Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Microvascular Changes in Pituitary Adenoma Correlate with Structural Measurements and Visual Field Loss
Authors Chen Y, Li X, Song X, Cong L, Zhang Y
Received 11 June 2023
Accepted for publication 16 November 2023
Published 6 December 2023 Volume 2023:19 Pages 2745—2754
DOI https://doi.org/10.2147/NDT.S425454
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Yaying Chen,1,2 Xiaojiao Li,2 Xiangyuan Song,2 Lin Cong,2 Yuyan Zhang1
1Department of Ophthalmology, Huadong Hospital, Fudan University School of Medicine, Shanghai, People’s Republic of China; 2Department of Ophthalmology, Huashan Hospital, Fudan University School of Medicine, Shanghai, People’s Republic of China
Correspondence: Yuyan Zhang, Department of Ophthalmology, Huadong Hospital, Fudan University School of Medicine, No. 221 West Yan’an Road, Shanghai, 200040, People’s Republic of China, Tel +86 13162899051, Fax +86 21 62483180, Email [email protected]
Purpose: This study aimed to determine the relationship among microvascular changes, retinal nerve fiber layer (RNFL) thickness, and visual field loss in pituitary adenoma (PA) patients.
Patients and Methods: Optic disc and macular vessel densities were measured, using optical coherence tomography angiography (OCTA) in the eyes from PA patients with radiographic chiasmal compression. Comparisons of retinal microvascular and structural parameters were conducted between PA patients and age/sex-matched healthy controls. The PA group was subdivided into PA with temporal visual field defects (perimetric PA) and PA without visual field defect (preperimetric PA) groups. The study determined correlation between microvascular parameters and optic nerve damage, including visual field and structural measurements. Subgroup analyses were performed to distinguish the different microcirculation characteristics of the perimetric PA eyes and preperimetric PA eyes.
Results: Forty-five eyes from 40 PA patients and 24 eyes from 24 healthy controls were recruited prospectively. Eyes in the perimetric PA group had significantly decreased optic disc vessel density but slightly increased macular vessel density at superficial retinal capillary plexus (SCP) level. Eyes in the preperimetric PA group had significantly increased macular vessel density at SCP level. Optic disc vessel density was inversely correlated with visual field mean deviation and positively correlated with RNFL thickness.
Conclusion: Significantly decreased optic disc vessel density in the perimetric stage but increased SCP macular vessel density in the preperimetric stage were found in PA patients. Our data suggest that increased SCP macular vessel density may serve as an early biomarker of preperimetric PA eyes, while decreased optic disc vessel density could be a late biomarker of perimetric PA eyes. Optic disc vessel density was correlated with RNFL thickness and visual field loss in PA eyes. OCTA is a useful tool to detect retinal microvascular changes and access the severity of neural impairments in chiasmal compression caused by PA.
Keywords: optical coherence tomography angiography, pituitary adenoma, optic disc vessel density, macular vessel density, chiasmal compression
Introduction
Pituitary adenoma (PA) is a benign, slow-growing intracranial tumor with a prevalence of 68–94 per 100,000 in the general population,1–4 being the third most common tumor type among all intracranial tumors.5 The clinical characteristic manifestations of PA include pituitary hormone hypersecretion and local space occupying effects, especially visual impairment.6,7 The most common visual impairment in PA patients is visual field defect, which is typically bitemporal hemianopia, suggesting an impairment of optic chiasm in the pathogenesis of the disease.8
Chiasmal compression by PA can result in visual dysfunction through several mechanisms including metabolic, ischemic, and mechanical insults.9,10 In the vascular theory, infra-sellar lesion may disturb the ventral blood vessels of chiasm, thereby selectively affecting the central crossed fibers.11,12 However, chiasmal vascular changes are difficult to be detected directly. Retinal microcirculation may serve as a surrogate marker for vascular pathology in the visual pathway and central nervous system.13,14 Recently, optical coherence tomography angiography (OCTA) has been developed as non-invasive retinal and choroidal vascular imaging with three-dimensional reconstruction, gaining popularity in optic neuropathy diagnosis.15,16 To date, a few studies have explored the retinal vessel densities of PA patients using OCTA, showing a reduction in vessel densities of the papillary region in PA eyes,17–20 but there is little knowledge about macular microvascular changes in PA eyes. In addition, no study has evaluated the early changes of retinal microcirculation before visual field defect occurs in PA.
In the present study, we evaluated the retinal microvascular changes of both optic disc and macular region in PA eyes. Meanwhile, subgroup analyses were performed to distinguish the different microcirculation characteristics of PA eyes with and without visual field defect. The aim of the present study was to determine the relationship among microvascular changes, retinal nerve fiber layer thickness, and visual field loss in PA patients.
Materials and Methods
Subjects
This was a cross-sectional and case-control study. PA patients with chiasmal compression and age-matched normal participants (control) were recruited in the Department of Ophthalmology at Huashan Hospital, Fudan University, between March 2017 and January 2018. The study was approved by the Huashan Hospital Institutional Review Board and all study conducted adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from every participant prior to enrollment in the study.
PA was diagnosis with brain magnetic resonance imaging (MRI) prior to surgery and confirmed with histopathology. All enrolled PA patients had chiasmal compression on MRI. Chiasmal compression was defined as at least one scanning plane, the highest point of the PA was in contact with the optic chiasm, with or without lifting displacement of the optic chiasm.
Exclusion criteria included the eyes co-existing other optic nerve diseases and/or any retinal and ocular diseases, including diabetic retinopathy, choroidal neovascularization, glaucoma, optic neuritis, high refractive hyperopia or myopia (more than +6 or −6 diopters), opacities of the ocular media, and ophthalmic surgical treatment. Control eyes were age-matched volunteers with a refractive error of less than 6 diopters and without any encephalic, retinal, and ocular diseases, as described.16
Automated static perimetry was performed using Octopus 101 perimeter (G2 programs, Haag-Streit, Inc., Koeniz, Switzerland) in PA patients. Abnormal visual field defect was defined as a cluster of three points with a probability <5% on the pattern deviation map, including at least one point with a probability of <1% or a cluster of two points with a probability of <1% with respect to the vertical meridian. Visual field defects were all confirmed on two consecutive, reliable (false-positive errors <15%, false-negative errors <15%, and fixation loss <20%) tests. According to the visual field test result, PA patients were divided into two subgroups: PA patients with temporal visual field defects (perimetric PA) and without visual field defect (preperimetric PA).
All subjects underwent complete ophthalmic examinations in the Department of Ophthalmology, Huashan Hospital, including best corrected visual acuity (BCVA), slit-lamp biomicroscopy, fundus examination, and OCTA examination (Optovue Inc., Fremont, California, USA; software version 2016.2.0.35). It was considered eligible for the current study that the OCTA images of the eyes kept reasonable still without significant movement or shadow artifacts and with a signal strength index score greater than Q6.
We recruited a total of 40 PA patients and 24 healthy controls in the current study. To avoid bias, we included only one eye for analysis in the control and perimetric PA groups. However, both eyes were included for analysis in the preperimetric PA group due to the small sample size. Finally, 30 eyes from 30 perimetric PA patients, 15 eyes from 10 preperimetric PA patients (five eyes had to be excluded because of poor scan quality), and 24 eyes from 24 normal controls were included in the present study. For the correlation and regression analysis, 40 eyes from 40 PA patients were included.
Image Acquisition and Processing
The RTVue-XR Avanti OCTA system (Optovue, Inc., Fremont, CA, USA) with split spectrum amplitude-decorrelation angiography (SSADA) software was utilized to detect the microvascular network of study subjects. This device has an A-scan rate of 70-kHz scans per second, using a light source centered on 840 nm. Four scans were obtained from each participant:
- OCTA imaging of the macula covering a 3×3-mm area centered on the fovea.
- OCTA imaging of the optic disc covering a 4.5×4.5-mm area centered on the optic disc.
- Crossline structural scan of the macula.
- ONH scan of the optic disc to generate retinal nerve fiber layer (RNFL) thickness analysis.
ImageJ software was utilized for quantification of the optic disc OCTA images (ImageJ 1.50g, Wayne Rasband, National Institutes of Health, USA). Four vascular layers (nerve head, vitreous, RPC, choroid) were derived from optic disc OCTA imaging, and the nerve head layer from selected eyes was analyzed. Each image was processed into a circle with a diameter of 270 pixels, aligning with the center of the optic nerve head. We used ImageJ software to extract a binary image of the blood vessels from the grayscale OCTA image, and then calculated the percentage of pixels occupied by blood vessels in the defined region (as described above). This binarization technique was previously validated, which involved duplicating the image and then using a filter on one and a local median threshold on the other.21,22 The percentage of pixels occupied by blood vessels was considered to be close to the average vessel density in the selected range.
The macular OCTA images were analyzed, using the built-in software (Optovue Inc., Fremont, CA, USA; software version 2016.2.0.35). Vessel density was measured in four vascular layers, including superficial retinal capillary plexus (SCP), deep retinal capillary plexus (DCP), outer retina, and choriocapillaris plexus (CC). SCP is defined as the blood flow information between the inner boundary membrane and 10 µm above the lower edge of the inner plexus layer. DCP is defined as the blood flow information between 10 µm above the lower edge of the inner plexus layer and 10 µm below the outer plexus layer. The outer retina is defined as between 10 µm below the outer plexiform layer and 10 µm above Bruch’s membrane. CC is defined as blood flow information between 10 µm above Bruch’s membrane and 30 µm below Bruch’s membrane. In particular, SCP and DCP constitute the retinal capillary network, which is responsible for nourishing the inner and middle retina.
Data Analysis
Statistical analysis was performed using IBM SPSS 23.0 software (Chicago, IL, USA). A Shapiro–Wilk test was utilized to test normal distribution for all continuous variables. Normally distributed variables were presented as the mean±standard deviation, and variables from non-normal distribution was presented as the median (25th percentile, 75th percentile). Independent t-tests, chi-square tests and non-parametric tests were used to compare data between PA and control groups. One-way analysis of variance (ANOVA) or analysis of covariance (ANCOVA) with gender as a covariate (in order to take account of the influence of gender on vascular parameters) was applied to compare variables between the perimetric PA, preperimetric PA, and control groups. To determine the relationship between retinal vessel density and optic nerve damage measurements, Pearson correlation (for variables with normal distributions) or Spearman correlation (for variables with non-normal distributions) was conducted. Multiple linear regression analysis was used to explore whether any of the structural and functional parameters were predictive for the retinal vessel density. A p value less than 0.05 was considered significant. To account for multiple testing, two-sided p values were adjusted according to the method of Benjamini/Hochberg to control the false discovery rate.
Results
General Characteristics
The general characteristics of enrolled subjects are presented in Table 1. Forty PA patients (10 preperimetric and 30 perimetric PAs) with a mean age of 45.5±10.6 years (24–69 years, 18 male and 22 female) were included in this study. In addition, 24 age-matched controls with a mean age of 50.3±12.8 years (22–67 years, 6 male and 18 female) were included. There were 4 (10%) adrenocorticotropic hormone-secreting, 11 (27.5%) growth hormone-secreting, 3 (7.5%) prolactin-secreting, 4 (10%) thyroid-stimulating hormone-secreting, and 18 (45%) non-functioning PAs. The average disease duration of all PA patients was 17.9±3.9 months and the average volume of tumor was 13.0±3.2 cm3.
 |
Table 1 Characteristics of PA Patients and Controls |
The median visual field mean defect of PA group was 1.8 dB. The BCVA in PA group was significantly lower than that of the controls (p<0.001). The average and nasal RNFL thickness were significantly lower in the PA group than that of the controls (p<0.05).
Optic Disc and Macular Vessel Density Outcome
Average optic disc vessel density (Table 2) was significantly reduced in the perimetric PA group compared with the controls (p<0.05). Neither the whole PA group nor preperimetric PA group was characterized by significant difference of average optic disc vessel densities, compared to that of the controls. Nasal and temporal sectorial analysis of vessel densities were also performed, showing that both the nasal and temporal optic disc vessel densities were significantly reduced in the whole PA group (p<0.05) and perimetric PA subgroup (p<0.01), compared to that of the controls.
 |
Table 2 Retinal Vessel Density of PA Eyes and Controls |
In the macular region (Table 2), the average parafoveal SCP vessel density was significantly increased in the whole PA group (p<0.05) and preperimetric PA subgroup (p<0.05), compared to that of the controls. The average parafoveal SCP vessel density in the perimetric PA subgroup was slightly higher than that of the controls without significant difference. There was no significant difference between PA and controls regarding vessel density at DCP levels.
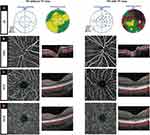
Representative cases of PA eyes with visual field loss and without visual field loss involving their OCTA and structural measurements are shown in Figure 1.
In addition, the ANOVA or ANCOVA p values and the post-hoc comparison p values based on ANOVA or ANCOVA analysis for variables among the preperimetric PA, perimetric PA, and control groups are presented in Supplementary Table 1.
Correlation and Regression Analysis
Forty eyes from the enrolled 40 PA patients were included in the correlation and regression analysis. Pearson or Spearman correlation analysis (Tables 3 and 4) was conducted between retinal vessel density and optic nerve damage measurements in PA eyes. Optic disc vessel density (Table 3 and Figure 2) was negatively correlated with visual field mean deviation (r=−0.602, p<0.001) and positively correlated with average RNFL thickness (r=0.367, p<0.05), as well as nasal RNFL thickness (r=0.518, p<0.01). There was no association between the macular vessel densities and optic nerve damage measurements in our study cohort (Table 4).
 |
Table 3 Correlation and Regression Analysis Between Average Optic Disc Vessel Density and Variation in PA Eyes |
 |
Table 4 Correlation Analysis Between Macular Vessel Densities and Variation in PA Eyes |
Multiple linear regression analysis illustrated that visual field mean deviation and nasal RNFL thickness were significant predictors of optic disc vessel density (adjusted R2=0.350, p<0.001) (Table 3). For every 1 dB difference in visual field mean deviation, optic disc vessel density decreased by an average of 0.416%. For every 1 μm difference in nasal RNFL thickness, optic disc vessel density increased by an average of 0.497%.
Discussion
The present study demonstrated a decreased optic disc vessel density and increased macular vessel density at SCP levels in the PA patients, using OCTA. These microvascular changes were associated with the severity of optic nerve damage, suggesting that OCTA may be a reliable method to detect retinal microvascular changes and access the severity of optic nerve damage in chiasmal compression caused by PA.
OCTA provides an objective approach to evaluate retinal microvasculature and distribution, particularly in microcirculation related optic nerve diseases, such as glaucoma,15 optic neuritis,23 and ischemic optic neuropathy24 via determining images in vivo. To date, a few studies have evaluated the potential role of OCTA for detecting microvascular changes in chiasmal compression. Dallorto et al reported decreased peripapillary retinal perfusion on OCT angiograms in PA patients.17 Lee et al found significantly reduced radial peripapillary capillary vessel density in patients with pituitary tumors.18 In addition, Cennamo et al showed a decrease in vessel density of the papillary region in PA eyes.19 Suzuki et al found that circumpapillary and macular vessel density were significantly reduced in eyes with temporal visual field defect and were strongly correlated with retinal neural and visual field loss.20 However, no study has evaluated the early changes of retinal microcirculation before visual field defect occurs in PA. In the present study, we were particularly interested in microvascular alterations of the optic disc or macular region in PA eyes without visual field defect. We divided the PA patients into two subgroups: PA patients with temporal visual field defects (perimetric PA) and without visual field defects (preperimetric PA).
In our current study, the most intriguing OCTA finding is that macular vessel density at the SCP layer was increased significantly in the eyes from PA with normal visual field. This result suggests that changes in macular vessel density of the inner retina may precede visual field changes, and therefore SCP macular vessel density derived by OCTA may be a potential early biomarker for PA eyes. SCP is responsible for the blood supply of the inner retina, including the retinal nerve fiber layer and ganglion cell layer. Increased macular microvasculature may be an auto-regulatory mechanism and compensatory reaction to match the metabolic demands of retinal ganglion cells in the early stage under conditions caused by visual pathway compression. Compensatory increase in macular capillary hemodynamic changes using OCTA have been reported both in eyes with pan-retinal photocoagulation therapy in proliferative diabetic retinopathy and in eyes with optic disc drusen.25,26 However, our finding contrasts with the other OCTA studies of chiasmal compression, showing that SCP vessel densities of eyes with chiasmal compression are significantly reduced compared with healthy controls.18,19 Such discrepancy may be due to the different characteristics of PA patients enrolled in each study. Compared to prior studies, our study enrolled more PA patients without visual field defect or with only mild visual field defects. Thus, further OCTA studies with a larger sample size are needed to validate increase in SCP macular vessel density as an early biomarker for development of visual dysfunction in PA patients.
Also, we demonstrated a reduction of optic disc vessel density in PA eyes with visual field defects. In line with previous OCTA studies,17–20 we confirmed that impaired optic disc perfusion is involved in compressive optic neuropathy, which might continue to cause further damage even after decompression.9 In addition, we found that average optic disc vessel density was strongly correlated with visual field mean deviation. This finding is consistent with Dallorto et al, showing a good correlation between peripapillary vessel density and visual field mean deviation in 16 eyes from nine PA patients.17 Several studies have demonstrated preoperative visual field changes as reliable indicators of visual impairment severity and powerful predictors of postoperative visual function recovery in chiasmal compression.27–29 The strong correlations between visual field and OCTA parameters found in our study support the claim that OCTA provides an adequate assessment of visual function loss in chiasmal compression caused by PA. Therefore, preoperative assessment of optic disc vessel density with OCTA might be important for the visual estimation after decompression surgery.
Furthermore, we demonstrated that there was a moderate correlation between OCTA parameters and RNFL thickness in PA eyes. Thinning of RNFL thickness is attributed to the long-term axonal compression, resulting in axial plasma stasis and retrograde lesions, which are generally permanent injuries.9 Whereas the decreased optic disc microvasculature is likely secondary loss or closure of capillaries at the area of RNFL atrophy, due to the decrease of metabolic demands of retinal ganglion cells.30 Our finding is consistent with previous studies evaluating the relationship between OCTA and OCT parameters in other optic neuropathies. Lee et al reported that the vascular impairment exactly coincided with the RNFL defect in terms of both the location and the extent.15 It was also detected that peripapillary retinal vessel density in chronic anterior ischemic optic neuropathy was significantly decreased, which is significantly correlated with visual field mean deviation and RNFL.31 Meanwhile, we showed that nasal RNFL thickness was a significant predictor of optic disc retinal vessel density, further supporting that the impaired optic disc microcirculation is secondary to the retrograde axonal injury caused by compression.29,30 Overall, a decrease in optic disc vessel density is an important biomarker to monitor functional and structural injury of PA eyes, but this likely occurs at a later stage in disease.
There are limitations in our study. This study is a cross-sectional study. Future studies with extended longitudinal follow-up of this cohort may provide additional substantive information, especially the prognostic value for visual function recovery of OCTA parameters. In addition, there are more women in the control group than in PA patients, and therefore sex may be a confounding factor in this study, although there was no significant difference in the ratio of males to females between the two groups.
Conclusion
In conclusion, our study demonstrated microvascular changes in PA patients presented as significantly decreased optic disc vessel density in the perimetric stage and significantly increased SCP macular vessel density in the preperimetric stage. Increase in SCP macular vessel density may be an early biomarker to detect early visual dysfunction in preperimetric PA eyes, while decreased optic disc vessel density is a late biomarker to monitor functional and structural injury in PA eyes. OCTA maybe a reliable approach to detect retinal microvascular changes and access the severity of visual loss in chiasmal compression caused by PA.
Acknowledgments
A preprint has previously been published.32 The authors would like to thank Professor Shisan Bao of Discipline of Pathology, School of Medical Science and Bosch Institute, Charles Perkins Centre, University of Sydney, Sydney, Australia, for assistance with the revision of this manuscript.
Funding
This study was funded by Science and Technology Commission of Shanghai Municipality (Technology project 18441901400).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Surawicz TS, McCarthy BJ, Kupelian V, Jukich PJ, Bruner JM, Davis FG. Descriptive. epidemiology of primary brain and CNS tumors: results from the central brain tumor registry of the United States, 1990–1994. Neuro Oncol. 1999;1(1):14–25. doi:10.1093/neuonc/1.1.14
2. Ezzat S, Asa SL, Couldwell WT, et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101(3):613–619. doi:10.1002/cncr.20412
3. Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A. High prevalence of pituitary adenomas: a cross-sectional study in the province of liege, Belgium. J Clin Endocrinol Metab. 2006;91(12):4769–4775. doi:10.1210/jc.2006-1668
4. Fontana E, Gaillard R. Epidemiology of pituitary adenoma: results of the first Swiss study. Rev Med Suisse. 2009;5(223):2172–2174.
5. Scheithauer BW, Gaffey TA, Lloyd RV, et al. Pathobiology of pituitary adenomas and carcinomas. Neurosurgery. 2006;59(2):341–353. doi:10.1227/01.NEU.0000223437.51435.6E
6. Aflorei ED, Korbonits M. Epidemiology and etiopathogenesis of pituitary adenomas. J Neurooncol. 2014;117(3):379–394. doi:10.1007/s11060-013-1354-5
7. Levy A. Pituitary disease: presentation, diagnosis, and management. J Neurol Neurosurg Psychiatry. 2004;75(3):47–52. doi:10.1136/jnnp.2004.045740
8. Kidd D. The optic chiasm. Clin Anat. 2014;27(8):1149–1158. doi:10.1002/ca.22385
9. Danesh-Meyer HV, Yoon JJ, Lawlor M, Savino PJ. Visual loss and recovery in chiasmal compression. Prog Retin Eye Res. 2019;73:100765. doi:10.1016/j.preteyeres.2019.06.001
10. Ito YA, Di Polo A. Mitochondrial dynamics, transport, and quality control: a bottleneck for retinal ganglion cell viability in optic neuropathies. Mitochondrion. 2017;36:186–192. doi:10.1016/j.mito.2017.08.014
11. Bergland R, Ray BS. The arterial supply of the human optic chiasm. J Neurosurg. 1969;31(3):327–334. doi:10.3171/jns.1969.31.3.0327
12. Lao Y, Gao H, Zhong Y. Vascular architecture of the human optic chiasma and bitemporal hemianopia. Chin Med Sci J. 1994;9(1):38–44.
13. Lee JY, Kim JP, Jang H, et al. Optical coherence tomography angiography as a potential screening tool for cerebral small vessel diseases. Alzheimers Res Ther. 2020;12(1):73. doi:10.1186/s13195-020-00638-x
14. Ye C, Kwapong WR, Tao W, et al. Characterization of macular structural and microvascular changes in thalamic infarction patients: a swept-source optical coherence tomography-angiography study. Brain Sci. 2022;12(5):518. doi:10.3390/brainsci12050518
15. Lee EJ, Lee KM, Lee SH, Kim TW. OCT angiography of the peripapillary retina in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2016;57(14):6265–6270. doi:10.1167/iovs.16-20287
16. Borrelli E, Balasubramanian S, Triolo G, Barboni P, Sadda SR, Sadun AA. Topographic macular microvascular changes and correlation with visual loss in chronic leber hereditary optic neuropathy. Am J Ophthalmol. 2018;192:217–228. doi:10.1016/j.ajo.2018.05.029
17. Dallorto L, Lavia C, Jeannerot AL, et al. Retinal microvasculature in pituitary adenoma patients: is optical coherence tomography angiography useful? Acta Ophthalmol. 2020;98(5):e585–e592. doi:10.1111/aos.14322
18. Lee GI, Park KA, Oh SY, Kong DS. Parafoveal and peripapillary perfusion predict visual field recovery in chiasmal compression due to pituitary tumors. J Clin Med. 2020;9(3):697. doi:10.3390/jcm9030697
19. Cennamo G, Solari D, Montorio D, et al. Early vascular modifications after endoscopic endonasal pituitary surgery: the role of OCT-angiography. PLoS One. 2020;15(10):e0241295. doi:10.1371/journal.pone.0241295
20. Suzuki ACF, Zacharias LC, Preti RC, Cunha LP, Monteiro MLR. Circumpapillary and macular vessel density assessment by optical coherence tomography angiography in eyes with temporal hemianopia from chiasmal compression. Correlation with retinal neural and visual field loss. Eye. 2020;34(4):695–703. doi:10.1038/s41433-019-0564-2
21. Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH. Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT362–OCT370. doi:10.1167/iovs.15-18904
22. Mansoori T, Sivaswamy J, Gamalapati JS, Agraharam SG, Balakrishna N. Measurement of radial peripapillary capillary density in the normal human retina using optical coherence tomography angiography. J Glaucoma. 2017;26(3):241–246. doi:10.1097/IJG.0000000000000594
23. Ulusoy MO, Horasanli B, Isik-Ulusoy S. Optical coherence tomography angiography findings of multiple sclerosis with or without optic neuritis. Neurol Res. 2020;42(4):319–326.
24. Pierro L, Arrigo A, Aragona E, Cavalleri M, Bandello F. Vessel density and vessel tortuosity quantitative analysis of arteritic and non-arteritic anterior ischemic optic neuropathies: an optical coherence tomography angiography study. J Clin Med. 2020;9(4):1094. doi:10.3390/jcm9041094
25. Fawzi AA, Fayed AE, Linsenmeier RA, Gao J, Yu F. Improved macular capillary flow on optical coherence tomography angiography after panretinal photocoagulation for proliferative diabetic retinopathy. Am J Ophthalmol. 2019;206:217–227. doi:10.1016/j.ajo.2019.04.032
26. Yan Y, Zhou X, Chu Z, et al. Vision loss in optic disc drusen correlates with increased macular vessel diameter and flux and reduced peripapillary vascular density. Am J Ophthalmol. 2020;218:214–224. doi:10.1016/j.ajo.2020.04.019
27. Barzaghi LR, Medone M, Losa M, Bianchi S, Giovanelli M, Mortini P. Prognostic factors of visual field improvement after trans-sphenoidal approach for pituitary macroadenomas: review of the literature and analysis by quantitative method. Neurosurg Rev. 2012;35(3):369–378. doi:10.1007/s10143-011-0365-y
28. Yu FF, Chen LL, Su YH, Huo LH, Lin XX, Liao RD. Factors influencing improvement of visual field after trans-sphenoidal resection of pituitary macroadenomas: a retrospective cohort study. Int J Ophthalmol. 2015;8(6):1224–1228. doi:10.3980/j.issn.2222-3959.2015.06.27
29. Gnanalingham KK. The time course of visual field recovery following transphenoidal surgery for pituitary adenomas: predictive factors for a good outcome. J Neurol Neurosurg Psychiatry. 2005;76(3):415–419. doi:10.1136/jnnp.2004.035576
30. Yu DY, Cringle SJ, Balaratnasingam C, Morgan WH, Yu PK, Su EN. Retinal ganglion cells: energetics, compartmentation, axonal transport, cytoskeletons and vulnerability. Prog Retin Eye Res. 2013;36:217–246. doi:10.1016/j.preteyeres.2013.07.001
31. Song Y, Min JY, Mao L, Gong YY. Microvasculature dropout detected by the optical coherence tomography angiography in nonarteritic anterior ischemic optic neuropathy. Lasers Surg Med. 2018;50(3):194–201. doi:10.1002/lsm.22712
32. Chen YY, Li XJ, Song XY, Cong L, Zhang YY. Microvascular changes and correlation with visual loss in pituitary adenoma, 02 May 2022, PREPRINT (Version 1); 2023.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.