Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Minimal Clinically Important Difference in Barthel Index Dyspnea in Patients with COPD
Authors Vitacca M , Malovini A, Balbi B, Aliani M, Cirio S , Spanevello A , Fracchia C, Maniscalco M, Corica G, Ambrosino N , Paneroni M
Received 5 June 2020
Accepted for publication 7 September 2020
Published 21 October 2020 Volume 2020:15 Pages 2591—2599
DOI https://doi.org/10.2147/COPD.S266243
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Michele Vitacca,1 Alberto Malovini,2 Bruno Balbi,3 Maria Aliani,4 Serena Cirio,5 Antonio Spanevello,6,7 Claudio Fracchia,8 Mauro Maniscalco,9 Giacomo Corica,10 Nicolino Ambrosino,8 Mara Paneroni1
1Istituti Clinici Scientifici Maugeri IRCCS, Respiratory Rehabilitation Unit of the Institute of Lumezzane, Brescia, Italy; 2Istituti Clinici Scientifici Maugeri IRCCS, Laboratory of Informatics and Systems Engineering for Clinical Research of the Institute of Pavia, Pavia, Italy; 3Istituti Clinici Scientifici Maugeri IRCCS, Respiratory Rehabilitation Unit of the Institute of Veruno, Novara, Italy; 4Istituti Clinici Scientifici Maugeri IRCCS, Respiratory Rehabilitation Unit of the Institute of Cassano Delle Murge, Bari, Italy; 5Istituti Clinici Scientifici Maugeri IRCCS, Respiratory Rehabilitation Unit of the Institute of Pavia, Pavia, Italy; 6Istituti Clinici Scientifici Maugeri IRCCS, Respiratory Rehabilitation Unit of the Institute of Tradate, Varese, Italy; 7University of Insubria, MACRO, Varese, Italy; 8Istituti Clinici Scientifici Maugeri IRCCS, Respiratory Rehabilitation Unit of the Institute of Montescano, Pavia, Italy; 9Istituti Clinici Scientifici Maugeri IRCCS, Respiratory Rehabilitation Unit of the Institute of Telese, Benevento, Italy; 10Istituti Clinici Scientifici Maugeri IRCCS, Health Directorate of the Institute of Lumezzane, Brescia, Italy
Correspondence: Michele Vitacca
Istituti Clinici Scientifici Maugeri IRCCS, Via Salvatore Maugeri, 4, Pavia 27100, Italy
Email [email protected]
Background: The Barthel Index dyspnea (BId) is responsive to physiological changes and pulmonary rehabilitation in patients with chronic obstructive pulmonary disease (COPD). However, the minimum clinically important difference (MCID) has not been established yet.
Aim: To identify the MCID of BId in patients with COPD stratified according to the presence of chronic respiratory failure (CRF) or not.
Materials and Methods: Using the Medical Research Council (MRC) score as an anchor, receiver operating characteristic curves and quantile regression were retrospectively evaluated before and after pulmonary rehabilitation in 2327 patients with COPD (1151 of them with CRF).
Results: The median post-rehabilitation changes in BId for all patients were − 10 (interquartile range = − 17 to − 3, p< 0.001), correlating significantly with changes in MRC (r = 0.57, 95% CI = 0.53 to 0.59, p< 0.001). Comparing different methods of assessment, the MCID ranged from − 6.5 to − 9 points for patients without and − 7.5 to − 12 points for patients with CRF.
Conclusion: The most conservative estimate of the MCID is − 9 points in patients with COPD, without and − 12 in those with CRF. This estimate may be useful in the clinical interpretation of data, particularly in response to intervention studies.
Keywords: activities of daily life, breathlessness, dyspnea, chronic respiratory failure, exercise training, health related quality of life, rehabilitation
Introduction
Breathlessness, “the subjective experience of breathing discomfort”1 is the main multidimensional symptom of chronic obstructive pulmonary disease (COPD), associated to reduced health related quality of life (HRQL), leading to distress in patients and families in all stages of disease.2 This experience can be affected by psychological factors and in different chronic respiratory diseases is based on different pathophysiological abnormalities with different qualities of respiratory discomfort, as defined by specific verbal descriptors.3 The severity of symptom cannot be predicted from lung function; therefore, dyspnea must be measured specifically in order to evaluate the effects of any treatment. Several instruments are commonly used to measure different domains of dyspnea such as sensory-perceptual experience, affective distress, symptom impact or burden.4
Pulmonary rehabilitation has stronger evidence of effectiveness to improve exercise capacity, dyspnea in activity of daily life (ADL), and HRQL than almost all other therapies in patients with COPD including those with very severe disease, complex comorbidities and different phenotypes.5–7 Therefore, current guidelines recommend pulmonary rehabilitation in these patients.8
In routine clinical practice, the Barthel Index is the most widely used scale to measure patients’ motor and functional disabilities in ADL.9 This index was developed for chronic and long-term hospital patients with neurological diseases examining their performance before and after treatment and predicting time needed for motor rehabilitation and amount of nursing aid required. However, motor disability in ADL of patients with COPD is different from that of patients with neuromotor difficulties, because it is influenced by dyspnea. In patients with chronic respiratory diseases, the Barthel Index dyspnea (BId), based on a modified Barthel Index was shown to be a reliable, sensitive, and adequate tool for measuring the level of dyspnea perceived in performing basic ADL. It has been shown to be responsive to pulmonary rehabilitation in patients with COPD with and without chronic respiratory failure (CRF).10
The minimum clinically important difference (MCID) is the smallest change in score that patients perceive as beneficial or detrimental, and it is useful to aid the clinical interpretation of an outcome measure in response to intervention. At difference with other tools to assess dyspnea such as the Medical Research Council (MRC) score,11,12 to our knowledge the MCID of the BId has not yet been described.
Therefore, we aimed to estimate the MCID of the BId in response to pulmonary rehabilitation in patients with COPD with and without CRF. We postulated that the BId score would improve with pulmonary rehabilitation, change in BId score would correlate significantly with changes in MRC score and that the MCID would be different between COPD patients with CRF and COPD patients without CRF.
Materials and Methods
The study was approved by the Istituti Clinici Scientifici (ICS) Maugeri Ethics Committee (registered: EC 1078). Patients give their informed consents for the use of scientific data as part of the routine assessment at admission. This study was conducted in accordance with the Declaration of Helsinki.
Participants
This retrospective study was conducted on the Automated Integrated Health-Care Record database of patients with COPD with or without CRF, consecutively admitted between January, the first, and August, the 30th, 2019 to the centers of ICS Maugeri (Lumezzane, Pavia, Tradate, Veruno, Montescano, Cassano delle Murge, Italy), referral hospitals for pulmonary rehabilitation, diagnosis and care of chronic patients.13 Patients were either transferred from acute care hospitals, after recovery from an acute exacerbation or admitted directly from home, on referral by their general practitioners, where they had not performed any pulmonary rehabilitation program.
Diagnosis and severity of COPD were confirmed by spirometry according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines.8 At the time of BId measure, all patients were in stable conditions as assessed by the absence of worsening in symptoms, ie, no change in the patient’s dyspnea, cough, and/or sputum beyond day-to-day variability which would have been sufficient to warrant a change in management, and stability in blood gas values (eg, no respiratory acidosis) compared to the conditions reported at home or at discharge from the referring hospital. All patients received their regular treatment according to current guidelines for their disease stage.8 For the purpose of this study, CRF was defined as the presence of at least one of the following conditions: i) need of long-term oxygen therapy (LTOT); ii) arterial oxygen tension (PaO2) < 55.0 mmHg at rest. Exclusion criteria were: obese patients with overlap syndrome, patients needing home mechanical ventilation, oncological, neurological, heart failure, ischemic cardiovascular diseases, inability or denial to perform evaluations or pulmonary rehabilitation.
Measurements
Demographic, comorbidities with the Cumulative Illness Rating Scale (CIRS),14 dynamic lung volumes, assessed according to standards,15 using the predicted values of Quanjer,16 and arterial blood gases were recorded at admission. Arterial blood gases of patients under LTOT were assessed under their usual inspiratory oxygen fraction (FiO2).
For the purpose of this retrospective study, we have used the MRC as anchor measure as the best and available measurement scale measuring dyspnea, therefore we analyzed the BId10 and the MRC scale.11
- Barthel Dyspnoea Index. The total BId score ranges from 0 (no dyspnea) to 100 (maximum level of dyspnea). A decrease in BId score represents an improvement, whereas an increase in score represents a worsening in symptom. The test–retest and inter-observer reliability and concurrent validity of the BId have recently been reported in patients with chronic respiratory diseases, mostly COPD.10
- A one-point decrease is usually considered the MCID for MRC.12
To assess the effectiveness of the pulmonary rehabilitation program used before and after the program we assessed also:
- Disease impact by the COPD assessment test (CAT)17
- Exercise tolerance by the six minutes walking distance test (6MWD)18
Pulmonary Rehabilitation
A multidisciplinary team consisting of chest physicians, nurses, physical therapists, dieticians, and psychologists offered care. Our standard inpatient multidisciplinary program includes the optimization of drug therapy, education, nutritional programs and psychosocial counselling when appropriate, and at least 22 sessions over a period of 3–4 weeks, of supervised incremental exercise training according to Maltais et al,19 until performing 30 min continuous cycling at 50–70% of the maximal load calculated on the basis of the baseline 6MWT according to Luxton et al.20 Peripheral limb muscle activities, shoulder, and full arm circling are also performed.
Supplemental oxygen for patients with CRF and interval training for most compromised patients were delivered. Pulse oximetry, arterial blood pressure and heart rate were monitored during exercise. The total daily time duration of activities was 2–3 hours, and the entire program was performed in the hospital.
Statistical Analysis
All statistical procedures were performed by the R statistical software tool version 3.6.1 (www.r-project.org). Quantitative variables distribution was described by median and interquartile range (IQR), categorical variables distribution by absolute frequencies (n) and relative frequencies (%).
We included in the analysis only patients with paired (at admission and discharge) BId and MRC scores. Since most of the numerical variables analyzed deviated from the normal distribution based on the visual inspection of histograms and quantile-quantile plots it was decided to apply non-parametric tests and to describe numerical variables distributions by median and IQR.
The two-tailed Wilcoxon test for paired samples was applied to test for statistically significant post-rehabilitation changes. The two-tailed Wilcoxon rank-sum test and the Pearson Chi-square test were applied to test for statistically significant differences in variables distribution between presence and absence of CRF.
Changes in BId and MRC were estimated as BId discharge – BId admission values and MRC discharge – MRC admission values, respectively.
The correlation between changes in BId and MRC was assessed by means of Spearman correlation coefficient r using functions implemented in the package “stats”.
The Receiver Operating Characteristics (ROC) curves, the corresponding Area Under the ROC curve (AUROC) and most informative BId change threshold were estimated by functions implemented in the package called “pROC” using a reduction in MRC of at least 1 point as positive reference (ie, patients with changes ranging from −4 to −1 were coded as “1” while patients with changes ranging from 0 to +4 were coded as “0”).
Quantile regression (tau = 0.50) was applied to estimate the difference in median BId change between patients with MRC change ≤-1 point (ie, ranging from −4 to −1) compared to those with MRC change >-1 point (ie, ranging from 0 to +4) as implemented in the package called “quantreg. The BId change was considered the response variable while the MRC change (binarized according to a threshold of ≤-1 as described above) was considered the independent variable: the MCID was assumed to correspond to the MRC regression coefficient.
The MCID values using ROC curve and quantile regression methods have been estimated on all patients, independently from their MRC change (ie, including patients with changes ranging from −4 to +4) and BId change (ie, including patients with changes ranging from −100 to +100). The same approach has been applied to the analysis of the whole cohort and of the subgroup of patients with and without CRF.
The BId at admission was discretized based on baseline MRC scores using the midpoint value between the 75th percentile of BId corresponding to each MRC score value and the 25th percentile of the closest higher MRC value. As an example, assuming a 75th percentile value of BId = 6 for MRC = 0 and a 25th percentile value of BId = 5 for MRC = 1, the BId threshold discriminating between the two MRC consecutive values was estimated as (6 + 5)/2 = 5.5 points.
The significance level was set to α = 0.05.
For a more detailed description of statistical methods, the reader is referred to the Supplementary Methods section.
Results
Out of 3267 patients admitted to the centers in the study period, 2558 suffered from COPD. Out of them, data of 2327 COPD patients (1151 of them with CRF; 1013 patients (88.0%) with CRF type 1 and 138 (12.0%) with CRF Type 2), with available paired BId and MRC values were included in the analysis. The number of patients admitted to each center ranged from 288 to 463. Table 1 shows the main characteristics of the patients.
 |
Table 1 Characteristics of Patients |
The rehabilitation program was effective as assessed also by improvement in other outcome measures such as the 6MWD [from median (IQR): 245 (160:330) to 310 (222:390) meters, p < 0.001 and from 330 (238:411) to 375 (295:460) meters, p < 0.001 in patients with and without CRF, respectively,] and CAT [from median (IQR): 23 (18:27) to 15 (11:20), p < 0.001 and from 18 (12:24) to 11 (6:15), p < 0.001 in patients with and without CRF, respectively,], without any statistically significant difference in post-rehabilitation changes between the two populations (p > 0.05).
Table 2 shows the post-rehabilitation changes in BId and MRC. Both BId and MRC values at admission differed significantly between patients with and without CRF (p < 0.001). A significant difference in BId, but not in MRC changes was observed between patients with and without CRF.
 |
Table 2 Distribution of Barthel Index Dyspnea (BId) and MRC Score at Admission, at Discharge and Post-Rehabilitation Changes |
Out of the 2327 patients of the analyzed cohort, 1903 (81.8%) showed an improvement in BId, 376 (16.2%) did not change their condition while 48 (2.1%) showed an increase in their BId. A total number of 1667 patients (71.6%) experienced a decrease in MRC, 637 (27.4%) did not change their condition while 23 (1.0%) increased their MRC. The frequency distribution of the variations in post-rehabilitation BId and MRC is reported in Supplementary material, Supplementary Figure 1 panel A and panel B, respectively.
Figure 1 shows that the highest changes in BId were observed in patients with the highest baseline values. Figure 2 describes the distribution of baseline BId according to the baseline MRC, with levels of severity for BId as function of MRC grading. The correlation coefficient between BId and MRC values at admission was 0.90 (95% CI = 0.89 to 0.91, p < 0.001).
 |
Figure 1 Post-rehabilitation changes in Barthel Index dyspnea (BId) by deciles of baseline values. |
 |
Figure 2 Distribution of baseline BId by MRC. |
Estimation of MCID of BId in All Patients
The Spearman correlation coefficient between changes in BId and MRC in the whole cohort was 0.57 (95% CI = 0.53 to 0.59, p< 0.001, Figure 3). An alternative visualization of changes in BId as function of the MRC changes is provided by the boxplots in Supplementary material, Supplementary Figure 2 panel A and panel B, showing a nearly linear increasing trend in median BId changes as function of MRC change, with a more evident trend when low-frequency categories (<10 observations) were pooled.
Out of 2327 patients, 1667 (71.6%) showed a post-program reduction in MRC scale less or equal to one point. The AUROC reached by the changes in BId in discriminating patients with variations in MRC less or equal to −1 point (ie, MRC changes ranging from −4 points to −1 point) from those with variations greater than −1 point (ie, MRC changes ranging from 0 points to +4 points) was 0.79 (95% CI = 0.77 to 0.81) while the best discriminating cut off value in BId change was −6.5, with sensitivity = 0.77 and specificity = 0.72 (Figure 4). With quantile regression, by use of a reduction in MRC score by 1 point as the threshold for MCID, the estimated MCID for BId representing the difference in median values between the two MRC populations (MRC changes ranging from −4 points to −1 point vs changes ranging from 0 points to +4 points) was −10 points (95% CI = −11 to −9) (Supplementary Table 1).
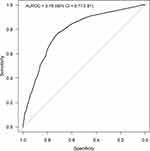 |
Figure 4 ROC curve describing the performances of the changes in BId in discriminating changes in MRC ≤ −1. |
Thus, considering the results from anchor based methods, the MCID of BId estimated in all COPD patients ranged from −6.5 to −10 points.
Estimation MCID of BId According to the Presence of CRF
The MCID was also assessed in patients without and with CRF independently. Results showed that the strength of the correlation between changes in BId and MRC scores estimated in the two subpopulations was similar (r = 0.58, 95% CI = 0.53 to 0.62 for patients without CRF - Supplementary material, Supplementary Figure 3 panel A - and r = 0.56, 95% CI = 0.52 to 0.60 for patients with CRF - Supplementary material, Supplementary Figure 3 panel B).
The AUROC estimates reached by the changes in BId in discriminating patients with reduction in MRC ≤ 1 point from the others were pretty the same: 0.79 (95% CI = 0.76 to 0.82) in patients without (Supplementary material, Supplementary Figure 4 panel A) and 0.79 (95% CI = 0.76 to 0.82) in patients with CRF (Supplementary material, Supplementary Figure 4 panel B). Based on the ROC curve, the best discriminating cut off values of BId changes was −6.5 for patients without CRF (sensitivity = 0.76, specificity = 0.72) and −7.5 for patients with CRF (sensitivity = 0.75, specificity = 0.73).
With quantile regression, by use of a reduction in MRC score by 1 point as the threshold for MCID, the estimated MCID for BId was −9 points (95% CI = −10 to −7) in patients without CRF while −12 (95% CI = −13 to −10) in patients with CRF (Supplementary Table 1).
Table 3 summarizes the results of the different methods used to estimate the MCID of BId. Thus, MCID ranged from −6.5 to −9 points for patients without and from −7.5 to −12 points for patients with CRF patients. The most conservative estimate of the MCID is −9 points in patients without and −12 in those with CRF.
 |
Table 3 Summary of the Results from Different Methods Used to Estimate the MCID of BId |
Discussion
Our study shows that the BId is responsive to changes after pulmonary rehabilitation and that changes in BId correlate significantly with changes in MRC. Furthermore, this study is the first to estimate the MCID for the BId in patients with COPD with and without chronic respiratory failure. As far as we are aware, our study is the largest so far to use the BId during pulmonary rehabilitation. We showed longitudinal validity of the BId by identifying significant correlations between changes in BId and in MRC. The most conservative estimate of the MCID was −10 points considering the whole cohort while −9 in patients without and −12 in patients with chronic respiratory failure. The pulmonary rehabilitation program was effective as assessed by the changes in other available outcome measures.
The determination of the MCID remains controversial without any firm consensus, but it is important in the validation of clinical instruments and the assessment of clinical studies. MCID after rehabilitation have been reported for other outcome measures of pulmonary rehabilitation,5 such as MRC,12 6MWD,18 CAT,21 St George Respiratory questionnaire.12 In COPD, validated MCID exist also for other tools to measure dyspnoea such as the Transitional Dyspnoea Index,22 University of California, San Diego Shortness of Breath Questionnaire23 and the Chronic Respiratory Questionnaire.24
We have estimated the MCID using anchor-based methods, relying on the comparison of the change in BId with another outcome measure with a known MCID such as MRC as the anchor or external criterion. However, this comparison is only relevant if there is an established association between the outcome of interest and the anchor. No consensus exists about the threshold to define sufficient evidence of association: some investigators have suggested a minimum correlation coefficient greater than 0.50, although others have suggested 0.30.25,26 The correlation coefficients between changes in BId and MRC reported in our study was 0.57 with a 95% confidence interval (0.53 to 0.59) that did not overlap neither thresholds, thus significantly higher than both values. Anchor based methods (ROC curve method and quantile regression) provided MCID estimates of −6.5 and −10 based on the whole cohort, while −6.5 and −9 for patients without CRF and −7.5 and −12 for patients with CRF. However, results from the ROC method should be cautiously interpreted due to the unbalanced distribution of patients who improved their condition and those who did not improved or worsened based on MRC MCID of −1 point (improved/not improved or worsened ratio: 2.53 in the whole cohort while 2.71 and 2.36 in patients without and with CRF, respectively).
In patients with severe COPD, the BId may therefore have a particular role in the assessment of poorly functioning or frail individuals. Typical scenarios might be the critical care setting, hospitalized patients, those recovering from exacerbation or housebound patients. Future prospective studies in different populations are needed to confirm this hypothesis.
Anchors are designed to detect changes in outcome but rarely take into account costs to the patient, for example, side effects of treatment. Changes in an outcome measure might be dependent on the baseline level. In our study the post-rehabilitation changes in BId increased with increased severity of baseline values (Figure 1). Although this does not affect the potential utility of the BId as a simple screening tool for dyspnea, the BId may be less useful when testing the effects of intervention in patients with low level of disability.
The observed improvement also in other outcome measures such as 6MWD and CAT in both populations studied is not surprising, previous studies and meta-analyses have shown the effectiveness of pulmonary rehabilitation also in most severe patients including those with CRF.6,7,27
The MCID are used in clinical trials. Factors such as trial duration, withdrawal rates, and baseline disease severity may affect the size of benefit relative to the MCID in clinical trials and MCID should be interpreted as indicative, rather than absolute.28
Limitations
This study has limitations. It is a retrospective analysis and due to missing data, only patients with coupled data were included. It could be argued that these missing data might bias our estimates of the MCID. However, the sample size was huge, covering a wide range of severity. No other tools for evaluation of dyspnea were assessed.
We have not used more outcome measures as anchor or reference instruments because in this retrospective study MRC was the only available specific tool to measure dyspnea. The MRC is a short 5 points scale and it has an inherently crude measure and therefore might be less sensitive to change. However, the MRC is the routinely available measurement scale measuring dyspnea in all rehabilitative settings. Despite the MCID has been assessed for outpatients with acute exacerbations of COPD,29 no formal study has assessed the MCID for MRC in stable patients. However, the value of 1 point has been widely used to assess the benefits of pulmonary rehabilitation.12
Other tools such as those assessing health status or ADL often include items related to symptoms but are not specific.30
Other potential limitations linked to the retrospective nature of this study is represented by the lack of multiple anchor scales and of additional clinical and demographic information not routinely collected. The availability of this kind of data could allow estimating more robust MCID values controlling for potential confounders, thus increasing the reproducibility and generalizability of the results.
Conclusions
This study shows that the BId is responsive to the effects of pulmonary rehabilitation in patients with COPD. MCID estimates provided by the different methods resulted quite variable, ranging from –6.5 to −10 in the whole cohort, from −6.5 to −9 in patients without CRF and from −7.5 to −12 in patients with CRF. The most conservative estimate of the MCID is −10 points considering the whole cohort while −9 in patients without and −12 in patients with chronic respiratory failure.
Ethics Approval and Informed Consent
The study was approved by the Istituti Clinici Scientifici (ICS) Maugeri Ethics Committee (registered: EC 1078).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the “Ricerca Corrente” Funding scheme of the Ministry of Health, Italy.
Disclosure
All authors report no conflict to disclose.
References
1. Parshall MB, Schwartzstein RM, Adams L, et al. American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–452. doi:10.1164/rccm.201111-2042ST
2. Ambrosino N, Fracchia C. Strategies to relieve dyspnoea in patients with advanced chronic respiratory diseases. A narrative review. Pulmonology. 2019;25(5):289–298. doi:10.1016/j.pulmoe.2019.04.002
3. Laviolette L, Laveneziana P. Dyspnoea: a multidimensional and multidisciplinary approach. European Respiratory Journal. 2014;43(6):1750–1762. doi:10.1183/09031936.00092613
4. Mahler DA. Measurement of dyspnea: clinical ratings. In: Mahler DA, O’Donnell DE, editors. Dyspnea: Mechanisms, Measurement, and Management.
5. Spruit MA, Singh SJ, Garvey C, et al. ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–e64. doi:10.1164/rccm.201309-1634ST
6. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;2:CD003793.
7. Paneroni M, Simonelli C, Vitacca M, Ambrosino N. Aerobic Exercise Training in Very Severe Chronic Obstructive Pulmonary Disease. Am J Phys Med Rehabil. 2017;96(8):541–548. doi:10.1097/PHM.0000000000000667
8. 2019 Global strategy for prevention, diagnosis and management of COPD. http://goldcopd.org/gold-reports/.
9. Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42(8):703–709. doi:10.1016/0895-4356(89)90065-6
10. Vitacca M, Paneroni M, Baiardi P, et al. Development of a Barthel Index based on dyspnea for patients with respiratory diseases. Int J Chron Obstruct Pulmon Dis. 2016;11:1199–1206. doi:10.2147/COPD.S104376
11. Fletcher CM. Standardised questionnaire on respiratory symptoms: a statement prepared and approved by the MRC Committee on the aetiology of chronic bronchitis (MRC breathlessness score). Br Med J. 1960;2(5213):1665.
12. De Torres JP, Pinto-Plata V, Ingenito E, et al. Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest. 2002;121(4):1092–1098. doi:10.1378/chest.121.4.1092
13. Maestri R, Bruschi C, Fracchia C, Pinna GD, Fanfulla F, Ambrosino N. Physiological and clinical characteristics of patients with COPD admitted to an inpatient pulmonary rehabilitation program: A real-life study. Pulmonology. 2019;25(2):71–78. doi:10.1016/j.pulmoe.2018.07.001
14. Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622–626. doi:10.1111/j.1532-5415.1968.tb02103.x
15. Miller MR, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
16. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi:10.1183/09031936.00080312
17. Jones PW, Harding G, Berry P, Wiklund I, Chen W-H, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509
18. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi:10.1183/09031936.00150314
19. Maltais F, LeBlanc P, Jobin J, et al. Intensity of training and physiologic adaptation in patients with chronic obstructive pulmonary disease.. Am J Respir Crit Care Med. 1997;155(2):555–561. doi:10.1164/ajrccm.155.2.9032194
20. Luxton N, Alison JA, Wu J, Mackey MG. Relationship between field walking tests and incremental cycle ergometry in COPD. Respirology. 2008;13(6):856–862. doi:10.1111/j.1440-1843.2008.01355.x
21. Kon S, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med. 2014;2(3):195–203. doi:10.1016/S2213-2600(14)70001-3
22. Mahler DA, Witek TJ. The MCID of the transition dyspnea index is a total score of one unit. COPD. 2005;2(1):99–103. doi:10.1081/COPD-200050666
23. Kupferberg DH, Kaplan RM, Slymen DJ, Ries AL. Minimal clinically important difference for the UCSD Shortness of Breath Questionnaire. J Cardiopulm Rehabil. 2005;25(6):370–377.
24. Schunemann HJ, Puhan M, Goldstein R, Jaeschke R, Guyatt GH. Measurement properties and interpretability of the Chronic Respiratory Disease Questionnaire (CRQ). COPD. 2005;2(1):81–89. doi:10.1081/COPD-200050651
25. Schunemann HJ. Evaluation of the minimal important difference for the feeling thermometer and the St. George’s Respiratory Questionnaire in patients with chronic airflow obstruction. J Clin Epidemiol. 2003;56(12):1170–1176. doi:10.1016/S0895-4356(03)00115-X
26. Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102–109.
27. Carone M, Patessio A, Ambrosino N, et al. Efficacy of pulmonary rehabilitation in chronic respiratory failure (CRF) due to chronic obstructive pulmonary disease (COPD): the Maugeri Study. Respir Med. 2007;101(12):2447–2453. doi:10.1016/j.rmed.2007.07.016
28. Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med. 2014;189(3):250–255. doi:10.1164/rccm.201310-1863PP
29. Oliveira A, Machado A, Marques MA. Minimal Important and Detectable Differences of Respiratory Measures in Outpatients with AECOPD †. COPD. 2018;15(5):479–488. doi:10.1080/15412555.2018.1537366
30. Almeida Gulart A, de Araujo CLP, Bauer Munari A, Schneider BF, Dal Lago P, Mayer AF. Minimal important difference for London Chest Activity of Daily Living scale in patients with chronic obstructive pulmonary disease. Physiotherapy. 2019;107:28–35.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

