Back to Journals » Journal of Pain Research » Volume 15
Mechanisms of Neuropathic Pain and Pain-Relieving Effects of Exercise Therapy in a Rat Neuropathic Pain Model
Authors Sumizono M , Yoshizato Y , Yamamoto R, Imai T, Tani A, Nakanishi K, Nakakogawa T, Matsuoka T, Matsuzaki R, Tanaka T, Sakakima H
Received 30 March 2022
Accepted for publication 14 June 2022
Published 13 July 2022 Volume 2022:15 Pages 1925—1938
DOI https://doi.org/10.2147/JPR.S367818
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Megumi Sumizono,1,2 Yushin Yoshizato,1 Ryohei Yamamoto,1 Takaki Imai,1 Akira Tani,2 Kazuki Nakanishi,2 Tomomi Nakakogawa,2 Teruki Matsuoka,2 Ryoma Matsuzaki,2 Takashi Tanaka,3 Harutoshi Sakakima2
1Department of Rehabilitation, Kyushu University of Nursing and Social Welfare, Kumamoto, Japan; 2Department of Physical Therapy, School of Health Sciences, Faculty of Medicine, Kagoshima University, Kagoshima, Japan; 3Department of Rehabilitation, Kumamoto Health Science University, Kumamoto, Japan
Correspondence: Megumi Sumizono, Department of Rehabilitation, Kyushu University of Nursing and Social Welfare, 888 Tominoo, Tamana, Kumamoto, 865-0062, Japan, Tel/Fax +81 968-75-1931, Email [email protected]
Purpose: Pain disrupts the daily and social lives of patients with neuropathic pain. Effective treatment of neuropathic pain is difficult. Pharmacological treatments for neuropathic pain are limited, and 40– 60% of patients do not achieve even partial relief of their pain. This study created a chronic constriction injury (CCI) model in rats to examine the effects of regular exercise on neuropathic pain relief, elucidate the mechanism, and determine the effects of neuropathic pain in the hippocampus.
Methods: CCI model rats were randomly divided into exercise (Ex) and no exercise (No-Ex) groups. Normal rats (Normal group) were used as controls. The Ex group exercised on a treadmill at 20 m/min for 30 min, 5 days per week for 5 weeks post-CCI. The 50% pain response threshold was assessed by mechanical stimulation. Using immunohistochemistry, we examined activation of glial cells (microglia and astrocytes) by CCR2 and TRAF6 expression in the spinal cord dorsal horn and DCX and PROX1 expression in the hippocampal dentate gyrus.
Results: The 50% pain response threshold was significantly lower in the Ex than in the No-Ex group at 5 weeks post-CCI, indicating pain relief. In the spinal cord dorsal horn, IBA1, CCR2, and TRAF6 expression was markedly lower in the Ex group than in the No-Ex group at 3 weeks post-CCI. IBA1, GFAP, CCR2, and TRAF6 expression was markedly lower in the Ex group than in the No-Ex group at 5 weeks post-CCI. In the hippocampus, DCX, but not PROX1, expression was significantly higher in the Ex group than in the No-Ex group at 3 weeks post-CCI. At 5 weeks post-CCI, both DCX and PROX1 expression was markedly increased in the Ex group compared to the No-Ex group.
Conclusion: Our findings suggest that regular exercise can improve the neuropathic pain-induced neurogenic dysfunction in the hippocampal dentate gyrus.
Keywords: CCR2, hippocampal dentate gyrus, microglia, neurogenesis, spinal dorsal horn, TRAF6
Introduction
The International Association for the Study of Pain defined pain as “An unpleasant sensory and emotional experience with actual or potential tissue damage, or described in terms of such damage” in 1979. However, almost 40 years thereafter, in 2020, pain was redefined as an unpleasant sensory and emotional experience related to or similar to actual or potential tissue damage.1 An accurate classification of chronic neuropathic pain in the International Classification of Diseases (ICD)-11 is necessary to document the public health needs and therapeutic challenges associated with chronic neuropathic pain, which is a major contributor to the global burden of disease. Chronic pain is the most important cause of current and future morbidity-associated disability worldwide, with significant increases in both morbidity and disability loss each year. Its prevalence is considered to be constant or increasing,2,3 at a population prevalence of 6.9‒10%.4
The clinical manifestations of neuropathic pain include pathological pain states, such as spontaneous pain, burning pain, and hyperalgesia. Patients with neuropathic pain frequently present with abnormal sensation or hypersensitivity in the skin areas where peripheral nerves have been damaged. Neuropathic pain patients have more difficulties in daily life and social life due to their pain. However, neuropathic pain is very difficult to treat effectively. Pharmacological treatment of neuropathic pain is limited, with 40‒60% of patients failing to achieve even partial relief of their pain.5–7 Various reports have addressed therapeutic effects and mechanisms of treatment in humans and animals, but many unclear issues remain.
Neuropathic pain is caused by central or peripheral nerve damage. It has been reported that neuronal damage activates microglia and astrocytes in the dorsal horn of the spinal cord, causing pain through the production of inflammatory substances and abnormal nerve cell function.8–10 Nerve injury essentially causes reorganization of the nervous system after peripheral afferent loss in peripheral nerve injury, triggering hypersensitive (central sensitization) pain memory.11 Microglia become activated upon nerve injury and are involved in the development of neuropathic pain. C-C motif chemokine receptor 2 (CCR2) is expressed on activated microglia, indicating that CC chemokine ligand 2 (CCL2)/CCR2-dependent mechanisms play an important role in the development of neuropathic pain.12 Furthermore, CCL2/CCR2 binding is involved in modulation of N-methyl-D-aspartate receptor (NMDAR)-mediated central sensitization, thereby contributing to the development of neuropathic pain.13
Furthermore, astrocytes are implicated in the development and maintenance of neuropathic pain. Tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6), which plays an important role in TNF receptor superfamily and interleukin (IL)-1 receptor superfamily signaling, is expressed on astrocytes in the late post-nerve injury period. Neuropathic pain may be maintained by coupling TNF-α and IL-1β signaling and activating the c-Jun N-terminal kinase (JNK)/CCL2 pathway in astrocytes.14
In the brain, chronic pain caused by peripheral nerve injury is associated with decreased neurogenesis and nerve cell proliferation, increased proinflammatory cytokine levels, and decreased neuroprotective microglia/macrophages in the hippocampus, which is a memory center. Chronic pain caused by neuroma, as a result of amputation, does not progress to the point of working memory impairment, but strangulated peripheral nerve injury, resulting in neuropathic pain, has been reported to cause signs of pathological inflammation in the dentate gyrus of the hippocampus, leading to decreased neurogenesis, with memory destruction.15 Prospero homeobox protein 1 (PROX1), a master transcription factor essential for lymphatic endothelial cell differentiation and cell function in the hippocampal dentate gyrus, is required for granule cell maturation in this brain region. PROX1 is required for maintenance of intermediate progenitor cells in adult neurogenesis; and lack of PROX1 leads to increased doublecortin (DCX) cell apoptosis and lack of neurogenesis in adults.16–18
The therapeutic effects of exercise on neuropathic pain have been examined by focusing on the brain and spinal cord separately, but have been simultaneously observed in the brain, spinal cord, and local areas over time. The mechanism by which exercise reduces neuropathic pain remains unclear. Although previous studies have often reported on the therapeutic effects of medication as a treatment for neuropathic pain and have examined the differences in the therapeutic effects of exercise intensity, the effectiveness of exercise intensity, duration, and frequency in alleviating neuropathic pain remains poorly understood, and reports on the effects of exercise remain scarce.19–23 We have previously examined the effects of different exercise frequencies in a rat model of neuropathic pain and reported that, despite differences in frequency, they alleviated neuropathic pain through activation of glial cells, expression of brain-derived neurotrophic factor (BDNF) in the ipsilateral spinal dorsal horn, and modulation of the endogenous opioid system.24 This study builds on our previous study and aimed to examine in more detail the effects of regular exercise on pain relief in a rat model of neuropathic pain to clarify the underlying mechanism and to clarify the effects of neuropathic pain on the hippocampus.
Materials and Methods
Animals
Male Sprague‒Dawley rats (8-week-old; n = 25; weight 274.3 ± 21.2 g [mean ± standard deviation]) were used in this experiment. The rats were subjected to a 12-h light/dark cycle under controlled room temperature (23.0 ± 1.0°C), with free access to solid food and water. The experiment was conducted with the minimum possible number of animals needed to collect the appropriate amount of information. This experiment was approved by the Animal Experiment Committee of the Kagoshima University, School of Medicine. The protocol complied with the Classification of Biomedical Experimental Processes Based on Ethical Considerations for Nonhuman Species (Consensus Recommendations for Effective Institutional Animal Experimentation).
Chronic Constriction Injury Model
To create a chronic constriction injury (CCI) model, rats were anesthetized by intraperitoneal injection of 4% chloral hydrate solution (10 mL/kg). The skin covering the right thigh was incised and carefully dissected to avoid injuring the sciatic nerve. The method reported by Bennett and Xie25 was modified slightly. Briefly, four ligature threads (4–0 silk) were loosely tied around the right sciatic nerve at intervals of about 1 mm. The length of the nerve affected by ligation was approximately 6 mm. The incision was closed in layers with 4–0 silk sutures and the animal was returned to the cage for recovery. The contralateral hindlimb was left untreated and was used as a type of control for mechanical sensitivity of the hindlimb. The rats were allowed 1 or 2 days of free cage recovery after CCI before starting exercise.
Regular Treadmill Exercise and Experimental Group
Treadmill exercise was started 2‒3 days after CCI. Exercise was performed on a treadmill (MK-680, MUROMACHI KIKAI CO, LTD, Tokyo, Japan), and environmental adaptation was performed for all rats at a speed of 20 m/min for 15 min and a voltage of 1.0 A for 3 days. After environmental adaptation, the rats were randomly divided into three groups: a CCI exercise group (Ex, n = 11), a CCI non-exercise group (No-Ex, n = 11), and a normal group (Normal, n = 3).
In the Ex group, rats exercised on a treadmill for 5 weeks at a rate of 20 m/min for 5 days. The duration of exercise was increased from 15 to 30 min after 1 day of exercise and this duration was maintained until 5 weeks after CCI. The work rate of the rats at this training rate was approximately 55% of their maximal oxygen consumption.22,23 Body weight was measured periodically to monitor the stress induced in the rats by the treadmill exercise. None of the animals were excluded.
Change in Mechanical Pain Stimulus Threshold
Mechanical susceptibility was assessed as the withdrawal response frequency upon stimulation using von Frey filaments (MUROMACHI KIKAI CO, LTD.) in both hind limbs. Measurements were performed before CCI and at 1, 2, 3, 4, and 5 weeks after CCI (between 2:00 p.m. and 5:00 p.m.). The rats were placed in individual transparent plastic cages on wire mesh grids that allowed full access to the ventral aspect of the hind limbs. The animals were then acclimated to the experimental environment (room and apparatus) for at least 20 min. A logarithmic series of 11 filaments (0.41‒28.84 g) was pressed vertically against the plantar surface of the hind paw until the filaments bent. The test was started with 3.63 g filaments. If the animal withdrew the foot, it was counted as a positive response, and a lower filament was then applied. If the animal did not respond, then a larger filament was applied. Filaments were applied until there was an initial change in response, followed by four additional filament applications. Measurements were taken before motor training, and tactile stimuli that produced 50% of the possible hindlimb withdrawal responses (50% gram threshold) were calculated using equations from top-down methods, referring to previous studies.26
Histology and Immunohistochemistry
The rats were sacrificed and histological and immunohistochemical analyses were performed at 3 weeks (Ex, n = 4; No-Ex, n = 4), and 5 weeks (Ex, n = 4; No-Ex, n = 4) after CCI. In addition, three rats from the Normal group were used as normal controls for histological and immunohistochemical analysis at the end of the experiment. Every effort was made to reduce the number of animals used.
Rats were first treated with 4% chloral hydrate (10 mL/kg, intraperitoneal overdose), before cardiac perfusion of heparin saline, 4% paraformaldehyde 0.1 M phosphate buffer (pH 7.4). The lumbar portion of the spinal cord and midbrain were then removed and fixed overnight at 4°C. After fixation, the tissues were dehydrated and embedded in paraffin. After preparing 5-μm-thick sections, sections were stained with hematoxylin and eosin (HE) to observe histological changes.
In addition, immunohistochemical changes after CCI were also analyzed. After deparaffinization, the sections were soaked in 3% H2O2 solution for 10 min to inactivate endogenous peroxidases. After rinsing sections in phosphate-buffered saline (PBS, pH 7.6) three times for 5 min each time, the sections were incubated with 10% skim milk in PBS to block specific sites. Sections were again rinsed in PBS three times for 5 min each time and were then incubated individually at 4°C with one of the following antibodies: rabbit anti-ionized calcium-binding adaptor molecule 1 (IBA1) antibody (1:2000, rabbit polyclonal, Wako Pure Chemical Industries, Osaka, Japan), a marker of microglia; rabbit anti-glial fibrillary acidic protein (GFAP) antibody (1:1000, rabbit polyclonal, Shima Research Institute, Tokyo), a marker of astrocytes; goat anti-CCR2 antibody (1:1500, goat polyclonal, Novus Biologicals, Littleton, CO, USA); anti-TRAF6 antibody (1:600, mouse monoclonal, Proteintech Group, Inc, Rosemont, IL, USA), a marker of immature neurons; anti-doublecortin (DCX) antibody (1:1500, rabbit polyclonal, Abcam plc, Cambridge, UK, ab18723); anti-PROX1 antibody (1:100, rabbit polyclonal, Proteintech Group, Inc). After the primary antibody incubation, the sections were rinsed three times with PBS for 5 min each time. The sections were then incubated for 60 min with goat anti-rabbit IgG conjugated to peroxidase-labeled dextran polymer (EnVision; Dako, Carpinteria, CA, USA). Sections incubated with anti-TRAF6 antibody were reacted with the ABC kit (Vector Laboratories, Burlingame, CA, USA), according to the manufacturer’s instructions. After three 5-min PBS washes, the immunoreactivity of the sections was visualized using diaminobenzidine (DAB) peroxide.
Double stainings with anti-IBA1 antibody (1:500) and anti-TRAF6 antibody (1:300) or anti-GFAP antibody (1:500) and anti-TRAF6 antibody in the dorsal horn of the spinal cord were performed to assess colocalization. In the hippocampal dentate gyrus, anti-DCX antibody (1:500) and anti-NeuN (a marker of neurons) antibody (1:500, mouse monoclonal, Abcam plc; ab104224) or anti-PROX1 antibody (1:100) and anti-NeuN antibody colocalization was examined by immunofluorescence staining.
After incubation with primary antibodies and PBS washing, sections were incubated with Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:200) and Alexa Fluor 546-conjugated goat anti-mouse IgG (1:200) for 60 min. Sections were counterstained with PBS and 4′ 6-diamidino-2-phenylindole for 10 min. Finally, sections were mounted with aqueous mounting medium. Immunofluorescence staining was observed with a fluorescence microscope (EVOS f1; AMG, Mill Creek, WA, US).
Quantitative Analysis of Immunostained Areas
Stained sections of lumbar vertebrae and the cerebrum were photographed at 10 × magnification using a digital camera for optical microscopy (DP21, Olympus Optical Co., Tokyo, Japan). The ratios of cell areas showing anti-IBA1, GFAP, CCR2, and TRAF6 immunoreactivity to the areas showing no reactivity in the dorsal horn of the spinal cord, including laminae I‒III, and the DCX and PROX1 immunoreactivity ratios in the hippocampal dentate gyrus region were quantitatively measured using Image-J software (NIH, Bethesda, MD, USA). Quantitative analysis of each immunostained section was performed by two or three persons who were informed of the treatment group. This method of analysis was employed because it was possible to quantify the percentage of immunostained areas.
Statistical Analysis
Data were expressed as mean ± standard error (SE). Two-way analysis of variance (group × time), followed by the Bonferroni post-hoc test for multiple comparisons, was used to analyze the time course of the 50% withdrawal threshold. Where appropriate, one-way analysis of variance followed by the Tukey post-hoc test was used to analyze the proportion of immunostained areas. Statistical significance was set at P < 0.05 and data were analyzed using SPSS version 25 (IBM SPSS Corp., Armonk, NY, USA).
Results
Regular Exercise Reduces Post-CCI Pain Hypersensitivity
The 50% pain response threshold (g) induced by the Von Frey test was evaluated over time in the Ex and No-Ex groups before CCI (n = 11, 11) and at 1 week (n = 11, 11), 2 weeks (n = 8, 8), 3 weeks (n = 8, 8), 4 weeks (n = 4, 4), and 5 weeks (n = 4, 4) post-CCI. The Ex group and the non-injured side of the Ex group were used as controls (Figure 1A).
The pain response threshold of the Ex group showed a trend toward improvement when compared to the No-Ex group, but there was no significant difference from 1 to 4 weeks post-CCI. However, at 5 weeks after CCI, the pain response threshold of the Ex group showed significant improvement (P > 0.01, F=17.7).
Compared with the control group, the Ex and No-Ex groups showed a significant decrease in pain withdrawal thresholds from the first week after CCI. The No-Ex group, on the other hand, showed no significant difference at week 4, but showed a prominent decrease in the pain threshold at 5 weeks.
IBA1 and GFAP, CCR2, TRAF6 in the Spinal Dorsal Horn are Suppressed by Exercise Therapy
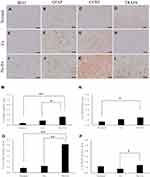
At 3 and 5 weeks post-CCI, IBA1 and GFAP, CCR2, TRAF6 positive cell area ratios were quantified in laminae I‒III of the dorsal horn of the spinal cord (Figure 1B).
The area ratio of IBA1-positive cells in the Normal group was 0.4 ± 0.1% (right side) and 0.6 ± 0.1% (left side) (Figure 2A and M), and the area ratio of the GFAP-positive cells was 0.7 ± 0.2% (right side) and 0.9 ± 0.4% (left side) (Figure 2B and N). The No-Ex group showed a significant increase in IBA1 and GFAP at both 3 and 5 weeks post-CCI, as compared to the Normal group (P < 0.01). The area ratio of CCR2-positive cells in the Normal group was 0.4 ± 0.1% (Figure 2C and O), and the area ratio of TRAF6-positive cells was 1.2 ± 0.2% (Figure 2D and P).
At 3 weeks post-CCI, the area ratio of IBA1-positive cells was 0.9 ± 0.1% (n = 4) in the Ex group and 1.6 ± 0.3% (n = 4) in the No-Ex group (Figure 2E, I and M). GFAP was significantly lower in the Ex group (P < 0.05) than in the No-Ex group (P < 0.05). For IBA1, the Ex group (P < 0.05) showed a significant decrease as compared to the No-Ex group, but there was no significant difference in GFAP (Figure 2F, J and N). Furthermore, at 3 weeks after CCI, the CCR2 area ratio was 1.2 ± 0.5% (n = 4) in the Ex group and 4.7 ± 0.7% (n = 4) in the No-Ex group. The value in the Ex group (P < 0.01) was significantly lower than that in the No-Ex group (Figure 2G, K and O). For TRAF6, the value was 0.8 ± 0.1% in the Ex group (n = 4) and 1.4 ± 0.2% in the No-Ex group (n = 4), showing a significant decrease in the Ex group (P < 0.05) as compared to the No-Ex group (Figure 2H, L and P).
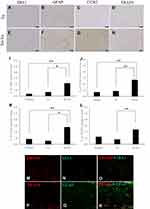
At 5 weeks post-CCI, IBA1 was 0.6 ± 0.1% (n = 4) in the Ex group and 2.2 ± 0.4% (n = 4) in the No-Ex group (Figure 3A, E and I). These values were 0.9 ± 0.2% (n = 4) in the Ex group and 2.7 ± 0.4% (n = 4) in the No-Ex group for GFAP. GFAP was significantly lower in the Ex group (P < 0.05) than in the No-Ex group (Figure 3B, F and J). Furthermore, at 5 weeks after CCI, CCR2 was 0.6 ± 0.2% (n = 4) in the Ex group and 2.8 ± 0.6% (n = 4) in the No-Ex group (Figure 3C, G and K). The value in the No-Ex group was significantly increased (P < 0.05), while the value in the Ex group was decreased significantly (P < 0.01) as compared to the Normal group. The value for TRAF6 was 0.8 ± 0.2% (n = 4) in the Ex group and 2.4 ± 0.4% (n = 4) in the No-Ex group, indicating a significant decrease in the Ex group (P < 0.05) as compared to the No-Ex group (Figure 3D, H and L).
Fluorescence immunostaining of IBA1 and TRAF6, and of GFAP and TRAF6 in the No-Ex group was performed at 5 weeks after CCI (Figure 3M‒R). The results showed co-expression and activation of GFAP and TRAF6 (Figure 3R).
DCX and PROX1 in the Hippocampal Dentate Gyrus are Activated by Exercise Therapy
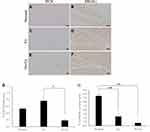
The cell area ratios positive for DCX and PROX1 in the hippocampal dentate gyrus were measured at 3 and 5 weeks after CCI (Figure 1C). These values were 0.8 ± 0.2% (n = 4) in the Ex group and 0.2 ± 0.0% (n = 4) in the No-Ex group at 3 weeks (Figure 4C, E and G). The Ex group showed a significant increase as compared to the No-Ex group (P <0.05). The No-Ex group showed no significant difference compared to the Normal group. The PROX1-positive cell area ratio was 2.3 ± 0.5% (n = 4) in the Ex group and 0.7 ± 0.1% (n = 4) in the No-Ex group (Figure 4D, F and H). The positive cell area ratio in the Normal group was 0.5 ± 0.0% for DCX (Figure 4A and G) and 7.5 ± 0.5% for PROX1 (Figure 4B and H).
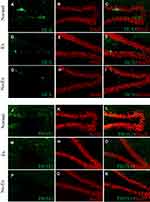
At 5 weeks post-CCI, the Ex group was 0.5 ± 0.1% (n = 4) and 0.1 ± 0.0% in the No-Ex group (n = 4) (Figure 5A, C and E). There was a significant increase in the Ex group (P <0.01) as compared with the No-Ex group, and there was a significant decrease in the No-Ex group as compared with the Normal group. In immunofluorescent staining (Figure 6A–I), DCX was more abundant in the Normal and Ex groups than in the No-Ex group (Figure 6A, D and G). The PROX1-positive cell area ratio was 4.5 ± 0.6% (n = 4) in the Ex group and 2.2 ± 0.3% (n = 4) in the No-Ex group (Figure 5B, D and F). The Ex group (P < 0.05) showed a significant increase as compared with the No-Ex group. In fluorescent staining (Figure 6J–R) of PROX1, larger granule cells were observed in the Normal and Ex groups than in the No-Ex group (Figure 6J, M and P). No simultaneous staining was observed in DCX and NeuN double-staining (Figure 6L, O and R).
Discussion
The purpose of this study was to investigate the effects of regular exercise on neuropathic pain relief, to elucidate the mechanism, and to determine the effects of neuropathic pain in the hippocampus. To this end, we investigated activation of spinal glial cells, expression of CCR2 and TRAF6, and the dynamics of DCX and PROX1 in the hippocampal dentate gyrus with and without regular exercise therapy in a rat CCI model of neuropathic pain. We showed that exercise led to a significantly lower pain response threshold at 5 weeks post-CCI, indicating pain relief. In the spinal cord dorsal horn, IBA1, CCR2, and TRAF6 expression was markedly lower in the Ex group than in the other groups at 3 weeks post-CCI, and these proteins as well as GFAP were also expressed significantly less in the Ex group than in the No-Ex group at 5 weeks post-CCI. Hippocampal DCX and PROX1 expression at 5 weeks post-CCI was markedly increased in the Ex group compared to the No-Ex and Ex groups.
Neuropathic pain is triggered by central or peripheral nerve injury and plays a fundamental role in the development of functional allodynia, nociceptive pain, and spontaneous pain by activating microglia and astrocytes in the dorsal horn of the spinal cord. These cells produce inflammatory substances and abnormal neuronal function in response to nerve cell damage.
CCL2/CCR2-dependent mechanisms play an important role in the development of neuropathic pain, in NMDAR-mediated central sensitization, and in the development and maintenance of neuropathic pain.12 Chemokines induce glial cell activation or promote excitatory synaptic transmission in spinal neurons and exaggerate central sensitization, depending on the differential distribution of ligand receptors in neurons and glial cells.27 In addition, nerve injury causes rapid microglial activation, and delayed and sustained astrocyte activation.28,29 In our experiments, activated microglia in the dorsal horn of the spinal cord at 3 weeks after CCI showed a significant increase in the No-Ex group as compared to the Normal group, due to the nerve injury. This continued to increase significantly by 5 weeks after CCI, indicating continued microglial activation. Astrocytes were also significantly increased in the No-Ex group as compared to the Normal group at both 3 and 5 weeks post-CCI. In addition, levels of CCR2, a chemokine receptor, was significantly increased in the No-Ex group as compared to the Normal group at both 3 and 5 weeks post-CCI.
TRAF6, which is expressed on astrocytes, maintains neuropathic pain by integrating TNF-α and IL-1β signaling and activating the JNK/CCL2 pathway.14 In this study, the No-Ex group showed a significant increase in astrocytes when compared to the Normal at both 3 and 5 weeks post-CCI, but the difference was not significant. These results suggested that microglia may be more involved in the development of neuropathic pain, and that astrocytes, but not microglial activation, may be more involved in its maintenance. Additionally, the expression of TRAF6 on astrocytes is important for the maintenance of neuropathic pain. The continued expression of TRAF6 may contribute to the activation of CCR2 receptors, which are receptors for CCL2 in primary terminals and microglia. CCR2 receptors on microglia may then activate the JNK/CCL2 pathway in astrocytes.12,30 TRAF6 is also likely to be an important therapeutic target as it links activation of these receptors to downstream signals, thereby performing important functions in a variety of physiological and pathological processes, including adaptive immunity, innate immunity, inflammation, and tissue homeostasis.31
Regarding the effect of exercise therapy on neuropathic pain, at 3 weeks post-CCI, microglia and astrocytes in the dorsal horn of the spinal cord showed a significant decrease in IBA1 in the Ex group, as compared to the No-Ex and Normal groups. CCR2 expression increased with increased expression of IBA1 after nerve injury, but a comparison of the No-Ex and Ex groups showed a significant relative decrease in the Ex group, indicating that exercise suppressed microglial activation and chemokine receptor inhibition. Astrocytes were activated by nerve damage, but no significant difference was observed between the No-Ex and Ex groups. However, TRAF6 showed a small but significant difference between the No-Ex and Ex groups. In the dorsal horn of the spinal cord at 5 weeks post-CCI, IBA1 showed a significant decrease in the Ex group as compared to the No-Ex, and microglial activation was suppressed by exercise. CCR2 also showed a significant decrease in the Ex group as compared to the No-Ex group at this time point. On the other hand, GFAP expression at 5 weeks post-CCI was significantly higher in the No-Ex group than in the Ex group at 3 weeks, confirming that it was suppressed by exercise. In addition, TRAF6, which is involved in astrocyte activation, was also significantly decreased in the Ex group as compared to the No-Ex group, indicating that its expression was suppressed in the Ex group. Regarding the effects of neuropathic pain on the hippocampal dentate gyrus, a recent study reported that the onset of neuropathic pain causes signs of pathological inflammation in the hippocampal dentate gyrus, leading to a decrease in neurogenesis with memory destruction, which leads to a decrease in DCX.15 Furthermore, a lack of PROX1, which is required for the maintenance of intermediate nerve progenitor cells in adult neurogenesis, has been reported to lead to increased apoptosis of DCX-expressing cells and lack of neurogenesis in adults.16
In this study, we investigated the effects of exercise on the expression of DCX, a neurogenic cell marker, and PROX1, which is required for the maintenance of intermediate progenitor cells in neurogenesis, in our CCI rat model. No significant difference was observed between the Normal and No-Ex groups at 3 weeks after CCI. On the other hand, comparison of the No-Ex and Ex groups showed a significant increase in neurogenesis. PROX1 showed highly significant differences among groups, with the No-Ex and Ex groups showing a decrease as compared to the Normal group. New neurons are generated in the adult dentate gyrus throughout life, and the mammalian brain undergoes a process called adult hippocampal neurogenesis. Hippocampal-mediated behavior, synaptic plasticity, and neurogenesis are abnormal in cases with neuropathic pain, where therapeutic approaches are particularly challenging.32 Our findings may indicate that neuropathic pain may have led to an inability to maintain intermediate progenitor cells by 3 weeks post-CCI. Furthermore, at 5 weeks post-CCI, the No-Ex group showed a significant decrease as compared to the Normal and No-Ex groups. In terms of PROX1, the Normal and No-Ex groups and the Normal and Ex groups continued to show highly significant differences, with expression being significantly higher in the Ex than in the No-Ex group.
Five weeks after CCI, DCX immunocytochemistry showed a significant decrease in neurogenesis in the No-Ex group as compared to the Normal. The Ex group showed a significant increase relative to the No-Ex group, indicating that neurogenesis was activated by the exercise intervention. The significant decrease in PROX1 in the No-Ex group as compared to the Normal and No-Ex groups at 5 weeks post-CCI may indicate that chronic neuropathic pain may be a result of difficulty maintaining neural intermediate progenitor cells. Comparing the No-Ex and Ex groups, the Ex group showed a significantly higher level, but this level was nevertheless significantly lower in the Ex than in the Normal group. This suggests that, although neuropathic pain affects the maintenance of intermediate progenitor cells, there may be a recovery trend due to exercise. It is possible that neurogenesis was promoted at 5 weeks post-CCI due to exercise, although it was somewhat difficult to maintain the intermediate progenitor cells necessary for neurogenesis due to neuropathic pain, as was observed at 3 weeks post-CCI.
The literature suggests that chronic pain impairs hippocampus-dependent memory formation, particularly spatial memory, and has been demonstrated in both humans and in rodent models. It has been reported that only chronic pain, but not acute pain, inhibits spatial memory formation.33 Clinical observations have indicated that many patients with chronic pain have increased anxiety and depression, making the emotional pain component important.32 Cognitive and emotional-behavioral impairments caused by the onset of neuropathic pain are associated with hippocampal neurogenic deficits.34 Neuropathic pain has also been reported to inhibit differentiation into mature neurons in the hippocampal dentate gyrus region in parallel with reduced survival of neoplastic cells.17 It has been reported that exercise works to prevent the onset of pain and eliminate pain after injury, and 58/64 (90%) articles published on the topic of exercise-induced analgesia showed a positive effect of exercise with only one activity.35 In our study, we also observed a decrease in neurogenic pain-induced expression of neurogenic cells at 3 weeks post-CCI. However, at 5 weeks post-CCI, when exercise is most likely to have an effect, we observed an increase in DCX, a marker of neurogenic cells, and PROX1, a marker of the intermediate progenitor cells necessary for neurogenesis. These findings suggest that regular exercise alleviates neuropathic pain and improves the neuropathic pain induced abnormal neurogenic function in the hippocampal dentate gyrus.
Conclusion
In this study, we found that the expression of spinal glial cells markers, CCR2 and TRAF6, and the dynamics of DCX and PROX1 in the hippocampal dentate gyrus were related to the effects of regular exercise therapy on the response to neuropathic pain. These results suggested that regular exercise suppresses microglial activation, which is involved in the development of neuropathic pain, in the dorsal horn of the spinal cord. It also suppresses expression of CCR2, a chemokine receptor that may be involved in central sensitization, and that of TRAF6, which integrates TNF-α and IL-1β signaling. Although often considered with respect to glial cells as a target for therapy, TRAF6 is a noteworthy target with respect to maintenance in neuropathic pain. In terms of the relationship between neuropathic pain and the hippocampus, exercise was found to activate DCX and PROX1 expression in the hippocampal dentate gyrus, suggesting that the onset of neuropathic pain inhibits neurogenesis, which may be accompanied by memory destruction.
Acknowledgments
We are grateful for the support from Professor Shigeyoshi Higo of Kyushu University of Nursing and Social Welfare. We also thank the Sumizono Seminar member (Masaya Koga, Shiho Ishimoto, Mai Kamoda, Shiori Koba, Nozomi Hidaka, Yusuke Ono, and Shoto Igawa) at Kyushu University of Nursing and Social Welfare for their support.
Funding
This work was supported by JSPS KAKENHI Grant Number JP19K19858.
Disclosure
The authors have no conflicts of interest to disclose in this study.
References
1. Raja SN, Carr DB, Cohen M, et al. The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982. doi:10.1097/j.pain.0000000000001939
2. Scholz J, Finnerup NB, Attal N, et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 2019;160(1):53–59. doi:10.1097/j.pain.0000000000001365
3. Rice ASC, Smith BH, Blyth FM. Pain and the global burden of disease. Pain. 2016;157(4):791–796. doi:10.1097/j.pain.0000000000000454
4. van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(9):654–662. doi:10.1016/j.pain.2013.11.013
5. Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237–251. doi:10.1016/j.pain.2007.08.033
6. Cavalli E, Mammana S, Nicoletti F, Bramanti P, Mazzon E. The neuropathic pain: an overview of the current treatment and future therapeutic approaches. Int J Immunopathol Pharmacol. 2019;33:2058738419838383. doi:10.1177/2058738419838383
7. Bannister K, Sachau J, Baron R, Dickenson AH. Neuropathic pain: mechanism-based therapeutics. Annu Rev Pharmacol Toxicol. 2020;60:257–274. doi:10.1146/annurev-pharmtox-010818-021524
8. Inoue K, Tsuda M. Microglia and neuropathic pain. Glia. 2009;57(14):1469–1479. doi:10.1002/glia.20871
9. Zhu KJ, Yang JS. Anti-allodynia effect of safranal on neuropathic pain induced by spinal nerve transection in rat. Int J Clin Exp Med. 2014;7(12):4990–4996.
10. Tsuda M. Microglia in the spinal cord and neuropathic pain. J Diabetes Investig. 2016;7(1):17–26. doi:10.1111/jdi.12379
11. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi:10.1146/annurev.neuro.051508.135531
12. Piotrowska A, Kwiatkowski K, Rojewska E, et al. Direct and indirect pharmacological modulation of CCL2/CCR2 pathway results in attenuation of neuropathic pain – in vivo and in vitro evidence. J Neuroimmunol. 2016;297:9–19. doi:10.1016/j.jneuroim.2016.04.017
13. Xian H, Jiang Y, Zhang H, Ma SB, Zhao R, Cong R. CCL2-CCR2 axis potentiates NMDA receptor signaling to aggravate neuropathic pain induced by brachial plexus avulsion. Neuroscience. 2020;425:29–38. doi:10.1016/j.neuroscience.2019.11.012
14. Lu Y, Jiang BC, Cao DL, et al. TRAF6 upregulation in spinal astrocytes maintains neuropathic pain by integrating TNF-α and IL-1β signaling. Pain. 2014;155(12):2618–2629. doi:10.1016/j.pain.2014.09.027
15. Egorova E, Starinets A, Tyrtyshnaia A, et al. Hippocampal neurogenesis in conditions of chronic stress induced by sciatic nerve injury in the rat. Cells Tissues Organs. 2019;207(1):58–68. doi:10.1159/000501236
16. Lavado A, Lagutin OV, Chow LM, Baker SJ, Oliver G. Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. PLOS Biol. 2010;8(8):e1000460. doi:10.1371/journal.pbio.1000460
17. Somelar K, Jürgenson M, Jaako K, et al. Development of depression-like behavior and altered hippocampal neurogenesis in a mouse model of chronic neuropathic pain. Brain Res. 2021;1758:147329. doi:10.1016/j.brainres.2021.147329
18. Abbott LC, Nigussie F. Adult neurogenesis in the mammalian dentate gyrus. Anat Histol Embryol. 2020;49(1):3–16. doi:10.1111/ahe.12496
19. Moisset X, Bouhassira D, Avez Couturier J, et al. Pharmacological and non-pharmacological treatments for neuropathic pain: systematic review and French recommendations. Rev Neurol. 2020;176(5):325–352. doi:10.1016/j.neurol.2020.01.361
20. Stagg NJ, Mata HP, Ibrahim MM, et al. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114(4):940–948. doi:10.1097/ALN.0b013e318210f880
21. Ghodrati-Jaldbakhan S, Ahmadalipour A, Rashidy-Pour A, Vafaei AA, Miladi-Gorji H, Alizadeh M. Low- and high-intensity treadmill exercise attenuates chronic morphine-induced anxiogenesis and memory impairment but not reductions in hippocampal BDNF in female rats. Brain Res. 2017;1663:20–28. doi:10.1016/j.brainres.2017.02.024
22. Bement MK, Sluka KA. Low-intensity exercise reverses chronic muscle pain in the rat in a naloxone-dependent manner. Arch Phys Med Rehabil. 2005;86(9):1736–1740. doi:10.1016/j.apmr.2005.03.029
23. Véras-Silva AS, Mattos KC, Gava NS, Brum PC, Negrão CE, Krieger EM. Low-intensity exercise training decreases cardiac output and hypertension in spontaneously hypertensive rats. Am J Physiol. 1997;273(6):H2627–H2631. doi:10.1152/ajpheart.1997.273.6.H2627
24. Sumizono M, Sakakima H, Otsuka S, et al. The effect of exercise frequency on neuropathic pain and pain-related cellular reactions in the spinal cord and midbrain in a rat sciatic nerve injury model. J Pain Res. 2018;11:281–291. doi:10.2147/JPR.S156326
25. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi:10.1016/0304-3959(88)90209-6
26. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi:10.1016/0165-0270(94)90144-9
27. Zhang ZJ, Jiang BC, Gao YJ. Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain. Cell Mol Life Sci. 2017;74(18):3275–3291. doi:10.1007/s00018-017-2513-1
28. Kubíčková L, Klusáková I, Dubový P. Bilateral activation of glial cells and cellular distribution of the chemokine CCL2 and its receptor CCR2 in the trigeminal subnucleus caudalis of trigeminal neuropathic pain model. Histochem Cell Biol. 2020;153(4):239–255. doi:10.1007/s00418-020-01850-4
29. Xu J, Dong H, Qian Q, et al. Astrocyte-derived CCL2 participates in surgery-induced cognitive dysfunction and neuroinflammation via evoking microglia activation. Behav Brain Res. 2017;332:145–153. doi:10.1016/j.bbr.2017.05.066
30. Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27(45):12396–12406. doi:10.1523/JNEUROSCI.3016-07.2007
31. Wu H, Arron JR. TRAF6, a molecular bridge spanning adaptive immunity, innate immunity and osteoimmunology. BioEssays. 2003;25(11):1096–1105. doi:10.1002/bies.10352
32. Grilli M. Chronic pain and adult hippocampal neurogenesis: translational implications from preclinical studies. J Pain Res. 2017;10:2281–2286. doi:10.2147/JPR.S146399
33. Xia SH, Hu SW, Ge DG, et al. Chronic pain impairs memory formation via disruption of neurogenesis mediated by mesohippocampal brain-derived neurotrophic factor signaling. Biol Psychiatry. 2020;88(8):597–610. doi:10.1016/j.biopsych.2020.02.013
34. Manzhulo I, Manzhulo O, Tyrtyshnaia A, et al. Modulation of hippocampal astroglial activity by synaptamide in rats with neuropathic pain. Brain Sci. 2021;11(12):1561. doi:10.3390/brainsci11121561
35. Lesnak JB, Sluka KA. Mechanism of exercise-induced analgesia: what we can learn from physically active animals. PAIN Rep. 2020;5(5):e850. doi:10.1097/PR9.0000000000000850
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Dexmedetomidine Alleviates Neuropathic Pain via the TRPC6-p38 MAPK Pathway in the Dorsal Root Ganglia of Rats
Xu S, Yi Y, Wang Y, Wang P, Zhao Y, Feng W
Journal of Pain Research 2022, 15:2437-2448
Published Date: 19 August 2022
Identification of Key Genes and Pathways in Oxaliplatin-Induced Neuropathic Pain Through Bioinformatic Analysis
Lou Y, Xu X, Wang R, Yao D
Journal of Pain Research 2024, 17:1639-1650
Published Date: 3 May 2024
Ligusticum chuanxiong Hort. Ameliorates Neuropathic Pain by Regulating Microglial M1 Polarization: A Study Based on Network Pharmacology
Cui S, Feng X, Xia Z
Journal of Pain Research 2024, 17:1881-1901
Published Date: 23 May 2024