Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Modified Patent Hemostasis Strategy Based on the Platelet Counts for Transradial Access Chemoembolization in Patients with Hepatocellular Carcinoma: A Prospective Single-Center Study
Authors Luo YG , Zhang XW, Tsauo JW, Li Y, Li JG, Peng Q, Li YW, Sun W, Zhao H, Li X
Received 3 March 2023
Accepted for publication 14 April 2023
Published 21 April 2023 Volume 2023:10 Pages 687—695
DOI https://doi.org/10.2147/JHC.S410952
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mohamed Shaker
Yin-Gen Luo, Xiao-Wu Zhang, Jiay-Wei Tsauo, Ying Li, Jin-Gui Li, Qing Peng, Ya-Wei Li, Wei Sun, He Zhao, Xiao Li
Department of Interventional Therapy, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Xiao Li; He Zhao, Department of Interventional Therapy, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, People’s Republic of China, Tel/Fax +86 010 8778 8502, Email [email protected]; [email protected]
Objective: This study aimed to investigate the shortest compression time to achieve hemostasis and the optimal hemostasis strategy in patients treated with transradial access chemoembolization (TRA-TACE).
Methods: From October 2019 to October 2021, 119 consecutive patients with hepatocellular carcinoma (HCC) who underwent 134 sessions of TRA-TACE were included in this prospective single-center study. The compression time was measured by decompressing the device for 30 min, and thereafter, every 10 min after the procedure until complete hemostasis was achieved.
Results: Technical success was achieved for all TRA procedures. None of the patients experienced major TRA-related adverse events. Minor adverse events occurred in 7.5% of the patients. The mean compression time was 31.8 ± 5.0 min. Factors that may impact hemostasis were analyzed by univariate and multivariate analyses, and a platelet count < 100× 109 /L (p = 0.016, odds ratio = 3.942) was found to be an independent factor that could predict the failure to achieve hemostasis within 30 min. For patients with a platelet count < 100× 109 /L, the compression time required to achieve hemostasis was 60 min. For patients with a platelet count ≥ 100× 109 /L, the compression time required to achieve hemostasis was 40 min.
Conclusion: To achieve hemostasis in patients with HCC treated with TRA-TACE, compression for 60 min is sufficient for those with a platelet count < 100× 109 /L, and compression for 40 min is sufficient for those with a platelet count ≥ 100× 109 /L.
Keywords: hepatocellular carcinoma, transarterial chemoembolization, transradial access, hemostasis
Introduction
Transradial access (TRA) and transfemoral access (TFA) are both well-accepted artery access sites for peripheral interventions. The advantages of TRA include early ambulation and swifter hospital discharge.1,2 Recent studies have shown that the satisfaction and quality of life in patients using TRA were significantly better than those using TFA, and bleeding complications at the access site were fewer in patients using TRA.3–5 Therefore, in recent years, TRA has been increasingly used in all types of peripheral interventions.6
The compression time to achieve hemostasis after TRA can affect patient satisfaction and the time to discharge.1 Compressing the puncture site for at least 1.5 h after the TRA procedure is the hemostasis strategy recommended for patients treated with peripheral interventions.4,5 In cardiac interventions, it has been shown that shorter compression time can reduce the rate of radial artery occlusion (RAO),7 which is the most common complication of TRA and restricts the use of the same radial artery for future procedures.8 However, to date, no study has investigated the shortest compression time for peripheral TRA interventions.
We hypothesized that the time for removal of the compression device could be shortened without increasing the incidence of bleeding complications after peripheral interventions. As the most common peripheral TRA procedure is transradial access chemoembolization (TRA-TACE), which is performed in 32–59% of all peripheral TRA procedures,5,9 we aimed to investigate the shortest compression time to achieve hemostasis in patients treated with TRA-TACE and explore the optimal hemostasis strategy for TRA in peripheral interventions.
Materials and Methods
Study Design
This prospective, single-center study was approved by our institutional review board and conducted in accordance with the guidelines of the Declaration of Helsinki. This study was registered in the Chinese Clinical Trial Registry (registration number: ChiCTR2200056326). Written informed consent was obtained from all patients. The study also adheres to the STROBE guidelines. From October 2019 to October 2021, patients diagnosed with hepatocellular carcinoma (HCC) who were scheduled to undergo TRA-TACE were considered eligible for this study. The inclusion criteria were as follows: (1) age ≥ 18 years, (2) clinically and/or pathologically diagnosed HCC,10,11 and (3) patients scheduled to be treated with TRA-TACE. The exclusion criteria were as follows: (1) radial artery diameter < 2 mm,4 (2) radial artery occlusion or congenital malformation,12 (3) systemic active infection or local active infection at the puncture site, (4) neurological or mental disorders, and (5) unwillingness to participate or provide written informed consent.
TRA-TACE and Patent Hemostasis
The procedure for TRA-TACE is described in a previous study.3 After insertion of a sheath (5-Fr radial access kit, Radiofocus, Terumo) in the left radial artery, patients were given a cocktail consisting of 5 mg of verapamil, 200 μg of nitroglycerine, and 3000 U of heparin diluted in 20 mL of their blood via the vascular sheath.3 The method to achieve hemostasis in the radial artery was based on the patent hemostasis strategy, which requires the maintenance of antegrade blood flow through the radial artery during hemostatic compression.13 The patent hemostasis strategy is briefly described as follows: (1) after removing the guiding catheter, pull out the sheath approximately 2–3 cm; (2) place the center of the radial compression device (Finale, Merit) 2–3 mm proximal to the skin entry site and tighten; (3) remove the sheath completely; (4) decompress the device until minimal bleeding is observed; (5) recompress the device at a level just enough to maintain patent hemostasis; (6) confirm the patency of the radial artery by the reverse Barbeau’s test; and (7) after at least 30 min, remove the device according to the strategy described below.
Study Endpoints
The compression time to achieve hemostasis is defined as the time between achieving patent hemostasis using a compression device and complete removal of the compression device.14 To examine the shortest compression time possible to achieve hemostasis, removal of the device was attempted 30 min after patent hemostasis for the first time by decompressing the device, and the attempt was repeated every 10 min thereafter until complete hemostasis was observed (Figure 1). The compression device was removed immediately after complete hemostasis was achieved. Technical success, number of puncture attempts, puncture time, and procedure time were recorded. Technical success was defined as successful chemoembolization of the targeted tumor-feeding arteries via the TRA.3 Puncture time was defined as the time between the beginning of the first puncture and the successful insertion of a guidewire. The diameter of the radial artery before and 24 h after the procedure was measured using ultrasound. Early and late RAO was evaluated at 24 h and 30 days after the procedure using ultrasound and the reverse Barbeau’s test, respectively. Other TRA-related adverse events were also recorded according to the Common Terminology Criteria for Adverse Events version 5.0.15
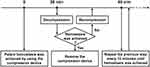 |
Figure 1 The process to measure time to achieve hemostasis for all of the 134 TRA-TACE procedures. |
Statistical Analysis
A two-sided p < 0.05 was considered as a statistically significant difference. Univariate and multivariate logistic regression analyses were performed to determine whether certain factors could predict hemostasis failure within 30 min after the procedure. SPSS statistical software (version 21.0; Chicago, IL, USA) was used for the statistical analyses.
Results
Patient Characteristics
One hundred and twenty-two patients with HCC were considered eligible according to the inclusion criteria. Two patients with small radial arteries (diameter < 2 mm) and one patient unwilling to participate in the study were excluded. Ultimately, 119 patients who underwent 134 TRA-TACE procedures were included in the analysis. Fifteen (12.6%) patients underwent TRA-TACE twice. The baseline characteristics of the 119 patients are shown in Table 1. Most patients were men (86.6%), and the most common etiology was hepatitis B virus infection (73.1%). According to the Barcelona Clinic Liver Cancer (BCLC) staging system, half of the patients were classified as BCLC stage B (52.1%). Factors that potentially affect the compression time to achieve hemostasis, such as hypertension (25.2%), diabetes (19.3%), platelet count (135.3 ± 64.5×109 /L), and international normalized ratio (1.09 ± 0.12), have also been reported. None of the patients received antiplatelet or anticoagulation therapy within two weeks before the procedure.
 |
Table 1 Clinical Characteristics of the 119 Patients with HCC Who Underwent Transradial Access Chemoembolization (TRA-TACE) |
TRA-Related Outcomes
Technical success was achieved in all TRA procedures (Table 2), and none of the patients required conversion to the femoral artery or contralateral radial artery. For most TRA procedures (83.6%), one puncture attempt was sufficient to successfully establish radial artery access using the conventional palpation method. The puncture time was usually less than 1 min (27.9 ± 25.6 s). The diameter of the radial artery evaluated before the procedure (2.7 ± 0.3 mm), 24 h after the procedure (2.8 ± 0.3 mm) and 30 days after the procedure (2.7 ± 0.3 mm) were not significantly different from each other. None of the patients experienced major TRA-related adverse events. Minor adverse events occurred in 7.5% of the patients, including pain (n = 6, 4.5%), ecchymosis (n = 4, 3.0%), hematoma (n = 2, 1.5%), and early RAO (n = 1, 0.7%). For two patients with a hematoma, more than one puncture was attempted, and the platelet count was 69×109 /L in one patient. However, the compression time to achieve hemostasis for both patients was 30 min. Early RAO was detected via ultrasound in one patient 24 h after the procedure; however, the radial artery spontaneously recanalized one month after the procedure without any treatment. For patients who underwent the first and the second TRA procedures, the incidence of minor adverse events was not significantly different between each other (7.6% vs 6.7%, p > 0.999).
 |
Table 2 Outcomes of the 134 TRA Procedures in the 119 Patients |
Compression Time to Achieve Hemostasis and Its Related Factors
The median and mean compression time were 30 (range: 30–60) and 31.8 (standard deviation: 5.0) min, respectively. The mean compression time between the first (n = 119) and the second (n = 15) TRA procedures was not significantly different (31.8 ± 5.2 min vs 32.0 ± 4.1 min, p = 0.865). Successful initial hemostasis was achieved in 117 TRA procedures (87.3%) at 30 min after the procedure, and the compression time for all procedures to achieve hemostasis was 60 min (Figure 2A). To investigate the factors that independently affect the hemostasis process, TRA procedures were divided into two groups: those that achieved hemostasis within 30 min after the procedure (n = 117), and those that did not achieve hemostasis within 30 min after the procedure (n = 17). Factors that may impact hemostasis were analyzed in the univariate and multivariate analyses (Table 3), and a platelet count < 100×109 /L (p = 0.016, odds ratio = 3.942, 95% confidence interval: 1.296–11.989) was found to be the only independent factor that could predict the failure to achieve hemostasis within 30 min. For patients with a platelet count < 100×109 /L, 34 of 45 (75.6%) TRA procedures achieved hemostasis at 30 min after the procedure; the time for all procedures to achieve hemostasis was 60 min (Figure 2B). However, for patients with a platelet count ≥ 100×109 /L, 83 of 89 (93.3%) TRA procedures achieved hemostasis at 30 min after the procedure; the time for all procedures to achieve hemostasis was 40 min (Figure 2C).
 |
Table 3 Univariate and Multivariate Logistic Regression Analyses for Factors Predicting the Time to Remove the Compression Device (> 30 min or not) |
Discussion
Patent hemostasis using a compression device has been considered the most efficient strategy to achieve hemostasis and decrease the occurrence of RAO, and is recommended for use in peripheral TRA interventions.13 However, to the best of our knowledge, the shortest compression time using the patent hemostasis strategy is still unknown in the field of peripheral interventions. In this study, we found that (1) the patent hemostasis strategy was safe for use in patients with HCC treated with TRA-TACE, (2) compression for 30 min was sufficient to achieve hemostasis in most patients, and (3) platelet count was independently related to the time to achieve hemostasis. Based on these findings, we propose a novel strategy to modify the patent hemostasis strategy in the field of peripheral interventions, especially in patients treated with TRA-TACE (Figure 3).
 |
Figure 3 Modified patent hemostasis strategy based on platelet counts for transradial access chemoembolization (TRA-TACE) in patients with hepatocellular carcinoma. Abbreviation: PLT, platelet count. |
In this study, the patent hemostasis strategy was safe and efficient in achieving complete hemostasis. Posham et al applied the patent hemostasis strategy in more than 1500 cases of non-coronary TRA procedures. Major adverse events and RAO were observed in 0.13% and 0.73% of the patients, respectively, and repeat TRA procedures (even up to 7 times) were not associated with complications.5 In a randomized trial using the patent hemostasis strategy, Yamada et al compared patient satisfaction with TRA and TFA in 36 patients with HCC, and no major adverse events or RAO were observed. The compression time was further shortened in the current study compared to that in previous studies.4,5 No serious adverse events or permanent RAO were observed and repeat TRA procedures (no more than twice) were shown to be safe. In cardiac interventions, the rate of RAO after using the patent hemostasis strategy was higher (2.8–11.7%) than that observed in peripheral interventions (0–0.73%).12,16–18 This could be because of the smaller sheath size (ie, ≤ 5 Fr) which is usually sufficient for most peripheral interventions; however, larger sheaths are more commonly needed in cardiac TRA interventions.19
In this study, compression for 30 min was sufficient for most patients (87.3%) to achieve hemostasis (mean time to achieve hemostasis: 31.8 ± 5.0 min). The compression time was as short as the time to achieve hemostasis using distal radial artery access (25–40 min).20 Roberts et al used the TR band (Terumo) to measure compression time in patients undergoing cardiac TRA interventions, and the compression time was 219 ± 82 min.21 In another study using a self-made device to compress the TRA after coronary angiography, the compression time was 68.3 ± 12.2 min.22 These results showed that the compression time for peripheral and cardiac interventions was different, even with the application of a patent hemostasis technique. As discussed, these differences could result from the use of different sizes of sheaths. These data thus suggest that a patent hemostasis strategy should be modified to fit the unique application scenario of peripheral interventions.
Therefore, we propose a modified hemostasis protocol based on platelet counts and a patent hemostasis strategy for peripheral interventions. Platelet count was used to modify the strategy because: (1) bleeding complications were fewer in TRA, which was recommended for use in patients with a low platelet count (< 50×109 /L) in peripheral interventions;23,24 (2) previous studies showed that antiplatelet therapy could increase the incidence of access-related adverse events (eg, hematomas); and (3) in this study, platelet count was found to be an independent predictor of whether or not patients could achieve complete hemostasis within 30 min after the TRA procedure. Additionally, to simplify the strategy, a platelet count of 100×109 /L was used as the cut-off value to stratify the patients into two groups. For patients with a platelet count of < 100×109 /L, a 60-min compression was sufficient to achieve complete hemostasis. However, for patients with a platelet count of ≥ 100×109 /L, a 40-min compression was sufficient to achieve complete hemostasis.
This study had some limitations. First, this was a single-center study with a small sample size, and the findings and strategy require further validation. Second, only one type of radial sheath (5-Fr radial access kit, Radiofocus, Terumo) and a single fine radial compression device (Finale, Merit) were used in this study. For centers using other TRA sheaths and compression devices, we advise modifying the strategy according to their observations. Third, patients enrolled in this study received TRA procedures no more than twice; hence, further studies to investigate the influence of the modified hemostasis strategy on repeat TRA procedures (more than twice) are warranted. Finally, patients who did not achieve hemostasis during the initial evaluation at 30 min experienced at least one additional bleeding event during the testing process. Consequently, the time to achieve hemostasis might have been shorter in these patients if only one test was performed at a later, more appropriate time.
Conclusion
In conclusion, to achieve hemostasis in patients with HCC treated with TRA-TACE, compression for 60 min is sufficient in patients with a platelet count < 100×109 /L, and compression for 40 min is sufficient for patients with a platelet count ≥ 100×109 /L. This strategy may improve patient satisfaction and efficiency of TRA procedures in the field of peripheral interventions.
Abbreviations
TRA-TACE, Transradial access chemoembolization; TRA, Transradial access; TFA, Transfemoral access; RAO, Radial artery occlusion; HCC, Hepatocellular carcinoma; BCLC, Barcelona Clinic Liver Cancer; PLT, Platelet count.
Data Sharing Statement
All data are available upon request from the corresponding authors on reasonable request.
Ethics Approval and Informed Consent
This prospective, single-center study was approved by Ethics Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (Approval No. 2018121413395002). Written informed consent was obtained from all patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors report no conflicts of interest in this work.
References
1. Van Meter C, Vasudevan A, Cuccerre JM, Schussler JM. Time to discharge following diagnostic coronary procedures via transradial artery approach: a comparison of Terumo band and StatSeal hemostasis. Cardiovasc Revasc Med. 2018;19(7Pt A):759–761. doi:10.1016/j.carrev.2018.03.009
2. Jolly SS, Yusuf S, Cairns J, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377(9775):1409–1420. doi:10.1016/S0140-6736(11)60404-2
3. Zhang X, Luo Y, Tsauo J, et al. Transradial versus transfemoral access without closure device for transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized trial. Eur Radiol. 2022;32(10):6812–6819. doi:10.1007/s00330-022-09038-1
4. Yamada R, Bracewell S, Bassaco B, et al. Transradial versus transfemoral arterial access in liver cancer embolization: randomized trial to assess patient satisfaction. J Vasc Interv Radiol. 2018;29(1):38–43. doi:10.1016/j.jvir.2017.08.024
5. Posham R, Biederman DM, Patel RS, et al. Transradial approach for noncoronary interventions: a single-center review of safety and feasibility in the first 1500 cases. J Vasc Interv Radiol. 2016;27(2):159–166. doi:10.1016/j.jvir.2015.10.026
6. Iezzi R, Posa A, Bilhim T, Guimaraes M. Most common misconceptions about transradial approach in interventional radiology: results from an international survey. Diagn Interv Radiol. 2021;27(5):649–653. doi:10.5152/dir.2021.20256
7. Rashid M, Kwok CS, Pancholy S, et al. Radial artery occlusion after transradial interventions: a systematic review and meta-analysis. J Am Heart Assoc. 2016;5(1):e002686. doi:10.1161/JAHA.115.002686
8. Fernandez RS, Lee A. Effects of methods used to achieve hemostasis on radial artery occlusion following percutaneous coronary procedures: a systematic review. JBI Database System Rev Implement Rep. 2017;15(3):738–764. doi:10.11124/JBISRIR-2016-002964
9. Thakor AS, Alshammari MT, Liu DM, et al. Transradial access for interventional radiology: single-centre procedural and clinical outcome analysis. Can Assoc Radiol J. 2017;68(3):318–327. doi:10.1016/j.carj.2016.09.003
10. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
11. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
12. Dai N, Xu DC, Hou L, Peng WH, Wei YD, Xu YW. A comparison of 2 devices for radial artery hemostasis after transradial coronary intervention. J Cardiovasc Nurs. 2015;30(3):192–196. doi:10.1097/JCN.0000000000000115
13. Bernat I, Aminian A, Pancholy S, et al. Best practices for the prevention of radial artery occlusion after transradial diagnostic angiography and intervention: an international consensus paper. JACC Cardiovasc Interv. 2019;12(22):2235–2246. doi:10.1016/j.jcin.2019.07.043
14. Pacchioni A, Mugnolo A, Sanz Sanchez J, et al. Radial artery occlusion after conventional and distal radial access: impact of preserved flow and time-to-hemostasis in a propensity-score matching analysis of 1163 patients. Catheter Cardiovasc Interv. 2022;99(3):827–835. doi:10.1002/ccd.30005
15. US Department of Health and Human Services; National Institutes of Health; National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
16. Petroglou D, Didagelos M, Chalikias G, et al. Manual versus mechanical compression of the radial artery after transradial coronary angiography: the MEMORY multicenter randomized trial. JACC Cardiovasc Interv. 2018;11(11):1050–1058. doi:10.1016/j.jcin.2018.03.042
17. Lavi S, Cheema A, Yadegari A, et al. Randomized trial of compression duration after transradial cardiac catheterization and intervention. J Am Heart Assoc. 2017;6(2):e005029. doi:10.1161/JAHA.116.005029
18. Pancholy SB, Bernat I, Bertrand OF, Patel TM. Prevention of radial artery occlusion after transradial catheterization: the PROPHET-II randomized trial. JACC Cardiovasc Interv. 2016;9(19):1992–1999. doi:10.1016/j.jcin.2016.07.020
19. Park JY, Kim JK, Cheon JE, Lee MC, Han HS. Meniscus stiffness measured with shear wave elastography is correlated with meniscus degeneration. Ultrasound Med Biol. 2020;46(2):297–304. doi:10.1016/j.ultrasmedbio.2019.10.014
20. Hadjivassiliou A, Cardarelli-Leite L, Jalal S, et al. Safety and efficacy of a truncated deflation algorithm for distal transradial access. J Vasc Interv Radiol. 2020;31(8):1328–1333. doi:10.1016/j.jvir.2020.02.027
21. Roberts JS, Niu J, Pastor-Cervantes JA. Comparison of hemostasis times with a chitosan-based hemostatic pad (Clo-Sur(Plus) Radial) vs mechanical compression (TR Band(R)) following transradial access: a pilot study. Cardiovasc Revasc Med. 2019;20(10):871–874. doi:10.1016/j.carrev.2018.11.026
22. Campos MAC, Alves CMR, Tsunemi MH, Peterlini MAS, Avelar AFM. Randomized clinical study on radial artery compression time after elective coronary angiography. Rev Lat Am Enfermagem. 2018;26:e3084. doi:10.1590/1518-8345.2584.3084
23. Titano JJ, Biederman DM, Marinelli BS, et al. Safety and feasibility of transradial access for visceral interventions in patients with thrombocytopenia. Cardiovasc Intervent Radiol. 2016;39(5):676–682. doi:10.1007/s00270-015-1264-3
24. Kis B, Mills M, Hoffe SE. Hepatic radioembolization from transradial access: initial experience and comparison to transfemoral access. Diagn Interv Radiol. 2016;22(5):444–449. doi:10.5152/dir.2016.15571
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

