Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Mepolizumab for Eosinophil-Associated COPD: Analysis of METREX and METREO
Authors Pavord ID , Chapman KR , Bafadhel M , Sciurba FC, Bradford ES, Schweiker Harris S, Mayer B, Rubin DB, Yancey SW, Paggiaro P
Received 9 December 2020
Accepted for publication 9 May 2021
Published 16 June 2021 Volume 2021:16 Pages 1755—1770
DOI https://doi.org/10.2147/COPD.S294333
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Video abstract presented by Ian D Pavord.
Views: 411
Ian D Pavord,1 Kenneth R Chapman,2 Mona Bafadhel,1 Frank C Sciurba,3 Eric S Bradford,4 Stephanie Schweiker Harris,4 Bhabita Mayer,5 David B Rubin,4 Steven W Yancey,4 Pierluigi Paggiaro6
1Nuffield Department of Medicine and Oxford Respiratory NIHR BRC, University of Oxford, Oxford, UK; 2Asthma & Airway Centre, UHN and University of Toronto, Toronto, ON, Canada; 3Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA; 4Respiratory Therapeutic Area, GSK, Research Triangle Park, NC, USA; 5Clinical Statistics, GSK, Brentford, Middlesex, UK; 6Department of Surgery, Medicine, Molecular Biology, and Critical Care, University of Pisa, Pisa, Italy
Correspondence: Ian D Pavord
NDM Research Building, Nuffield Department of Medicine and Oxford Respiratory NIHR BRC, University of Oxford, Old Road Campus, Roosevelt Drive, Oxford, OX3 7FZ, UK
Tel +441865 612897
Email [email protected]
Background: A pre-specified meta-analysis of individual patient data from the 52-week METREX and METREO trials, which investigated mepolizumab for chronic obstructive pulmonary disease (COPD) in patients with blood eosinophil counts ≥ 150 cells/μL (screening) or ≥ 300 cells/μL (prior year) and frequent exacerbations, enables more robust characterization of mepolizumab efficacy in COPD and exploration of the relationship between blood eosinophil count and treatment responses.
Methods: In METREX (117106/NCT02105948) and METREO (117113/NCT02105961), randomized patients received mepolizumab or placebo added to existing inhaled corticosteroid (ICS)–based triple maintenance therapy. The annual rate of moderate/severe exacerbations (primary endpoint) was compared between subcutaneous (SC) mepolizumab 100 mg versus placebo (primary comparison of interest) and all doses (100 mg and 300 mg SC) versus placebo in patients with blood eosinophil counts ≥ 150 cells/μL at screening or ≥ 300 cells/μL in the prior year. Secondary/other endpoints included time to first moderate/severe exacerbation, exacerbations leading to emergency department visit/hospitalization and health-related quality of life (HRQoL). A predictive model of the relationship between screening blood eosinophil counts and exacerbation rates included data from all randomized patients.
Results: In total, 1510 patients were randomized in METREX and METREO and 1136 patients were included in the pre-specified meta-analysis. From the meta-analysis, mepolizumab 100 mg SC significantly reduced annual moderate/severe exacerbation rates versus placebo by 18% (rate ratio: 0.82; 95% confidence interval: 0.71, 0.95; p=0.006) and delayed time to first moderate/severe exacerbation (hazard ratio: 0.80 [0.68, 0.94]; p=0.006). Mepolizumab 100 mg SC versus placebo numerically reduced exacerbations leading to ED visits/hospitalization and improved HRQoL. A modelling approach demonstrated increasing efficacy for moderate/severe exacerbations with increasing screening blood eosinophil count; this relationship was more pronounced for exacerbations requiring oral corticosteroids (post hoc). The all-doses comparison had similar results.
Conclusion: Mepolizumab reduces exacerbations in patients with eosinophil-associated COPD. Results suggest that blood eosinophil counts (≥ 150 cells/μL at screening or ≥ 300 cells/μL in the prior year) allow for identification of patients with COPD who experience exacerbations while treated with maximal ICS-based triple maintenance therapy who are likely to benefit from mepolizumab.
Keywords: mepolizumab, COPD, eosinophil, exacerbation
Plain Language Summary
Chronic obstructive pulmonary disease (COPD) is a lung condition. Some patients with COPD can have lots of flares of their symptoms (also called exacerbations) even if they take their usual inhalers. These flares can require extra treatment or visits to the hospital. Some patients with COPD also have an increased number of a type of white blood cell called the eosinophil. Mepolizumab is a drug that works by reducing the number of eosinophils in the blood. This may help reduce the number of flares. Therefore, mepolizumab may help patients with COPD and an increased number of eosinophils in the blood. METREX and METREO were studies that looked at how well mepolizumab works in these patients. As analyses with more patients can strengthen the results, the analysis in our paper combined the data from both studies.
This analysis showed that mepolizumab reduced the number of COPD flares that needed antibiotics or steroid tablets. The number of patients who went to hospital was also reduced by about one fifth. Quality of life was improved. Mepolizumab worked better in patients who had higher numbers of eosinophils in the blood before they were given mepolizumab. Mepolizumab also worked better for patients whose flares were treated with steroid tablets compared with antibiotics.
These results suggest that mepolizumab works in patients with COPD who have flares despite taking their usual inhalers. They also suggest that the number of eosinophils in a patient’s blood may help doctors decide if mepolizumab will work for them.
Introduction
Eosinophils may be involved in the pathogenesis of chronic obstructive pulmonary disease (COPD) and associated exacerbations.1–3 Sputum eosinophilia (>3% non-squamous cells) has been observed in approximately one-third of patients during an exacerbation,3 and sputum eosinophil counts are associated with COPD severity.4,5 Sputum eosinophils can also identify patients more likely to respond to corticosteroid treatment.1,6
Blood eosinophil counts correlate positively with sputum eosinophil counts, with counts of <2% (equivalent to approximately <150 cells/µL)7 having a high negative predictive value in stable and exacerbating COPD.8–10 Although correlations between blood and sputum eosinophil counts are reported to be lower in COPD than asthma, potentially due to the presence of comorbidities,11 patients with higher blood eosinophil counts have a greater response to corticosteroid therapy in COPD.12–14 This indicates that measurement of blood eosinophil counts may be useful biomarker of the efficacy of therapies targeting eosinophilic inflammation.14
Mepolizumab is a humanized monoclonal antibody that binds to and inactivates interleukin-5, reducing blood eosinophils to normal levels.15,16 Clinical trials of mepolizumab in severe eosinophilic asthma demonstrated reduced exacerbation frequency and improvements in disease burden, particularly in patients with higher blood eosinophil counts.17–21 In COPD, the METREX and METREO trials investigated mepolizumab in patients with COPD who continued to experience exacerbations despite receiving maximal inhaled corticosteroid (ICS)–based triple maintenance therapy for ≥12 months prior to the study.21 In both trials, compared with placebo, mepolizumab 100 mg subcutaneously (SC) added to triple therapy produced clinically meaningful reductions (18–20%)22 in mean annual moderate/severe exacerbation rates; these differences were statistically significant in METREX, but not in METREO following the pre-specified correction for multiplicity.21 The effect of mepolizumab on other key outcomes, such as exacerbations leading to emergency department (ED) visits/hospitalization and St George’s Respiratory Questionnaire (SGRQ) total score versus placebo, varied between trials.21 Mepolizumab treatment effects on moderate/severe exacerbations were found to increase with increasing blood eosinophil counts; the relationship between other study outcomes and blood eosinophil counts remains to be determined.
We performed a pre-specified meta-analysis of patient level data from METREX and METREO of individuals with COPD and blood eosinophil counts ≥150 cells/µL at screening or ≥300 cells/µL in the prior year. Mepolizumab 100 mg SC was the proposed licensed dose; its comparison with placebo is the primary focus of this manuscript. The aims were to provide more robust estimates of the effect of mepolizumab compared with placebo on COPD outcomes including exacerbation rates and quality of life, and to fully explore the relationship between blood eosinophil count and treatment responses.
Methods
Study Design
METREX (117106/NCT02105948) and METREO (117113/NCT02105961) were Phase III, placebo-controlled, randomized, double-blind, multicenter trials. Detailed information regarding the trials, including study designs (Figure E1) has been published previously (see also Supplementary Materials).21 Briefly, eligible patients were randomized 1:1 in METREX to receive mepolizumab 100 mg or placebo SC, and 1:1:1 in METREO to receive mepolizumab 100 or 300 mg, or placebo SC every 4 weeks for 52 weeks (final dose at Week 48), while continuing existing standard of care: triple inhaled therapy consisting of high-dose ICS (≥500 μg/day of fluticasone propionate or equivalent), a long-acting β2-agonist (LABA) and a long-acting muscarinic-receptor antagonist (LAMA). This meta-analysis included patients from both trials with blood eosinophil counts ≥150 cells/µL at screening or ≥300 cells/µL in the prior year. Inclusion of data from METREX and METREO was pre-specified for this meta-analysis. Consequently, data from a pilot trial of mepolizumab in patients with COPD and chronic bronchitis was not included as the eligibility criteria were different to METREX/METREO.23
Patients
Eligible patients were aged ≥40 years with a documented diagnosis of COPD for ≥1 year,24 required a pre- and post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity ratio <0.70 and a post-bronchodilator FEV1 >20–≤80% of predicted and a history of ≥2 moderate (requiring antibiotics and/or systemic corticosteroids) or ≥1 severe (leading to hospitalization) exacerbations in the prior year despite being on ICS-based therapy. Full eligibility criteria have been published previously.21
Endpoints and Assessments
The primary meta-analysis endpoint was the annual rate of moderate or severe exacerbations. Secondary and other endpoints included annual rates of exacerbations leading to ED visit/hospitalization; annual rates of severe exacerbations; time to first moderate/severe exacerbation; time to first exacerbation leading to ED visit/hospitalization; health-related quality of life (HRQoL) including Week 52 change from baseline in COPD-specific SGRQ total (domain [symptoms, activity and impacts]) and COPD Assessment Test (CAT) scores, and the proportion of SGRQ and CAT (post hoc) responders (≥4-point and ≥2-point decrease from baseline, respectively; the minimal clinically important difference [MCID] for each measure);25,26 patient- and clinician-rated response to therapy and change from baseline in pre-bronchodilator FEV1.
Endpoints were assessed by screening blood eosinophil count thresholds: <150 (≥300 in prior year), ≥150, ≥300, ≥500 cells/µL and categories: <150 (≥300 in prior year), ≥150−<300, ≥300−<500, ≥500 cells/µL. A post hoc analysis of moderate/severe exacerbations requiring antibiotics (with and without OCS) and exacerbations requiring OCS (with and without antibiotics) by eosinophil category was also performed. Statistical modelling of the relationship between screening blood eosinophils and treatment effect on moderate/severe exacerbations and the post hoc analysis of exacerbations requiring corticosteroids included all randomized patients from METREX and METREO. Analyses were pre-specified unless otherwise stated.
Statistical Analyses
The primary analysis population for the meta-analysis was the modified intent-to-treat population: all randomized patients receiving ≥1 dose of study medication with a blood eosinophil count ≥150 cells/µL (screening) or ≥300 cells/µL (prior year). This meta-analysis evaluated mepolizumab 100 mg SC (primary comparison of interest), and all doses (100 and 300 mg SC combined; described in Supplementary Materials), versus placebo. A two-sided unadjusted significance level of α=0.05 was employed. Exacerbations were analyzed using negative binomial (rate/year) and Cox proportional hazards (time to first) models, SGRQ and CAT using mixed model repeated measures (change from baseline) and logistic regression (responders), patient- and clinician-rated responses using multinomial (ordered) logistic regression. Predictive modelling of exacerbation rate reductions and eosinophils used post hoc fractional polynomial modelling. Additional details are available in Supplementary Materials.
Results
Patient Population
In total, of the 1510 patients randomized in METREX and METREO, 1136 patients with blood eosinophil counts ≥150 cells/µL at screening or ≥300 cells/µL in the prior year were included in the meta-analysis (mepolizumab 100 mg: N=456; mepolizumab 300 mg SC: N=225; placebo: N=455). Results for the all-dose comparison including patients receiving the 300 mg dose of mepolizumab are presented in the Supplementary Materials. For the modelling analysis, the additional 374 patients from METREX with eosinophil count <150 cells/µL at screening and no evidence of ≥300 cells/µL in the previous year were also included (N=1510).
From the meta-analysis, 90 (8%) patients prematurely discontinued treatment and remained in the trial (mepolizumab 100/300 mg SC: 31 [7%]/19 [8%]; placebo: 40 [9%]). Patients had a high disease burden, with ≥94% classified as having disease meeting the criteria for GOLD (Global Initiative for Chronic Obstructive Lung Disease) group D COPD, a mean annual exacerbation rate (prior year) of 2.6 events/year and mean baseline SGRQ and CAT scores of 53.0–54.7 and 18.6–19.5, respectively (Table 1, Table E1). The proportions of non-smokers (~3%) were similar between groups; in a post hoc analysis, baseline blood eosinophil counts were similar for patients who were current smokers (geometric mean [standard deviation logs]: 260 [0.592] cells/μL) compared with those who were ex-smokers or who had never smoked (240 [0.805] cells/μL). Results presented below were similar when non-smokers were excluded (data not shown).
 |
Table 1 Patient Demographics and Clinical Characteristics at Baseline |
Exacerbation Rates
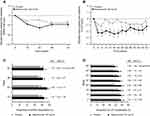
Mean annual moderate/severe exacerbation rates were 1.32 and 1.61 events/year following treatment with mepolizumab 100 mg SC and placebo, respectively, added to triple inhaled therapy. Mepolizumab 100 mg SC significantly reduced annual moderate/severe exacerbation rates by 18% versus placebo (rate ratio [RR]: 0.82; 95% confidence interval [CI]: 0.71, 0.95; p=0.006) (Figure 1, Table E2). In total, 18% and 22% of patients in the mepolizumab 100 mg SC and placebo groups, respectively, experienced ≥1 exacerbation leading to ED visit/hospitalization. The RR with mepolizumab 100 mg SC versus placebo was 0.85 (95% CI: 0.61, 1.18; p=0.328) for exacerbations leading to ED visit/hospitalization, and 0.88 (95% CI: 0.62, 1.25; p=0.475) for severe exacerbations.
Time to first moderate/severe exacerbation was significantly longer with mepolizumab 100 mg SC than placebo (Kaplan−Meier median time to first moderate/severe exacerbation: 218 vs 155 days, respectively; hazard ratio [HR]: 0.80; 95% CI: 0.68, 0.94; p=0.006) (Figure 2, Table E2). Time to first exacerbation leading to ED visit/hospitalization was numerically longer with mepolizumab 100 mg SC than placebo (HR: 0.81; 95% CI: 0.61, 1.09; p=0.170) (Figure 2, Table E2).
HRQoL
Improvements from baseline in SGRQ total score were initially greater with mepolizumab 100 mg SC versus placebo (Week 12 difference: −1.5; 95% CI: −3.1, 0.0; Week 24 difference: −3.4; 95% CI: −5.2, −1.6) but this was not sustained at Week 52 (difference: −0.7; 95% CI: −2.7, 1.3; p=0.486) (Figure 3, Table E3). A similar trend was observed with the activity and impact domains (Table E3). Symptom domain scores were significantly improved from baseline with mepolizumab 100 mg SC at all time points except Week 32 (Week 52 difference: −2.9; 95% CI: −5.3, −0.5; p=0.020) (Table E3). There were more SGRQ responders with mepolizumab 100 mg SC than with placebo at all time points; at Week 52: 42% versus 32% (odds ratio [OR]: 1.23; 95% CI: 0.94, 1.61; p=0.138) (Figure 3, Table E3).
Improvements from baseline in CAT score were greater with mepolizumab 100 mg SC versus placebo throughout the study (Figure 3), with a significant difference at Week 52 (difference: −0.9; 95% CI: −1.8, 0; p=0.039) (Table E3); the proportion of CAT responders was also greater throughout the study, with 39% and 33% of responders at Week 52, respectively (OR: 1.39; 95% CI: 1.04, 1.85; p=0.024) (Figure 3, Table E3).
Patient- and Clinician-Rated Response to Therapy
At Week 52, 56% and 50% of patients in the mepolizumab 100 mg SC and placebo groups rated themselves improved (OR: 1.26; 95% CI: 0.97, 1.63; p=0.077) and 53% and 44% were rated improved by their clinicians (OR: 1.41; 95% CI: 1.09, 1.83; p=0.008), respectively (Table E4).
Lung Function
Changes from baseline in pre-bronchodilator FEV1 were small throughout the study with mepolizumab 100 mg SC and placebo (difference: 5–31 mL across all time points) (Figure E2, Table E5).
Stratification by Eosinophil Thresholds
Treatment benefits with mepolizumab 100 mg SC versus placebo increased with increasing screening blood eosinophil counts (Figures 4 and 5) for the annual rate of moderate/severe exacerbations, exacerbations leading to ED visit/hospitalization, SGRQ total scores and SGRQ responders. This relationship was apparent to a lesser extent for severe exacerbations, CAT score and CAT responders (Figures 4 and 5). The analysis of endpoints by blood eosinophil categories yielded broadly consistent results (Tables E6–8).
In total, 1510 patients (mepolizumab 100 mg: N=640; mepolizumab 300 mg: N=225; placebo: N=645; [836 from METREX (including patients with <150 cells/µL at screening and no evidence of ≥300 in prior year) and 674 from METREO]) were included in the eosinophil response modelling. Predicted annual moderate/severe exacerbation rates increased with increasing screening blood eosinophil counts in the placebo group and remained relatively stable across screening blood eosinophil counts in the mepolizumab 100 mg group (Figure 6). Predicted exacerbation rate reductions were greater with mepolizumab 100 mg versus placebo as screening blood eosinophil counts increased, with a predicted 14% reduction (RR: 0.86; 95% CI: 0.75, 0.99) at 300 cells/µL, a 23% reduction (RR: 0.77; 95% CI: 0.63, 0.93) at 500 cells/µL and a 30% reduction (RR: 0.70; 95% CI: 0.55, 0.90) at 750 cells/µL (Figure 6). RR predictions were consistent with blood eosinophil category results (Table E9).
For exacerbations requiring treatment with corticosteroids, predicted annual moderate/severe exacerbation rates can be seen in Figure 7A and eosinophil response modelling–predicted rate reductions were 22% at 300 cells/µL, 35% at 500 cells/µL and 43% at 500 cells/µL (Figure 7B). For the analysis of moderate/severe exacerbations requiring antibiotics, no clear relationship between treatment effect and blood eosinophil category was observed (Figure 7C), so further modelling was not carried out.
Endpoints for the Mepolizumab All-Doses Group
Similar results to mepolizumab 100 mg SC versus placebo were observed in the mepolizumab all-doses group versus placebo for all endpoint comparisons (Tables E2–E8) and the analysis of exacerbations by treatment type (data on file).
Discussion
Overall, the results of this meta-analysis demonstrated a progressive increase in mepolizumab efficacy versus placebo with increasing blood eosinophil counts for most outcome measures, particularly in patients with ≥300 cells/μL. Building on the eosinophil subgroup analysis reported previously,21 predictive modelling analysis demonstrated rate reductions with mepolizumab versus placebo for moderate/severe exacerbations that increased with increasing blood eosinophil counts, consistent with previous studies.27,28 This is also consistent with an analysis of two large studies of benralizumab in COPD, the latter of which found that blood eosinophil counts ≥220 cells/µL were predictive of reduced exacerbation frequency, particularly in patients with ≥3 exacerbations in the prior year.19,29 Together, these results support the utility of blood eosinophil counts to identify patients with COPD responsive to anti–IL-5 treatment, although this population may require further refinement to determine the patients most likely to be responsive to treatment.
In general, larger mepolizumab versus placebo reductions were predicted for exacerbations requiring treatment with corticosteroids with increasing blood eosinophil count, whereas exacerbations requiring antibiotics showed no clear trend. Previously, a cluster analysis identified biologically distinct infection-related and eosinophil-associated exacerbation types distinguishable by the presence of raised blood eosinophil counts.3 Moreover, differential treatment responses to bronchodilator therapy with OCS-treated and antibiotic-treated exacerbations have also been demonstrated.30 With the current results, this suggests that exacerbation subtypes may be differentially responsive to specific inhibition of eosinophilic airway inflammation; future studies should investigate this possibility.
In the meta-analysis, mepolizumab treatment in addition to triple inhaled therapy resulted in an 18% reduction in annual moderate/severe exacerbation rates and delayed the time to first moderate/severe exacerbation versus placebo. This was coupled with mepolizumab-treated patients being significantly more likely to report clinically important improvements in CAT score and numerically more likely to report a clinically important improvement in SGRQ score and be in a higher improvement response category based on clinician assessment than placebo-treated patients. These findings suggest that mepolizumab treatment has clinical benefits in patients with eosinophil-associated COPD who have a history of exacerbations despite maximal ICS-based triple maintenance therapy and limited further treatment options.
All exacerbation types reported were numerically reduced with mepolizumab over placebo by 12–18%, which is consistent with the range of exacerbation rate reductions observed in previous COPD studies (8–35%).31–37 Exacerbation reductions in this meta-analysis should be considered in the context of being additional to reductions in exacerbations from ICS/LABA/LAMA triple therapy, which was a protocol-required background treatment for all patients. Previous studies have demonstrated moderate/severe exacerbation reductions with monotherapy versus placebo of 11–18%, dual therapy versus placebo of 25–29%, dual therapy versus monotherapy of 9–30% and triple therapy versus dual therapy of 8–35%.31–37 These results can be considered clinically meaningful in the context of the suggested MCID for COPD exacerbation reduction of 11%.22
The findings highlight the utility of meta-analyses in gaining further insights from observed data. METREX and METREO were designed on the basis of treatment effects observed in previous studies of patients with severe eosinophilic asthma,17,20 with each study being powered at 90% based on an expected placebo rate of 2.0 moderate/severe exacerbations/year and a true population reduction of 35% with mepolizumab treatment. In METREX and METREO, the observed reductions in moderate/severe exacerbation rates with mepolizumab 100 mg SC of 18% and 20%, respectively and the exacerbation rates with placebo (1.71 and 1.49/year, respectively) were lower than expected.21 Additionally, moderate/severe exacerbations were reduced by 14% with mepolizumab 300 mg SC versus placebo in METREO, suggesting no additional benefit with mepolizumab doses greater than 100 mg SC.21 This meta-analysis has allowed for greater precision in estimating the rate reductions in moderate/severe exacerbations by blood eosinophil count and evaluating treatment effects with mepolizumab 100 mg SC for more infrequent events, such as exacerbations leading to ED visits/hospitalization and severe exacerbations.
Exacerbations can have a significant impact on HRQoL; mepolizumab-treated patients showed improvements from baseline in HRQoL from the earliest post-baseline assessments, and maximum reductions were reached within 24 weeks, as noted in other studies.38,39 By Week 52, only changes in CAT score were significant compared with placebo. Differences between SGRQ and CAT results are likely a reflection of differences in assessment and specificity of the two measures.40,41 Decreased differentiation between treatment groups of HRQoL responses over time may be partly related to the disproportionate patient dropout rate and times in the placebo versus the mepolizumab 100 mg SC group, as has been previously reported in the 3-year TORCH trial.42 Unexpectedly, SGRQ improvements from baseline approached the MCID at Week 52 in both the placebo (−3.1 points) and mepolizumab (−3.8 points) groups;25 these were similar to improvements (−1.9 to −5.5 points) observed in previous 52-week studies of bronchodilator and ICS combination therapy in patients with severe COPD.34,37 When assessing the number of patients reaching the MCID, mepolizumab- versus placebo-treated patients were significantly more likely to be CAT responders and rated objectively by clinicians to be in a higher improvement-response category at Week 52. Overall, these results are supportive of the potential for mepolizumab to reduce aspects of disease burden.
Trends for improvements in severe exacerbation frequency and CAT scores with increasing blood eosinophil counts were less clear than for other endpoints. This effect is likely to be attributable to the rarity of severe exacerbations and that changes in CAT score may be less differentiable by screening blood eosinophil count. Interestingly, patients who exhibited screening eosinophil counts <150 cells/μL and entered the trials with a historical count ≥300 cells/μL in the past year exhibited the greatest reductions in annual rates of moderate/severe exacerbations and greatest HRQoL improvements. This suggests that a historical increase in eosinophils may be important in determining mepolizumab-responsive individuals.
Limitations of this meta-analysis relate to limitations of the original trials. The possibility of a clinical-trial effect leading to increased adherence to background ICS-based triple inhaled therapy due to monthly study visits, and symptom monitoring via daily diary that may lead to earlier identification of exacerbations, could explain the observed reductions in moderate/severe exacerbation rates/year in the placebo group during the study compared with the prior year. This would make the impact of mepolizumab on infrequent exacerbation events, such as exacerbations leading to an ED visit/hospitalization, more difficult to detect. The smoking criteria employed were also different from most COPD studies, where smoking history is generally an eligibility requirement. As approximately 20% of patients with COPD have no history of smoking,43 METREX and METREO allowed non-smokers in the study to better represent the real-world COPD population. All patients were required to meet accepted spirometry parameters for COPD, have a documented history of COPD exacerbations in the year prior to study initiation and required confirmation by the study physician that there was no history of asthma. Therefore, it is unlikely that the ~3% of the total study population who were non-smokers would have had undiagnosed asthma. Furthermore, treatment outcomes with mepolizumab were similar when these patients were excluded. Finally, the inclusion of patients in this analysis who prematurely discontinued treatment may have biased results, although, as the total percentage of the study population who discontinued was small (8%) and balanced across treatment groups (7–9% per group), this is unlikely to have influenced results.
In summary, COPD is a heterogenous disease and, without biomarkers, it is difficult to tailor treatments to patients. Data from this meta-analysis show that the use of blood eosinophil counts allows for the identification of patients with COPD who continue to experience exacerbations while treated with maximal ICS-based triple maintenance therapy who are responsive to anti–IL-5 treatment. Mepolizumab treatment benefits on reducing exacerbations and decreasing aspects of disease burden were clearest in patients with blood eosinophil counts ≥300 cells/μL, suggesting that this population requires further investigation.
Data Sharing Statement
Data are available upon reasonable request: Anonymized individual participant data and study documents for the original METREX and METREO studies can be requested for further research from www.clinicalstudydatarequest.com.
Ethics Approval and Informed Consent
All the patients provided written informed consent for METREX and METREO. Both METREX and METREO were conducted in accordance with the ethical principles of the Declaration of Helsinki, the International Conference on Harmonization. Good Clinical Practice guidelines and the applicable country-specific regulatory requirements. A full list of the ethics committees for each study is provided in the Supplementary Materials.
Acknowledgments
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Alex Lowe, PhD, and Mary E. Morgan, PhD, of Fishawack Indicia Ltd, UK, Part of Fishawack Health, and was funded by GlaxoSmithKline (GSK). Abstracts describing the results of this analysis were presented at the American Thoracic Society and European Respiratory Society International Conferences in 2018 as a poster presentation. The posters’ abstracts were published in the American Journal of Respiratory and Critical Care Medicine (Bafadhel et al, Am J Respir Crit Care Med 2018;197:A5912. Chapman et al, Am J Respir Crit Care Med 2018;197:A4230) and the European Respiratory Journal (Pavord et al, Eur Respir J. 2018;52(Suppl 62):PA1992).
Author Contributions
SSH, BM, DBR and SWY made substantial contributions to study concept and design; MB, KRC, IDP, FCS, SSH, BM and PP made substantial contributions to data acquisition; MB, KRC, IDP, FCS, ESB, SSH, BM, DBR, SWY and PP made substantial contributions to data analysis and interpretation. All authors took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
GlaxoSmithKline (GSK meta-analysis ID 207478, Integrated Summary of Efficacy [ISE] 207577, and the individual parent studies [117106/NCT02105948 and 117113/NCT02105961]).
Disclosure
MB has received honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK and Novartis and has received independent/institutional grant support from Pfizer and AstraZeneca. KRC reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, CSL Behring, Grifols, Sanofi, Genentech, Kamada, Mereo Biopharma, Regeneron, Roche and Novartis; grants from Baxter, GSK, Vertex, and Amgen; and personal fees from Merck, Takeda. He also holds a Canadian Institute of Health Research-GSK research chair in Respiratory Health Care Delivery at the University Health Network. In the last 5 years, IDP received funding from the NIHR as a Senior Investigator; he has received speaker’s honoraria for speaking at sponsored meetings from AstraZeneca, Boehringer Ingelheim, Aerocrine, Almirall, Menarini, Novartis, Teva, Chiesi and GSK and payments for organizing educational events from AstraZeneca and Teva. He has received honoraria for attending advisory panels with Genentech, Regeneron, AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Teva, Merck, Sanofi, Circassia, Chiesi and Knopp. He has received sponsorships to attend international scientific meetings from Boehringer Ingelheim, GSK, AstraZeneca, Teva and Chiesi. He has also received a grant from Chiesi to support a Phase II clinical trial in Oxford. In addition, IDP has a patent Leicester Cough Questionnaire with royalties paid. FCS received grants from the National Institutes of Health, Patient-Centered Outcomes Research Institute, the COPD Foundation, the US Department of Defense, Astellas, AstraZeneca, Boehringer Ingelheim, GSK, Phillips Respironics, PulmonX, PneumRx and Spiration; and personal fees from Circassia, Boehringer Ingelheim, GSK and PneumRx. ESB was an employee of GSK and holds GSK stock/shares during the conduct of the study, his current affiliation is Aeglea BioTherapeutics, Austin, Texas. SWY is an employee of GSK and hold GSK stock/shares. DBR was an employee of GSK and holds GSK stocks/shares during the conduct of the study, his current affiliation is DBR Consulting LLC, Raleigh NC. SSH was an employee of GSK and holds GSK stocks/shares during the conduct of the study. All authors received non-financial support from GSK in the form of editorial support. PP reports personal fees from GlaxoSmith Kline, during the conduct of the study; grants and/or personal fees from AstraZeneca, Chiesi, Novartis, and Sanofi, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Brightling CE, Monteiro W, Ward R, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2000;356(9240):1480–1485. doi:10.1016/S0140-6736(00)02872-5
2. Saha S, Brightling CE. Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1(1):39–47. doi:10.2147/copd.2006.1.1.39
3. Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671. doi:10.1164/rccm.201104-0597OC
4. Bafadhel M, McCormick M, Saha S, et al. Profiling of sputum inflammatory mediators in asthma and chronic obstructive pulmonary disease. Respiration. 2012;83(1):36–44. doi:10.1159/000330667
5. Bartoli ML, Costa F, Malagrino L, et al. Sputum inflammatory cells in COPD patients classified according to GOLD 2011 guidelines. Eur Repir J. 2016;47(3):978–980. doi:10.1183/13993003.00784-2015
6. Brightling CE, McKenna S, Hargadon B, et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax. 2005;60(3):193–198. doi:10.1136/thx.2004.032516
7. Singh D, Kolsum U, Brightling CE, et al. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697–1700. doi:10.1183/09031936.00162414
8. Kolsum U, Damera G, Pham TH, et al. Pulmonary inflammation in patients with chronic obstructive pulmonary disease with higher blood eosinophil counts. J Allergy Clin Immunol. 2017;140(4):1181–1184.e1187. doi:10.1016/j.jaci.2017.04.027
9. Bafadhel M, McKenna S, Pickering S, et al. The sensitivity and specificity of peripheral blood eosinophilia to predict sputum eosinophilia in COPD subjects. Am J Respir Crit Care Med. 2009;129:A1479.
10. Negewo NA, McDonald VM, Baines KJ, et al. Peripheral blood eosinophils: a surrogate marker for airway eosinophilia in stable COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1495–1504. doi:10.2147/COPD.S100338
11. Pignatti P, Visca D, Cherubino F, et al. Do blood eosinophils strictly reflect airway inflammation in COPD? Comparison with asthmatic patients. Respir Res. 2019;20(1):145. doi:10.1186/s12931-019-1111-1
12. Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186(1):48–55. doi:10.1164/rccm.201108-1553OC
13. Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord I. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442. doi:10.1016/S2213-2600(15)00106-X
14. Pavord ID, Agusti A. Blood eosinophil count: a biomarker of an important treatable trait in patients with airway disease. Eur Respir J. 2016;47(5):1299–1303. doi:10.1183/13993003.00055-2016
15. Varricchi G, Bagnasco D, Borriello F, Heffler E, Canonica GW. Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: evidence and unmet needs. Curr Opin Allergy Clin Immunol. 2016;16(2):186–200. doi:10.1097/ACI.0000000000000251
16. Hartl S, Breyer MK, Burghuber OC, et al. Blood eosinophil count in the general population: typical values and potential confounders. Eur Respir J. 2020;55(5):1901874. doi:10.1183/13993003.13901874-13992019.
17. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi:10.1016/S0140-6736(12)60988-X
18. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390–400. doi:10.1016/S2213-2600(17)30125-X
19. Ortega HG, Yancey SW, Mayer B, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4(7):549–556. doi:10.1016/S2213-2600(16)30031-5
20. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi:10.1056/NEJMoa1403290
21. Yancey SW, Ortega HG, Keene ON, et al. Meta-analysis of asthma-related hospitalization in mepolizumab studies of severe eosinophilic asthma. J Allergy Clin Immunol. 2017;139(4):1167–1175.e1162. doi:10.1016/j.jaci.2016.08.008
22. Chapman KR, Bergeron C, Bhutani M, et al. Do we know the minimal clinically important difference (MCID) for COPD exacerbations? COPD. 2013;10(2):243–249. doi:10.3109/15412555.2012.733463
23. Dasgupta A, Kjarsgaard M, Capaldi D, et al. A pilot randomised clinical trial of mepolizumab in COPD with eosinophilic bronchitis. Eur Respir J. 2017;49(3):1602486. doi:10.1183/13993003.13902486-13992016.
24. Celli BR, MacNee W. ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi:10.1183/09031936.04.00014304
25. Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J. 2002;19(3):398–404. doi:10.1183/09031936.02.00063702
26. Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med. 2014;2(3):195–203. doi:10.1016/S2213-2600(14)70001-3
27. Yun JH, Lamb A, Chase R, et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141(6):2037–2047.e2010. doi:10.1016/j.jaci.2018.04.010
28. Zeiger RS, Tran TN, Butler RK, et al. Relationship of Blood Eosinophil Count to Exacerbations in Chronic Obstructive Pulmonary Disease. J Allergy Clin Immunol in Practice. 2018;6(3):944–954.e945. doi:10.1016/j.jaip.2017.10.004
29. Criner GJ, Celli BR, Singh D, et al. Predicting response to benralizumab in chronic obstructive pulmonary disease: analyses of GALATHEA and TERRANOVA studies. Lancet Respir Med. 2020;8(2):158–170. doi:10.1016/S2213-2600(19)30338-8
30. Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26. doi:10.1164/rccm.200707-973OC
31. Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387(10030):1817–1826. doi:10.1016/S0140-6736(16)30069-1
32. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi:10.1056/NEJMoa063070
33. Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1(3):210–223. doi:10.1016/S2213-2600(13)70040-7
34. Lipson DA, Barnacle H, Birk R, et al. FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(4):438–446. doi:10.1164/rccm.201703-0449OC
35. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. doi:10.1056/NEJMoa1516385
36. Vestbo J, Leather D, Diar Bakerly N, et al. Effectiveness of fluticasone furoate-vilanterol for COPD in clinical practice. N Engl J Med. 2016;375(13):1253–1260. doi:10.1056/NEJMoa1608033
37. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
38. Kerwin E, Hebert J, Gallagher N, et al. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 study. Eur Respir J. 2012;40(5):1106–1114. doi:10.1183/09031936.00040712
39. Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):199–209. doi:10.1016/S2213-2600(13)70052-3
40. Morishita-Katsu M, Nishimura K, Taniguchi H, et al. The COPD assessment test and St George’s Respiratory Questionnaire: are they equivalent in subjects with COPD? Int J Chron Obstruct Pulmon Dis. 2016;11:1543–1551. doi:10.2147/COPD.S104947
41. Tsiligianni IG, Alma HJ, de Jong C, et al. Investigating sensitivity, specificity, and area under the curve of the Clinical COPD Questionnaire, COPD Assessment Test, and Modified Medical Research Council scale according to GOLD using St George’s Respiratory Questionnaire cutoff 25 (and 20) as reference. Int J Chron Obstruct Pulmon Dis. 2016;11:1045–1052. doi:10.2147/COPD.S99793
42. Vestbo J, Anderson JA, Calverley PM, et al. Bias due to withdrawal in long-term randomised trials in COPD: evidence from the TORCH study. Clin Respir J. 2011;5(1):44–49. doi:10.1111/j.1752-699X.2010.00198.x
43. Terzikhan N, Verhamme KM, Hofman A, Stricker BH, Brusselle GG, Lahousse L. Prevalence and incidence of COPD in smokers and non-smokers: the Rotterdam Study. Eur J Epidemiol. 2016;31(8):785–792. doi:10.1007/s10654-016-0132-z
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.