence potential, and patients with S. aureus ST121 infection often require longer hospitalization and extended duration of antimicrobial therapy.9 S. aureus CC5 induces blood-related diseases. All of the above suggests that analysis of the molecular characteristics and virulence effects of S. aureus is essential to predict the prognosis of patients with S. aureus infections.
There are many techniques to study the molecular characteristics of S. aureus, including multilocus sequence typing (MLST), staphylococcal protein A (spa) typing, staphylococcal cassette chromosome mec (SCC mec) typing, helper gene regulator (agr) typing and toxin gene profiling.10 The primary and most widely used molecular typing method is multilocus sequence typing. However, the molecular characteristics of S. aureus vary between countries, with ST5, ST8, ST36, and ST45 being the most common subtypes in North America.11 ST5, ST8, and ST22 are the most common subtypes in Europe. Studies have shown that Asia has the highest rate of S. aureus infections globally;12 and thus, the typing results have received widespread attention. In Korea, ST5 and ST239 are the two main MRSA clonal types in hospitals. In Japan, ST5 is the most common type in hospitals. The most common hospital types in China are ST5 and ST188.13 The molecular characteristics of S. aureus have changed over time and in different geographic regions. For example, the major types are ST188 in Wenzhou, China; ST398 in Hainan, China;14 ST5 in Dalian, China and Shenyang, China, and ST59 in Chengdu, China.15 Ongoing molecular surveillance is essential for epidemic prevention of Staphylococcus aureus isolates in healthcare facilities.
Staphylococcus aureus can adhere to the surface of medical devices and form a multi-layered structure called a “biofilm” that is difficult to treat.16 More virulent S. aureus produce thicker biofilms. Biofilms are resistant to antimicrobial therapy and host defense.17 Infections associated with biofilms are difficult to treat with antibiotics and require timely biofilm removal. PIA and rSesC serve as two relevant macromolecules in the biofilm formation process; the current putative vaccine candidates have been practically studied and PIA is evaluated as the most prominent candidate for vaccine development to prevent biofilm formation. In addition to biofilm determining the virulence intensity of S. aureus, hemolysis is an important phenotype. S. aureus can lyse blood cells for hemolysis. In this study, to visualize the virulence intensity of S. aureus, the virulence of S. aureus was determined mainly by hemolysis test.
Many national and international researchers have studied the epidemiology of S. aureus in different regions. Various techniques have been used to gain insight into the isolates’ drug resistance, molecular characteristics, and virulence.18 Herein, to better understand Staphylococcus aureus-associated infections and reduce the cost of treatment,19 genomic epidemiology, drug resistance analysis, and hemolytic assays were performed on S. aureus detected in the Anhui Provincial Hospital, which is essential to update the information on local S. aureus epidemiology and prevention and control of S. aureus.
Methods
Bacterial Isolates
From August 2021 to January 2022, 214 S. aureus isolates were collected from clinical patients at the First Affiliated Hospital, University of Science and Technology of China, Hefei, Anhui, China. All isolates were stored in a −80°C refrigerator. Patient information involved in this study was anonymized to protect patient privacy.
Antibiotic Susceptibility Testing (AST)
AST was performed using a VITEK2 Compact System 3. AST was done on all 214 isolates of S. aureus. The antibiotics included: penicillin (PEN), ceftaroline fosamil (CPT), oxacillin (OXA), vancomycin (VAN), teicoplanin (TEC), daptomycin (DAP), gentamicin (GEN), erythromycin (ERY), levofloxacin (LVX), moxifloxacin (MFX), clindamycin (CLI), paediatric compound sulfamethoxazole tablets (SXT), rifampin (RIF), linezolid (LNZ), and tigecycline (TGC).
Multilocus Sequence Typing (MLST)
MLST was performed as described previously and included internal fragments of the following seven housekeeping genes: arcC (carbamate kinase), aroE (manganate dehydrogenase), glpF (glycerol kinase), gmk (guanylate kinase), pta (phosphate acetyltransferase), tpi (triphosphate isomerase), and yqiL (acetyl coenzyme A acetyltransferase).20 PCR amplification of these seven housekeeping genes was performed with primers (Table 1). The sequencing results need to be analyzed in SanpGene, and the sequences are compared with those of the corresponding allele profiles in the MLST database (http://saureus.mlst.net) to determine the sequence type, and Search by locus combinations is used to determine the ST type and clone groups. After determining the typing, the Phyloviz online website21 (http://online.phyloviz.net/index) was applied and the differentiation tree data and auxiliary data (7 house keeping genes and clonal complexes were included within the data) were submitted in the system to build a phylogenetic tree and to see the population structure map more visually.
 |
Table 1 Primers Used in This Study |
Hemolysis Assay
The frozen S. aureus stored at −80 °C was activated after thawing, the plate was coated overnight, and six single clones were inoculated into six shaking tubes. S. aureus was cultured overnight in TSB liquid medium at 37°C for 220 rpm. Overnight cultures were diluted to an OD600 of 6 and centrifuged at 9015 g for 2 minutes. Approximately 100 μL of supernatant, was separated, 900 μL (3:100) of PBS, was added, and incubated for 10 min. The suspension was centrifuged at 13,523 g for 1 min and the OD543 value was measured by spectrophotometer. (Negative control: PBS; Positive control: ddH2O).
Statistical Analysis
The statistical analyses were performed using SPSS Statistics 26.0 for Windows (IBM Corp, Armonk, NY, USA). A chi-square test was used to compare the resistance rates between MSSA and MRSA isolates. A p < 0.05 was considered statistically significant.
Results
Patient Age, Gender, Departmental Distribution and Source of Specimens
From August 2021 to January 2022, the average age of patients in this study was 52 years (range:1–102 years), and the sex distribution (male/female) was 68%/32% (Table 2 and Table S1). A survey of the age and gender of patients infected with S. aureus revealed that the highest number of male elderly patients were infected with S. aureus; this may be related to the history of other diseases and low body resistance of elderly patients.
 |
Table 2 Patient and Gender Distribution Information in Different Wards |
In this study, 214 clinical isolates of S. aureus were mainly derived from sputum (50%) and purulent secretions (21%), with drainage fluid (13.1%) and blood (6.5%) being the following most common sources (Figure 1).
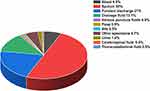 |
Figure 1 The distribution of sample sources. |
To facilitate physicians in setting effective treatment protocols, it is necessary to determine the status of S. aureus infections in different departments. The 214 isolates of S. aureus originated from 18 departments, each with different numbers of MRSA and MSSA. Statistical analysis (p < 0.05) was performed for 12 departments (n > 3), the number of patients infected with MRSA and MSSA in different departments was different and statistically significant (p < 0.05), the number of patients infected with MRSA in department of Critical Care Medicine and the department of General Geriatrics than the number of MSSA22 (Figure 2). In summary, it was observed that S. aureus infections were mainly distributed in the intensive care medicine department, and specimens were mainly sourced from sputum.
 |
Figure 2 Distribution of MRSA and MSSA in different departments (n>3) (**Indicates significant level). |
Antibiotic Resistance in Anhui Province
All 214 isolates of S. aureus were tested for antimicrobial resistance and the most resistant antibiotic was penicillin (PEN), 95.8%, followed by erythromycin (ERY), 56.1%, clindamycin (CLI), 54.7%, oxacillin (OXA), 45.3%, levofloxacin (LVX), 27.1%, moxifloxacin (MFX), 23.8%, gentamicin (GEN), 8.4%, levofloxacin (LVX), 27.1%, moxifloxacin (MFX), 23.8%, paediatric compound sulfamethoxazole tablets (SXT), 5.1%, rifampin (RIF), 3.3%, ceftaroline fosamil (CPT), 1.9%, and daptomycin (DAP), 0.5%. Among them, 214 isolates of S. aureus were not resistant to Vancomycin (VAN), Teicoplanin (TEC), Linezolid (LNZ), and tigecycline (TGC). Among the 214 isolates of S. aureus, there were 117 MRSA and 97 MSSA. Currently, according to the data cloud (www.chinets.com), the detection rate of methicillin resistance in 2022 is 30.5%, and the rate of methicillin resistance among the 214 isolates of S. aureus in this study was 53.9%, which is 1.8 times higher than the national reported average. Drug sensitivity testing is a standard method to distinguish MRSA from MSSA. In general, MRSA isolates showed much higher resistance rates to the tested antibiotics than MSSA isolates, consistent with the present study.23 However, SXT, and RIF did not exhibit this phenomenon (Table 3).
 |
Table 3 The Distribution of Antimicrobial Resistance of MRSA, MSSA and S. aureus Isolates and Its Statistical Significance |
Various antimicrobial drugs, such as clindamycin, linezolid, and tigecycline, have been recommended for empirical outpatient treatment of community-associated skin and soft tissue infections that may be attributable to MRSA.24,25 In our study, more than 90% of MRSA isolates were susceptible to linezolid and tigecycline drugs. Similarly, more than 90% of MRSA isolates were sensitive to rifampicin. Despite the rapid onset of resistance to rifampicin monotherapy, rifampicin in combination with other antibiotics has been shown to eradicate MRSA colonization and has been recommended for treating MRSA infections in the community.
Through the statistics of the MRSA detection rate in Anhui Province, the national MRSA detection rate, and national antimicrobial drug use intensity from 2015 to 2020, it is concluded that the MRSA detection rate in Anhui Province has been higher than the national MRSA detection rate, which shows that the prevalence of MRSA in Anhui Province needs to be controlled. The national trend of antimicrobial drug use intensity has been relatively stable in the past five years. However, the intensity of antimicrobial drug use is relatively high, which needs to be paid attention to by relevant departments to avoid the emergence of more drug-resistant bacteria (Figure 3).
 |
Figure 3 2015–2020 MRSA detection rate and intensity of antimicrobial drug use. |
MLST Typing results, Corresponding ST Type Resistance, and the Relationship Between Typing and Patient Age
S. aureus populations in Anhui Province had unique molecular characteristics, and 40 ST typologies were identified among the 214 clinical S. aureus isolates (Figure 4).
 |
Figure 4 The part between cloning complex (CC) and ST types. |
S. aureus isolates identified five or more than seven MLST-based housekeeping loci, referred to as the clonal complex (CC). Five loci identical are defined as a group and isolates can be divided into many groups, the first group is the largest and includes 1699 STs, including ST1, ST5, ST8, and ST15. ST5 is the founder, consistent with the minimum spanning tree (Figure 5). The relationship between sequence types in the minimal biochemical tree, illustrated in the Figure 5, shows the structure of the extremely diverse S. aureus population. The most common were CC181, accounting for 15%, and CC59, accounting for 13.6%. Statistical analyses were performed to determine whether the different CC clonal complexes were associated with the sex and age of the patients. The likelihood of CC5 infection increased with age (p<0.001), the clonal complex of CC22 was associated with age but not with sex, and the clonal complex of CC59 was associated with sex but not with age. However, it is possible that clonal complexes are associated with both factors, and due to the limited number of patients investigated, it was not possible to determine the association of different types of clonal complexes with age and sex. (Table 4).
 |
Table 4 Distribution of the Most Common CCs (N>3) in S. aureus Carriers by Sex and Age |
 |
Figure 5 Minimum spanning tree for ST types Minimum spanning tree generated from fractal data using the phyloviz website (http://online.phyloviz.net/index). Each sphere or node represents a unique ST type. The size of each node indicates the number of S. aureus isolates of each ST type. The length between two nodes reflects the genetic distance between the two bounding ST types. |
The resistance rates of different MLST subtypes were different. The 23 isolates of ST59 were identified, among which 22 isolates of S. aureus were resistant to OXA, which indicates that ST59 was highly resistant to OXA. The 9 isolates of ST764, and 9 isolates were resistant to ERY, LVX, MFX, and CLI. Therefore, speculation indicates strong resistance to ST764 (Table 5).
 |
Table 5 The Distribution of Antimicrobial Resistance of S. aureus with Different ST Types and Its Statistical Significance |
MLST typing distribution in different departments, is necessary to determine the rate and type of S. aureus infection in different departments to propose a better-differentiated treatment plan. Different hospital departments have different MLST typing distributions, and the departments with high clinical S. aureus detection rates are: critical care medicine, comprehensive geriatrics, and respiratory medicine. Statistical analysis of the MLST typing distribution in different departments revealed that the departments of critical care medicine, general surgery, respiratory medicine, comprehensive geriatrics, emergency medicine, gastroenterology, and pediatric surgery were statistically significant (p < 0.05), with the highest detection rate of ST764 in critical care medicine, and ST6697 in general (Table 6).
 |
Table 6 ST-Type Distribution of Different Hospital Departments (N>3) |
Hemolytic Phenotypes of Different ST Typing Isolates
An essential feature of S. aureus is the ability to secrete many toxin proteins, including α-toxin, which can cause lysis of various cells, such as erythrocytes, platelets, endothelial cells, epithelial cells, and some leukocytes. The α-toxin interacts with the host cells by targeting them in a receptor-mediated manner.26
Herein, the hemolysis experiment was performed with the supernatant of 214 S. aureus clinical isolates using aseptically treated sheep blood (Figure S1). To determine the hemolytic ability of different S. aureus types; the common ST types (ST1, ST5, ST6, ST7, ST8, ST15, ST22, ST25, ST59, ST72, ST88, ST188, ST239, ST630, ST764, ST5529, and ST6697) of S. aureus were analyzed for hemolytic values (Figure 6). We found that ST59 had the most robust hemolytic ability, among which ST7 and ST6697 also had the robust hemolytic ability.
 |
Figure 6 Hemolysis box diagram of different subtypes (A): ST1, ST5, ST6, ST7, ST8, ST15, ST22, ST25; (B) ST59, ST72, ST88, ST188, ST239, ST630, ST764, ST5529, ST6697). |
Discussion
In recent years, survey statistics have shown that S. aureus is one of the top three pathogens causing clinical infections in 2018–2021. This study mainly investigated the epidemiology of S. aureus in Anhui Province, and according to recent statistics (http://www.carss.cn) performed in 2020, the detection rate of S. aureus in Anhui Province was 8.37%, lower than the previous two years, which may be related to clinical treatment and control.
According to the National Bacterial Drug Resistance Surveillance Network, sputum samples accounted for the most significant proportion of the specimens in 2020, 38.35%, followed by urine. The distribution of sputum specimens in Anhui Province in 2020, accounted for the most significant proportion of 39.56%, with S. aureus presumably mainly causing respiratory tract infections in hospitals, or for some reason the patient’s swallowing reflex, cough reflex weakened, and cough weakness. The mucosal damage caused by the incision of the trachea was related.27
Among the 214 isolates of S. aureus collected in this study, resistance rate of 45.3%, 3.3%, 0%, and 0% was found against benzocillin, rifampicin, vancomycin, and linezolid; in the United States, the average resistance rates against benzocillin, rifampicin, vancomycin, and linezolid were 45%, 1%, 0%, and 0%, respectively; In India, the average resistance rates were 68%, 9%, 1% and 1% against benzocillin, rifampicin, vancomycin, and linezolid, respectively, and in Australia, the average resistance rates were 18%, 0%, 1% and 0% against benzocillin, rifampicin, vancomycin and linezolid, respectively (resistancemap.cddep.org/Antibiotic Resistance.php). S. aureus isolates from Anhui showed similar resistance to some antibiotics in other countries. However, there were differences in the resistance rates to benzocillin, suggesting that antibiotic resistance characteristics may be related to local antibiotic usage trends. There were no vancomycin-resistant isolates, but in recent years, a heterogeneous vancomycin-resistant S. aureus was found clinically.28 Therefore, the clinical use of vancomycin should be carefully selected according to the patient’s drug sensitivity results to prevent the emergence of VRSA (vancomycin-resistant staphylococcus aureus) in China.
Since the 1980s, treating MRSA has become a challenging clinical problem. In this study, after statistical analysis of the drug sensitivity tests of MRSA and MSSA, it was concluded that the drug resistance rate of MRSA was greater than that of MSSA. In 2020, the detection rate in Anhui Province was 38.1%, and the detection rate of MRSA in this study was 53.9%, which was higher than the average and deserved attention. From a geographical perspective, the MRSA detection rates of clinical isolates of S. aureus in Shanghai and Jiangsu, China, were similar to the MRSA detection rates in this study. However, the MRSA detection rates in other regions differed from this study, and the reasons for the differences may be related to local antibiotic use.29,30
To confirm the origin and affinity of the 214 isolates mentioned above, we used the MLST molecular typing method to explore the clonal spread of S. aureus between wards. Due to the diversity of isolates and the influence of clinical care, the number of ST types of S. aureus in the MLST database is increasing, and there are currently 4800 identifiable STs. Forty ST types and 12 clonal clusters (CCs) were found among the 214 clinical isolates in this study, with ST59 and ST6697 occupying the top two spots and the complex clonal CC5 having the highest number (independent of age-sex distribution), where ST5 was also detected at a high rate. Previous data suggested that the hospital-associated methicillin-resistant S. aureus (HA-MRSA) spectrum in China is characterized by multiple antibiotic-resistant isolates ST239 and ST5, which are widely circulating in several hospitals.31 The predominant ST59 and ST5 among MRSA isolates in this study overlaps with previous findings, but the high prevalence of ST59 and other ST types. However, the high prevalence of ST59 and other ST types indicates that Anhui Province has unique molecular characteristics.32 Doing resistance analysis of isolates with different ST typing, we found that ST5 clinical isolates were sensitive to rifampin, but more resistant to cephalosporin antibiotics than ST239 clinical isolates; therefore, when choosing treatment for the disease, appropriate drugs should be selected to reduce the stockpiling of resistant bacteria.33
The same ST was associated with different virulence profiles, and the expression of virulence genes encoded by the core genome differed between discrete molecular types of S. aureus.34 As an exotoxin of S. aureus, one of the virulence factors in S. aureus infections,35 hemolysin can lead to cell lysis and death.36 Hemolysin destroys red blood cell membranes and causes hemolysis in PBS sheep blood mixture. In this study, hemolysis experiments were performed on the 214 clinical isolates of S. aureus and the hemolytic ability of the isolates was compared by measuring OD values. Among them, the clinical isolates of ST59 had the most robust hemolytic capacity. Previous studies showed that the CA-MRSA ST59 isolates were significantly more virulent than geographically matched HA-MRSA clones ST5 and ST239 in various animal infection models.37 The PSMα peptide in ST59 CA-MRSA strongly affected neutrophil and erythrocyte lysis,38 echoing the present study’s findings. However, the sample size taken in this study was limited, and the conclusions drawn need further validation.
Conclusions
S. aureus isolated from Anhui Province had unique molecular characteristics. And the differences between the antibiotic resistance of MRSA and MSSA were observed, further validating that MRSA is more resistant than MSSA. However, the available data on MRSA in Anhui Province is limited, and future surveillance and molecular epidemiological studies on MRSA need to be strengthened. Meanwhile, specific measures should be used to monitor the clonal expansion and transmission of highly virulent clinical isolates of S. aureus after analyzing the survey results of hospital departments and patients infected with S. aureus.
Abbreviations
MLST, Multilocus sequence typing; MRSA, Methicillin-resistant Staphylococcus aureus; MSSA, Methicillin Staphylococcus aureus; VRSA, Vancomycin-resistant Staphylococcus aureus.
Data Sharing Statement
The datasets used during the study can be obtained from the corresponding author upon reasonable request. Two-way genome fragment sequencing to pinpoint seven housekeeping genes was performed. The whole genome sequences generated in the current study are available in the NCBI database (Bio Project: PRJNA824222).
Ethics Approval and Consent to Participate
All research studies on humans (individuals, samples or data) must have been performed in accordance with the principles stated in the Declaration of Helsinki. The study complies with the national exemptions for requesting informed consent and meets the four conditions of “it is impractical or impossible to obtain informed consent”, “the study is not greater than minimal risk”, “the subject’s privacy can be well protected” and “the subject’s rights will not be infringed”. Also for informed consent waivers, use of previously obtained samples/information, use of residual samples from clinical tests, and retrospective analysis using case information. All isolates in this study were collected during the bacteriological analysis in the clinical microbiology laboratory of a public hospital, and patients were treated anonymously and therefore did not require ethical approval.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the National Key Research and Development Program of China (2021YFC2300300), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB29020000), grants from the National Natural Science Foundation of China (32070132), and the Fundamental Research Funds for the Central Universities (YD9100002013).
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Gould D, Chamberlaine A. Staphylococcus aureus: a review of the literature. J Clin Nurs. 1995;4(1):5–12. doi:10.1111/j.1365-2702.1995.tb00004.x
2. Cheung GYC, Bae JS, Otto M. Pathogenicity and virulence of Staphylococcus aureus. Virulence. 2021;12(1):547–569. doi:10.1080/21505594.2021.1878688
3. Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339(8):520–532. doi:10.1056/NEJM199808203390806
4. Turner NA, Sharma-Kuinkel BK, Maskarinec SA, et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 2019;17(4):203–218. doi:10.1038/s41579-018-0147-4
5. Hassoun A, Linden PK, Friedman B. Incidence, prevalence, and management of MRSA bacteremia across patient populations-A review of recent developments in MRSA management and treatment. Crit Care. 2017;21(1):211. doi:10.1186/s13054-017-1801-3
6. Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111(9):1265–1273. doi:10.1172/JCI18535
7. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505–520. doi:10.1128/CMR.10.3.505
8. Fu Y, Xiong M, Li X, et al. Molecular characteristics, antimicrobial resistance and virulence gene profiles of Staphylococcus aureus isolates from Wuhan, Central China. Infect Drug Resist. 2020;13:2063–2072. doi:10.2147/IDR.S249988
9. Rao Q, Shang W, Hu X, et al. Staphylococcus aureus ST121: a globally disseminated hypervirulent clone. J Med Microbiol. 2015;64(12):1462–1473. doi:10.1099/jmm.0.000185
10. Zhang H, Zheng Y, Gao H, et al. Identification and characterization of Staphylococcus aureus strains with an incomplete hemolytic phenotype. Front Cell Infect Microbiol. 2016;6:146. doi:10.3389/fcimb.2016.00146
11. Chatterjee SS, Otto M. Improved understanding of factors driving methicillin-resistant Staphylococcus aureus epidemic waves. Clin Epidemiol. 2013;5:205–217. doi:10.2147/CLEP.S37071
12. Chen CJ, Huang YC. New epidemiology of Staphylococcus aureus infection in Asia. Clin Microbiol Infect. 2014;20(7):605–623. doi:10.1111/1469-0691.12705
13. Li X, Fang F, Zhao J, et al. Molecular characteristics and virulence gene profiles of Staphylococcus aureus causing bloodstream infection. Braz J Infect Dis. 2018;22(6):487–494. doi:10.1016/j.bjid.2018.12.001
14. Li X, Huang T, Xu K, et al. Molecular characteristics and virulence gene profiles of Staphylococcus aureus isolates in Hainan, China. BMC Infect Dis. 2019;19(1):873. doi:10.1186/s12879-019-4547-5
15. Lu PL, Chin LC, Peng CF, et al. Risk factors and molecular analysis of community methicillin-resistant Staphylococcus aureus carriage. J Clin Microbiol. 2005;43(1):132–139. doi:10.1128/JCM.43.1.132-139.2005
16. Mirzaei B, Babaei Rvalinejad S. Staphylococcal Vaccine Antigens related to biofilm formation. Hum Vaccin Immunother. 2021;17(1):293–303. doi:10.1080/21645515.2020.1767449
17. Shahmoradi M, Faridifar P, Shapouri R, et al. Determining the biofilm forming gene profile of Staphylococcus aureus clinical isolates via multiplex colony PCR method. Rep Biochem Mol Biol. 2019;7(2):181–188.
18. Tabaja H, Hindy JR, Kanj SS. Epidemiology of methicillin-resistant Staphylococcus aureus in Arab Countries of the Middle East and North African (MENA) Region. Mediterr J Hematol Infect Dis. 2021;13(1):e2021050. doi:10.4084/MJHID.2021.050
19. Lee BY, Singh A, David MZ, et al. The economic burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). Clin Microbiol Infect. 2013;19(6):528–536. doi:10.1111/j.1469-0691.2012.03914.x
20. Enright MC, Day NP, Davies CE, et al. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38(3):1008–1015. doi:10.1128/JCM.38.3.1008-1015.2000
21. Ribeiro-Goncalves B, Francisco AP, Vaz C, et al. PHYLOViZ Online: web-based tool for visualization, phylogenetic inference, analysis and sharing of minimum spanning trees. Nucleic Acids Res. 2016;44(W1):W246–W251. doi:10.1093/nar/gkw359
22. Chen K, Huang Y, Song Q, et al. Drug-resistance dynamics of Staphylococcus aureus between 2008 and 2014 at a tertiary teaching hospital, Jiangxi Province, China. BMC Infect Dis. 2017;17(1):97. doi:10.1186/s12879-016-2172-0
23. Chen Z, Han C, Huang X, et al. A molecular epidemiological study of methicillin-resistant and methicillin-susceptible Staphylococcus aureus contamination in the airport environment. Infect Drug Resist. 2018;11:2363–2375. doi:10.2147/IDR.S178584
24. Popovich KJ, Hota B. Treatment and prevention of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Dermatol Ther. 2008;21(3):167–179. doi:10.1111/j.1529-8019.2008.00188.x
25. Martinez-Aguilar G, Hammerman WA, Mason EO
26. Berube BJ, Bubeck Wardenburg J. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins. 2013;5(6):1140–1166. doi:10.3390/toxins5061140
27. Yu Y, Yao Y, Weng Q, et al. Dissemination and molecular characterization of Staphylococcus aureus at a Tertiary Referral Hospital in Xiamen City, China. Biomed Res Int. 2017;2017:1367179. doi:10.1155/2017/1367179
28. Liu P, Hao Z, Liu M, et al. Genetic mutations in adaptive evolution of growth-independent vancomycin-tolerant Staphylococcus aureus. J Antimicrob Chemother. 2021;76(11):2765–2773. doi:10.1093/jac/dkab260
29. Dadashi M, Nasiri MJ, Fallah F, et al. Methicillin-resistant Staphylococcus aureus (MRSA) in Iran: a systematic review and meta-analysis. J Glob Antimicrob Resist. 2018;12:96–103. doi:10.1016/j.jgar.2017.09.006
30. Aires de Sousa M, de Lencastre H. Evolution of sporadic isolates of methicillin-resistant Staphylococcus aureus (MRSA) in hospitals and their similarities to isolates of community-acquired MRSA. J Clin Microbiol. 2003;41(8):3806–3815. doi:10.1128/JCM.41.8.3806-3815.2003
31. Teixeira LA, Resende CA, Ormonde LR, et al. Geographic spread of epidemic multiresistant Staphylococcus aureus clone in Brazil. J Clin Microbiol. 1995;33(9):2400–2404. doi:10.1128/jcm.33.9.2400-2404.1995
32. Li T, Song Y, Zhu Y, et al. Current status of Staphylococcus aureus infection in a central teaching hospital in Shanghai, China. BMC Microbiol. 2013;13:153. doi:10.1186/1471-2180-13-153
33. Dai Y, Liu J, Guo W, et al. Decreasing methicillin-resistant Staphylococcus aureus (MRSA) infections is attributable to the disappearance of predominant MRSA ST239 clones, Shanghai, 2008–2017. Emerg Microbes Infect. 2019;8(1):471–478. doi:10.1080/22221751.2019.1595161
34. Kim T, Yi J, Hong KH, et al. Distribution of virulence genes in spa types of methicillin-resistant Staphylococcus aureus isolated from patients in intensive care units. Korean J Lab Med. 2011;31(1):30–36. doi:10.3343/kjlm.2011.31.1.30
35. Hildebrand A, Pohl M, Bhakdi S. Staphylococcus aureus alpha-toxin. Dual mechanism of binding to target cells. J Biol Chem. 1991;266(26):17195–17200. doi:10.1016/S0021-9258(19)47358-4
36. Du Y, Liu L, Zhang C, et al. Two residues in Staphylococcus aureus alpha-hemolysin related to hemolysis and self-assembly. Infect Drug Resist. 2018;11:1271–1274. doi:10.2147/IDR.S167779
37. Wang Y, Liu Q, Liu Q, et al. Phylogenetic analysis and virulence determinant of the host-adapted Staphylococcus aureus lineage ST188 in China. Emerg Microbes Infect. 2018;7(1):45. doi:10.1038/s41426-018-0048-7
38. Wang R, Braughton KR, Kretschmer D, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13(12):1510–1514. doi:10.1038/nm1656
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
