Back to Journals » Journal of Pain Research » Volume 15
Dexmedetomidine Alleviates Neuropathic Pain via the TRPC6-p38 MAPK Pathway in the Dorsal Root Ganglia of Rats
Authors Xu S, Yi Y, Wang Y, Wang P, Zhao Y, Feng W
Received 17 June 2022
Accepted for publication 15 August 2022
Published 19 August 2022 Volume 2022:15 Pages 2437—2448
DOI https://doi.org/10.2147/JPR.S378893
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qi Fang
Songchao Xu,1 Yusheng Yi,2 Yanting Wang,1 Pei Wang,1 Yang Zhao,1 Wei Feng1
1Department of Anesthesiology, Affiliated Hospital of Qingdao University, Qingdao University, Qingdao, People’s Republic of China; 2Department of Algology, Affiliated Hospital of Qingdao University, Qingdao University, Qingdao, People’s Republic of China
Correspondence: Yang Zhao; Wei Feng, Email [email protected]; [email protected]
Purpose: Neuropathic pain is a chronic intractable disease characterized by allodynia and hyperalgesia. Effective treatments are unavailable because of the complicated mechanisms of neuropathic pain. Transient receptor potential canonical 6 (TRPC6) is a nonselective calcium (Ca2+)-channel protein related to hyperalgesia. Dexmedetomidine (Dex) is an alpha-2 (α 2) adrenoreceptor agonist that mediates intracellular Ca2+ levels to alleviate pain. However, the relationship between TRPC6 and Dex is currently unclear. We speculated that the α 2 receptor agonist would be closely linked to the TRPC6 channel. We aimed to investigate whether Dex relieves neuropathic pain by the TRPC6 pathway in the dorsal root ganglia (DRG).
Methods: The chronic constriction injury (CCI) model was established in male rats, and we evaluated the mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL). The expression of TRPC6 and Iba-1 in the DRG were analyzed using quantitative real-time polymerase chain reaction, Western blot, and immunofluorescence assay. The levels of inflammatory cytokines were measured using an enzyme-linked immunosorbent assay.
Results: Compared with the CCI normal saline group, both the MWT and TWL were significantly improved after 7 days of Dex administration. Results demonstrated that TRPC6 expression was increased in the DRG following CCI but was suppressed by Dex. In addition, multiple administrations of Dex inhibited the phosphorylation level of p38 mitogen-activated protein kinase and the upregulation of neuroinflammatory factors.
Conclusion: The results of this study demonstrated that Dex exhibits anti-nociceptive and anti-inflammatory properties in a neuropathic pain model. Moreover, our findings of the CCI model suggested that Dex has an inhibitory effect on TRPC6 expression in the DRG by decreasing the phosphorylation level of p38 in the DRG.
Keywords: neuropathic pain, CCI model, microglia, TRPC6, dexmedetomidine, neuroinflammation
Introduction
Neuropathic pain is a common chronic pain condition encountered in clinical practice that is typically caused by a lesion or disease of the somatosensory nervous system.1,2 Its main clinical manifestations in patients include spontaneous pain, allodynia, and hyperalgesia.3 Neuropathic pain has become a widespread public health concern, affecting 6–8% of the population, and roughly a quarter of these patients experience chronic pain.4 The pathophysiological mechanisms underlying neuropathic pain are currently uncertain. Ion channels have been shown to play an important role at different stages of the disease. For example, altered expressions of ion channels in primary afferent neurons, such as voltage-gated sodium, potassium, and calcium (Ca2+) channels,5,6 either instigate abnormal electrical activity that contributes directly to spontaneous pain or accelerate the release of neurotransmitters in the synapse, which affects the excitability of the neurons.7 Furthermore, proliferating microglia and neuroinflammation play significant roles in the initiation and maintenance of chronic pain.8 Indeed, it has been confirmed that inhibiting microglial activation is an effective strategy for alleviating neuropathic pain.
The dorsal root ganglia (DRG) are located along the spinal cord and contain a variety of neuronal types and cell bodies of sensory neurons. The DRG is considered a critical structure in sensory transmission and modulation, such as the processing of nociceptive and thermal stimuli.9 Moreover, a variety of receptors (eg, ion channel receptors) in the DRG may be involved in neuropathic pain.10 Thus, it is critical to gain a thorough understanding of the receptors found in the DRG in neuropathic pain.
The transient receptor potential (TRP) superfamily of channels comprises seven subfamilies of ligand-gated ion channels.11 TRP channels participate in multiple biological processes by sensing different physicochemical stimuli.12,13 Several family members are expressed in the nervous system and are involved in the modulation of chronic neuropathic pain in the DRG and spinal cord.14 TRP canonical 6 (TRPC6) is a nonselective Ca2+ channel protein that is directly activated by diacylglycerol15 and is expressed in various tissues, such as the kidney, smooth muscle cells, placenta, and brain.13 The physiological role of TRPC6 in the kidney has been extensively studied. For example, TRPC6 can be used to balance the Ca2+ concentration of podocytes16 and is susceptible to mechanical stimuli and noxious temperatures.17 The TRPC6 channel acts synergistically with TRPV4 and TRPC1 to regulate mechanical hyperalgesia and nociceptor sensitization.18 TRPC6 blockers induce strong analgesic actions by suppressing microglia activation and increasing the level of proinflammatory cytokines via the p38 signaling pathway.19 Therefore, inhibiting the TRPC6 receptor may be a potential therapeutic strategy for patients with neuropathic pain.
Dexmedetomidine (Dex), an alpha-2 (α2) adrenoceptor agonist, is an ideal intravenous drug that has been used primarily as an adjunct to clinical anesthesia and ICU sedation.20 It has also been applied to the treatment of neuropathic pain, complex pain syndrome, and multimodal analgesia.21,22 However, the molecular mechanism underlying its analgesic property remains unclear.20 Previous study have reported that Dex induces an analgesic effect by inhibiting spinal P2X7R expression and ERK phosphorylation.23 Zhao et al24 demonstrated that Dex alleviates neuropathic pain by inhibiting HMGB1-mediated astrocyte activation and the TLR4/NF-κB signaling pathway. More importantly, Lee et al showed that Dex relieves neuropathic pain by inhibiting the expression of TRPV1 and capsaicin-induced Ca2+ responses in the DRG.25 Dex effectively reverses cytosolic Ca2+ ion accumulation in the neurons via the TRPV1 and TRPM2 pathways.26 Therefore, we speculated that the α2 receptor agonist will be closely related to the TRPC6 channel. In the present study, we aimed to investigate whether Dex alleviates neuropathic pain via the TRPC6 pathway in the DRG.
Methods
Animals
We used adult male Sprague-Dawley rats weighing 200–250 g (Jinan Pengyue Experimental Animal Breeding Co., Ltd., Jinan, China) in this study. Rats were housed under constant conditions of a room temperature of 23 ± 1.5 °C, a humidity level of 50%–60%, and a 12 h light-dark cycle. The animals were provided with sufficient food and water. The experiments were approved by the Ethics Committee of Qingdao University (Animal Ethical Committee approval number: AHQU-MAL20211015) and were performed according to the ethical guidelines for animal experimentation of Qingdao University and the IASP’s ethical guidelines.
Neuropathic Pain Model and Drug Administration
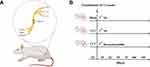
The rats were randomly divided into three groups: the sham-normal saline (NS) group, the chronic constriction injury (CCI)-NS group, and the CCI-Dex group (Figure 1B). The groups were further divided into subgroups (six rats per group) according to timepoint: 1 day before surgery group, 3 days after surgery group, 7 days after surgery group, and 14 days after surgery group. Rats were allowed to acclimate to the environment before initiating the experiment. The CCI model was established under anesthesia with 2% isoflurane. Blunt separation was performed in the middle of the left thigh to expose the sciatic nerve trunk. Four ligatures were loosely tied (~1-mm spacing) around the sciatic nerve until a slight tremor was observed (Figure 1A).27,28 The sciatic nerve was then carefully placed into the original position. Finally, the skin was sutured, and antibiotic cream was applied. The sham-NS group underwent the same surgical procedure as the experimental group but without sciatic nerve ligation. Following previous study,29 the CCI-Dex group was injected intraperitoneally with Dex (Jiangsu Hengrui Medicine Co., Ltd.) at a dose of 30 μg/kg once a day from days 1 to 14 after surgery. The CCI-NS group received an identical volume of saline injection.
Behavioral Testing
Behavioral tests were performed 2 h after drug delivery. To evaluate nociceptive behavior, we evaluated the mechanical withdrawal threshold (MWT) in response to mechanical allodynia and thermal withdrawal latency (TWL) in response to radiant heat. For the MWT testing, animals were allowed to habituate to their testing environment for at least 30 min before the test. Mechanical pain thresholds were determined using the Von Frey filament up-down method.30 A suitable starting filament was applied perpendicularly to the plantar surface of the left hind paw for 2–3 s. A positive reaction was defined as a sudden withdrawal, shaking, or licking of the hind paw in response to the stimulus. The smallest force that produced a positive reaction was recorded as the MWT. This test was repeated twice with a 5-min interval. The hot plate test was used to determine TWL. The rats were placed on a hot plate (55 ± 1 °C) and removed as soon as they exhibited positive behaviors, such as jumping or licking their paws. The time from being placed on the hot plate to exhibiting positive behaviors was recorded. To avoid tissue damage, the maximum heating time was limited to 25s. Two measurements were obtained with a 2-min interval.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from the lumbar L4–L6 DRG using the RNA Isolater Total RNA Extraction Reagent (Vazyme) according to the standard isolation protocol. Subsequently, the total RNA was reverse transcribed using the HiScript III 1st Strand cDNA Synthesis Kit (+gDNA wiper) (Vazyme). The Cham Q Universal SYBR qPCR Master Mix (Vazyme) was used to amplify the reverse transcription products for the PCR. The sequences for the qRT-PCR primers were as follows: TRPC6, forward, 5’-GCTCTCATATACTGGTGTGCTCCTT-3’, reverse, 5’-GGAGCTTGGTGCCTTCAAATC-3’; GAPDH, forward, 5’-ATGCCGCCTGGAGAAACC-3’, reverse, 5’-GCATCAAAGGTGGAAGAATGG-3’. Relative expression was analyzed using the 2^ddCt method.
Western Blot Analysis
DRG tissues were homogenized in RIPA lysis buffer (Beyotime), supplemented with proteinase inhibitor (PMSF, Beyotime). After centrifugation at 12,000 rpm for 15 min at 4 °C, the supernatants were collected, and the total protein concentration was detected by BCA assay (BCA Protein Quantification Kit, Vazyme). Following denaturation, proteins were separated using polyacrylamide gel electrophoresis. The in-gel proteins were then transferred to PVDF membranes (Millipore). Subsequently, the membrane was blocked for 2 h with 5% nonfat dry milk in 1X TBST buffer (Solarbio). The membranes were incubated overnight (12–16 h) at 4 °C with the following primary antibodies: rabbit anti-TRPC6 (1:1000, Bioss), rabbit anti-Iba-1 (1:500, ABcam), rabbit anti-GAPDH (1:10,000, ABcam), and rabbit anti-β actin (1:1000, ABcam). The membrane was incubated for 1 h at room temperature with a second antibody (1:5000, goat anti-rabbit, Elabscience) the next day. Protein bands were visualized using an Omni-ECL™Pico Light Chemiluminescence Kit (Epizyme), and images were acquired using the Automatic chemiluminescence image analysis system (Tannon). The data were analyzed using the Image J software (Image J 1.4, NIH, USA).
Immunofluorescence
Immunofluorescence staining was performed on the DRG tissues. Sections of DRG tissue were fixed in 4% paraformaldehyde. The fixed tissues were then embedded in paraffin and sectioned. Paraffin sections were deparaffinized and rehydrated. Double immunofluorescence staining was performed as per the standard protocol. After washing three times with PBS, the DRG sections were blocked in 3% bovine serum albumin for 30 min. Sections were incubated overnight at 4 °C with the primary antibodies of rabbit anti-TRPC6 (1:100, Proteintech) and rabbit anti-Iba-1 (1:3000, Abcam), followed by goat anti-rabbit antibody marked with HRP (Servicebio, 1:500) for 50 min at room temperature. The slides were incubated with TSA-FITC (Servicebio, 1:500) solution for 10 min in the dark. Microwave treatment was applied to remove the primary and secondary antibodies combined with the tissue. The sections were then incubated with an anti-NeuN antibody (Servicebio, 1:500) overnight at 4 °C. Subsequently, the sections were incubated in the dark for 50 min with goat anti-rabbit TSA-CY3 (Servicebio, 1:300), followed by DAPI staining for 10 min. The images were acquired at 400× magnification using a fluorescence microscope (Nikon, NIKON ECLIPSE C1) and scanned with Pannoramic DESK (Budapest, Hungary).
Enzyme-Linked Immunosorbent Assay (ELISA)
Inflammatory cytokines (TNF-α, IL-1β) were quantified using an ELISA kit for TNF-α and IL-1β rats (Boster). Fresh DRG tissues were homogenized, and the supernatant was collected. The procedure was performed according to the instructions included with the ELISA kit. Finally, the absorbance values of each well were measured at 450 nm using a microplate reader (Thermo Scientific).
Statistical Analysis
GraphPad Prism 8 (Graph Pad Software Inc., San Diego, USA) and Microsoft Excel 2019 (Microsoft, Redmond, USA) were used for all statistical analyses. All individual data in the figures are presented as means ± standard errors of the mean. The normality of data was tested using the D’Agostino and Pearson omnibus normality test. MWTs and TWLs at various time periods were analyzed using a two-way analysis of variance (ANOVA). The Bonferroni test was used for multiple comparisons. For qRT-PCR, Western blot, immunofluorescence, and ELISA data, significant differences between the three groups were determined by one-way ANOVAs with Dunnett’s multiple comparisons. A p ≤ 0.05 was considered significant.
Results
Dex Attenuated CCI-Induced Mechanical and Thermal Hyperalgesia
The baseline threshold of mechanical allodynia and thermal hyperalgesia were measured 1 day before the CCI operation. A decrease in hypersensitivity was observed (3.26 ± 0.24, p < 0.001, vs sham group) in the CCI group 7 days after the operation (Figure 2A). The day after the surgery, the heat pain threshold of the CCI group decreased to 11.3 ± 0.5 (p < 0.001, vs sham group) (Figure 2B). Dex was administrated intraperitoneally once per day for 14 consecutive days. The behavioral tests were performed 2 h after Dex administration to avoid the confound of sedation. Compared with the CCI group, both mechanical and heat pain thresholds of the Dex group were higher after the administration of Dex. Seven days after modeling, Dex significantly increased the mechanical pain threshold to 5.972 ± 0.82 g (p < 0.05 vs CCI-NS group). In addition, the heat pain threshold rose to 9.19 ± 0.37 s (p < 0.01 vs CCI group). The was a clear continuous increase in mechanical and thermal thresholds after 7 days of Dex administration.
Dex Inhibited CCI-Induced TRPC6 Upregulation in the DRG
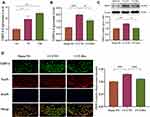
In subsequent experiments, TRPC6 mRNA expression was analyzed by qRT-PCR at different time points after the operation. As shown in Figure 3A, the expression of TRPC6 gradually increased over time. Compared with the −1 day CCI-NS group, TRPC6 expression in the CCI-NS group at 7 days increased by 1.92 ± 0.28 fold (p < 0.01), and that in the CCI-NS group at 14 days increased by 2.46 ± 0.16 fold (p < 0.001). The upregulation of TRPC6 (1.99 ± 0.12, p<0.001, vs sham-NS group) mRNA significantly decreased after 7 days of drug treatment (1.54 ± 0.13, p < 0.05, vs CCI-NS group; Figure 3B). In addition, protein expression of TRPC6 was verified by Western blot analysis (Figure 3C). A 106-kDa band was detected in the DRG tissue after 7 days. The level of TRPC6 in the CCI group increased by 1.41 ± 0.07 fold (p < 0.01 vs sham-NS group), and TRPC6 expression decreased significantly to 1.03 ± 0.09 (p < 0.05 vs CCI-NS group). We were also interested in the expression location of the TRPC6 protein. Seven days after the CCI operation, we performed double-immunofluorescence staining on the DRG tissues for TRPC6 and NeuN. TRPC6/NeuN labeling revealed that TRPC6 was predominately expressed in neurons (Figure 3D). TRPC6 fluorescence intensity increased by 1.3 ± 0.02 fold (p < 0.001) compared with that of the sham-NS group, and following Dex administration, the fluorescence intensity decreased by 1.11 ± 0.04 fold (p < 0.001, vs CCI-NS group).
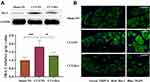
DRG Microglia Proliferation Was Inhibited by Dex Administration
Peripheral neuroimmune cells play an important role in chronic pain. In the present study, the effect of Dex on the activation of peripheral immune cells was examined using the microglial Iba-1 marker in the DRG 7 days after the CCI operation. The Western blot results showed that Iba-1 expression in the CCI-NS group increased by 1.80 ± 0.19 fold (p < 0.01) compared with that in the sham-NS group. Similarly, Iba-1 protein expression in the CCI-Dex group decreased significantly (1.27 ± 0.1, p < 0.001, vs CCI-NS group; Figure 4A). Considerable proliferation was observed around the neurons in the CCI-NS group, whereas microglia were barely visible in the CCI-Dex group (indicated by the arrow in Figure 4B). These findings suggested that immune cell proliferation around neurons was inhibited in the CCI-Dex group.
Proinflammatory Cytokines Were Downregulated After Dex Administration
The imbalance of cytokines has been associated with neuroinflammation in neuropathic pain. After 7 days, we investigated the CCI-induced changes in TNF-α and IL-1β and the influence of Dex on cytokines. The ELISA results are shown in Figure 5. Expression levels of TNF-α (596.1 ± 67.82 pg/mL) and IL-1β (1367 ± 109.5 pg/mL) in the DRG of the CCI group were significantly higher than in the sham-NS group (TNF-α: 310.5 ± 36.72 pg/mL, p < 0.001; IL-1β: 707.1 ± 25.91 pg/mL, p < 0.001). The inflammatory cytokines were attenuated after 7 days of Dex treatment (TNF-α: 361.3 ± 17 pg/mL, p < 0.01, vs CCI-NS group; IL-1β: 869.6 ± 62.53 pg/mL, p < 0.001, vs CCI-NS group).
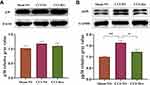
Effects of Dex on the Downstream p38 Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway
Investigations focusing on the downstream signaling pathway have suggested an intimate link between the MAPK pathway and neuropathic pain.31 However, Western blot analysis showed that normalized p38 expression did not significantly change in any of the three groups (Figure 6A). Further experiments revealed that normalized phosphorylate p38 was significantly increased (1.59 ± 0.09, p < 0.01 vs sham-NS group) in the CCI-NS group and decreased by Dex administration (1.21 ± 0.1, p < 0.05 vs CCI-NS group; Figure 6B). This suggested that Dex inhibits p38 phosphorylation, rather than p38 total protein, in the DRG, which is associated with TRPC6 expression in neuropathic pain.
Discussion
In the present study, we demonstrated that consecutive intraperitoneal administration of Dex alleviates CCI-induced mechanical allodynia and thermal hyperalgesia by suppressing TRPC6 expression and reducing the neuroinflammatory response in the DRG.
Neuropathic pain is a common chronic pain condition that is characterized by spontaneous pain and hyperalgesia and is challenging to treat.32 Constant pain has a significant impact on quality of life and often contributes to the development of emotional disorders, such as depression, anxiety, and insomnia.33,34 The primary causes of neuropathic pain are nerve injuries caused by infection, diabetes, cancer, chemotherapy, and surgery.35 The mechanism underlying neuropathic pain is complex and includes nociceptor autosensitization,36 excitation–inhibition imbalance in neural circuits,37 neuroimmune–glia interactions38 (eg, macrophages39 and microglia40), and synaptic plasticity.41 Moreover, the most commonly used analgesics are not effective. Therefore, developing more potential therapeutic candidates for the treatment of neuropathic pain is crucial.
Noxious stimuli are initially detected by peripheral nociceptors and subsequently transmitted to primary afferent neurons, the spinal cord, and finally to specific brain regions.42 It is becoming increasingly clear that there is a close connection between TRP channels and Somatosensation.10 TRP channels are nonselective cation channels with a six transmembrane domain topology. The current study confirmed that TRP expression is associated with the production of pain, including cold allodynia and mechanical and thermal hyperalgesia.43 TRPC6, a Ca2+ permeable mechanosensitive cation channel, is ubiquitously expressed in the nervous system, smooth muscle tissues, kidneys, and immune cells.44 TRPC6 is involved in the regulation of a multitude of biological processes, such as smooth muscle contraction,45 glomerular filter integrity,46 and mechanical hyperalgesia.47 Table 1 shows the role of the TRPC6 ion channel in models of chronic pain. The TRP ion channel family has recently gained attention because of its ability to modulate intracellular Ca2+ concentrations and cellular electrical activity.48 Wang et al demonstrated that intrathecal larixyl acetate induces analgesic and anti-inflammatory action by suppressing TRPC6 and p38 signaling of the spinal cord following spared nerve injury.19 The TRPC6 channel also plays an essential role in chemotherapy-induced neuropathic pain and the diabetic neuropathic pain model.17,49 Our behavioral results showed that intraperitoneal injection of Dex effectively alleviated mechanical and thermal hyperalgesic reactions for 7 days. Moreover, the qRT-PCR, Western blot, and immunofluorescence results demonstrated that TRPC6 was partly suppressed by Dex. Therefore, Dex can decrease TRPC6 expression in the DRG, which may reflect the mechanism underlying the analgesic effect.
 |
Table 1 The Role of TRPC6 Ion Channel in Models of Chronic Pain |
Microglia are involved in the development and maintenance of neuropathic pain.8 Furthermore, the activation of microglia is associated with an increase in intracellular Ca2+ ion concentration.51–53 Ca2+ homeostasis plays a critical role in the neuroprotective effects and inhibition of microglial activation.54 Pro-brain-derived neurotrophic factor regulates Ca2+ ion concentration in microglia, which alleviates the inflammatory response in the brain.55 It has also been shown that TRPC6 is expressed in microglia and upregulated by LPS stimulation.19 In addition, microglia are present in the DRG as well as the central nervous system.56 Thus, we used Iba-1 to mark microglia. Notably, we found significant microglia proliferation around the neurons in the CCI group. Peripheral immune cells, including macrophages, astrocytes, and microglia, contribute to neuropathic pain. However, in the DRG, Iba-1 was not used as an exclusive marker of microglia. Therefore, our findings demonstrated that the proliferation of peripheral immune cells was inhibited by Dex. Further investigations are warranted to understand the specific cell type that has the potential to relieve pain by Dex administration.
Neuropathic pain is associated with neuroinflammation. Pro-inflammatory cytokines, such as TNF-α and IL1-β, play an essential role in inflammation. Moreover, the p38 MAPK pathway has been shown to be involved in the neuroinflammatory response.57 The level of phosphorylated p38 has been linked to microglia activation and the development and maintenance of chronic pain.31,58 Several studies have found that both sanguinarine and acupuncture attenuate neuropathic pain by inhibiting the p38 MAPK pathway.59,60 Furthermore, TRPC6 may be implicated in inflammation via the p38 MAPK pathway.61,62 In our study, we suggest that the analgesic effect of Dex was related to the inhibition of p38 phosphorylation and proinflammatory cytokines.
Our study has several limitations worth mentioning. First, to reveal the underlying mechanisms of Dex, we used a single dose based on previous literature. The appropriate Dex dose to prevent neuropathic pain will be evaluated in future studies. Second, the mechanisms underlying the influence of Dex on TRPC6 have not yet been clarified. We speculate that α2-adrenergic receptor activation affects Ca2+ influx and microglial proliferation via the TRPC6 channel. We plan to use whole-cell patch-clamp techniques to detect the opening rate of the TRPC6 channel. Furthermore, because the DRG is composed of various types of neurons, determining the neuronal type that is primarily affected is important. Sex differences also need to be considered. Overall, this field of research is still in the nascent stages, and further studies are needed.
In conclusion, our study suggested that Dex alleviates mechanical allodynia and thermal hyperalgesia in the CCI rat model by inhibiting the expression of TRPC6 in the DRG, which is associated with reduced p38 phosphorylation. Moreover, Dex administration attenuated the inflammatory response and proliferation of peripheral immune cells. Additional research is required to better understand the mechanism underlying neuropathic pain.
Acknowledgments
We thank the Medical Research Center of Qingdao University for providing the experimental platform. We thank Profs Huirong Han and Kerui Gong for their advice and support. Figure 1 was created using BioRender.com (Publication License: FC2479NMCQ). We also thank the Charlesworth Group (http://charlesworth-group.com) and Home for Researchers (www.home-for-researchers.com) for editorial assistance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (81873729) and the Youth Research Fund of Affiliated Hospital of Qingdao University (awarded to YZ).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Jensen TS, Baron R, Haanpää M, et al. A new definition of neuropathic pain. Pain. 2011;152(10):2204–2205. doi:10.1016/j.pain.2011.06.017
2. Dzung H, De Leon-Casasola OA. Neuropathic pain: an updated grading system for research and clinical practice. Essence Analg Analg. 2010;157(8):30–37. doi:10.1017/CBO9780511841378.007
3. Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014;13(9):924–935. doi:10.1016/S1474-4422(14)70102-4
4. Mulvey MR, Bennett MI, Liwowsky I, Freynhagen R. The role of screening tools in diagnosing neuropathic pain. Pain Manag. 2014;4(3):233–243. doi:10.2217/pmt.14.8
5. Hargus NJ, Patel MK. Voltage-gated Na+ channels in neuropathic pain. Expert Opin Investig Drugs. 2007;16(5):635–646. doi:10.1517/13543784.16.5.635
6. Smith PA. K+ channels in primary afferents and their role in nerve injury-induced pain. Front Cell Neurosci. 2020;14. doi10.3389/fncel.2020.566418
7. Gong N, Park J, Luo ZD. Injury-induced maladaptation and dysregulation of calcium channel α 2 δ subunit proteins and its contribution to neuropathic pain development. Br J Pharmacol. 2018;175(12):2231–2243. doi:10.1111/bph.13930
8. Inoue K, Tsuda M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci. 2018;19(3):138–152. doi:10.1038/nrn.2018.2
9. Abraira VE, Ginty DD. The sensory neurons of touch. Neuron. 2013;79(4):618–639. doi:10.1016/j.neuron.2013.07.051
10. Julius D. TRP channels and pain. Ann Rev Cell Dev Biol. 2013;29:355–384. doi:10.1146/annurev-cellbio-101011-155833
11. Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi:10.1146/annurev.biochem.75.103004.142819
12. Clapham DE. TRP channels as cellular sensors. Nature. 2003;426(6966):517–524. doi:10.1038/nature02196
13. Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12(3). doi:10.1186/gb-2011-12-3-218
14. De Caro C, Russo R, Avagliano C, et al. Antinociceptive effect of two novel transient receptor potential melastatin 8 antagonists in acute and chronic pain models in rat. Br J Pharmacol. 2018;175(10):1691–1706. doi:10.1111/bph.14177
15. Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397(6716):259–263. doi:10.1038/16711
16. Greka A, Mundel P. Balancing calcium signals through TRPC5 and TRPC6 in podocytes. J Am Soc Nephrol. 2011;22(11):1969–1980. doi:10.1681/ASN.2011040370
17. Miao B, Yin Y, Mao G, et al. The implication of transient receptor potential canonical 6 in BDNF-induced mechanical allodynia in rat model of diabetic neuropathic pain. Life Sci. 2021;273:119308. doi:10.1016/j.lfs.2021.119308
18. Alessandri-Haber N, Dina OA, Chen X, Levine JD. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J Neurosci. 2009;29(19):6217–6228. doi:10.1523/JNEUROSCI.0893-09.2009
19. Wang J, Zhao M, Jia P, et al. The analgesic action of larixyl acetate, a potent TRPC6 inhibitor, in rat neuropathic pain model induced by spared nerve injury. J Neuroinflammation. 2020;17(1):1–20. doi:10.1186/s12974-020-01767-8
20. Zhao Y, He J, Yu N, Jia C, Wang S. Mechanisms of dexmedetomidine in neuropathic pain. Front Neurosci. 2020;14:1–11. doi:10.3389/fnins.2020.00330
21. Mannelli L, Micheli L, Crocetti L, Giovannoni M, Vergelli C, Ghelardini C. α 2 adrenoceptor: a target for neuropathic pain treatment. Mini Rev Med Chem. 2016;17(2):95–107. doi:10.2174/1389557516666160609065535
22. Helander EM, Menard BL, Harmon CM, et al. Multimodal analgesia, current concepts, and acute pain considerations. Curr Pain Headache Rep. 2017;21(1). doi:10.1007/s11916-017-0607-y
23. Lin JP, Chen CQ, Huang LE, et al. Dexmedetomidine attenuates neuropathic pain by inhibiting P2X7R Expression and erk phosphorylation in Rats. Exp Neurobiol. 2018;27(4):267–276. doi:10.5607/en.2018.27.4.267
24. Zhao E, Bai L, Li S, et al. Dexmedetomidine alleviates CCI-induced neuropathic pain via inhibiting HMGB1-mediated astrocyte activation and the TLR4/NF-κB signaling pathway in rats. Neurotox Res. 2020;38(3):723–732. doi:10.1007/s12640-020-00245-6
25. Lee BM, Jang Y, Park G, et al. Dexmedetomidine modulates transient receptor potential vanilloid subtype 1. Biochem Biophys Res Commun. 2020;522(4):832–837. doi:10.1016/j.bbrc.2019.11.146
26. Akpinar H, Naziroǧlu M, Övey IS, Çiǧ B, Akpinar O. The neuroprotective action of dexmedetomidine on apoptosis, calcium entry and oxidative stress in cerebral ischemia-induced rats: contribution of TRPM2 and TRPV1 channels. Sci Rep. 2016;6:1–13. doi:10.1038/srep37196
27. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces d … [Pain. 1988] - PubMed result. Pain. 1988;33:87–107. doi:10.1016/0304-3959(88)90209-6
28. Jaggi AS, Jain V, Singh N. Animal models of neuropathic pain. Fundam Clin Pharmacol. 2011;25(1):1–28. doi:10.1111/j.1472-8206.2009.00801.x
29. Liu Y, Wang S, Wang Z, et al. Dexmedetomidine alleviated endoplasmic reticulum stress via inducing ER-phagy in the spinal cord of neuropathic pain model. Front Neurosci. 2020;14:1–13. doi:10.3389/fnins.2020.00090
30. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi:10.1016/0165-0270(94)90144-9
31. Lin X, Wang M, Zhang J, Xu R. p38 MAPK: a potential target of chronic pain. Curr Med Chem. 2014;21(38):4405–4418. doi:10.2174/0929867321666140915143040
32. Beydoun A. Neuropathic pain: from mechanisms to treatment strategies. J Pain Symptom Manage. 2003;25(5 Suppl):1–114. doi:10.1016/S0885-3924(03)00063-0
33. De Gregorio D, McLaughlin RJ, Posa L, et al. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain. 2019;160.(1):136–150. doi:10.1097/j.pain.0000000000001386
34. Descalzi G, Mitsi V, Purushothaman I, et al. Neuropathic pain promotes adaptive changes in gene expression in brain networks involved in stress and depression. Sci Signal. 2017;10:471. doi:10.1126/scisignal.aaj1549
35. Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52(1):77–92. doi:10.1016/j.neuron.2006.09.021
36. Wang GQ, Cen C, Li C, et al. Deactivation of excitatory neurons in the prelimbic cortex via Cdk5 promotes pain sensation and anxiety. Nat Commun. 2015;6. doi:10.1038/ncomms8660
37. Foster E, Wildner H, Tudeau L, et al. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron. 2015;85(6):1289–1304. doi:10.1016/j.neuron.2015.02.028
38. Ji R, Chamessian A, Zhang Y. Role in chronic pain maintenance (2). For exam- ple, fibromyalgia, a widespread chronic pain syn-. Pain Res. 2016;354(6312):572–577.
39. Yu X, Liu H, Hamel KA, et al. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun. 2020;11(1):1–12. doi:10.1038/s41467-019-13839-2
40. Guan Z, Kuhn JA, Wang X, et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci. 2015;19(1):94–101. doi:10.1038/nn.4189
41. Ma X, Du W, Wang W, et al. Persistent Rheb-induced mTORC1 activation in spinal cord neurons induces hypersensitivity in neuropathic pain. Cell Death Dis. 2020;11(9). doi:10.1038/s41419-020-02966-0
42. Yam MF, Loh YC, Tan CS, Adam SK, Manan NA, Basir R. General pathways of pain sensation and the major neurotransmitters involved in pain regulation. Int J Mol Sci. 2018;19(8):2164. doi:10.3390/ijms19082164
43. Marwaha L, Bansal Y, Singh R, Saroj P, Bhandari R, Kuhad A. TRP channels: potential drug target for neuropathic pain. Inflammopharmacology. 2016;24(6):305–317. doi:10.1007/s10787-016-0288-x
44. Dietrich A, Gudermann T. Trpc6. Handb Exp Pharmacol. 2007;179:125–141. doi:10.1007/978-3-540-34891-7_7
45. Tsvilovskyy VV, Zholos AV, Aberle T, et al. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology. 2009;137(4):1415–1424. doi:10.1053/j.gastro.2009.06.046
46. Kim JH, Xie J, Hwang KH, et al. Klotho may ameliorate proteinuria by targeting TRPC6 channels in podocytes. J Am Soc Nephrol. 2017;28(1):140–151. doi:10.1681/ASN.2015080888
47. Jin H, Sun Y-T, Guo G-Q, et al. Spinal TRPC6 channels contributes to morphine-induced antinociceptive tolerance and hyperalgesia in rats. Neurosci Lett. 2017;639:138–145. doi:10.1016/j.neulet.2016.12.062
48. Pozsgai G, Bátai IZ, Pintér E. Effects of sulfide and polysulfides transmitted by direct or signal transduction-mediated activation of TRPA1 channels. Br J Pharmacol. 2019;176(4):628–645. doi:10.1111/bph.14514
49. Khan R, Patay Z, Klimo P, et al. Ce pt us cr ip t Ac ce pt us cr. J Gerontol Ser a Biol Sci Med Sci. 2018;0813:1–11.
50. Roa-Coria JE, Pineda-Farias JB, Barragán-Iglesias P, et al. Possible involvement of peripheral TRP channels in the hydrogen sulfide-induced hyperalgesia in diabetic rats 11 medical and health sciences 1109 neurosciences. BMC Neurosci. 2019;20(1):1–17. doi:10.1186/s12868-018-0483-3
51. Gilbert DF, Stebbing MJ, Kuenzel K, et al. Store-operated Ca2+ entry (SOCE) and purinergic receptor-mediated Ca2+ homeostasis in murine bv2 microglia cells: early cellular responses to ATP-mediated microglia activation. Front Mol Neurosci. 2016;9:1–15. doi:10.3389/fnmol.2016.00111
52. Thangthaeng N, Poulose SM, Fisher DR, Shukitt-Hale B. Walnut extract modulates activation of microglia through alteration in intracellular calcium concentration. Nutr Res. 2018;49:88–95. doi:10.1016/j.nutres.2017.10.016
53. Dolga AM, Letsche T, Gold M, et al. Activation of KCNN3/SK3/KCa2.3 channels attenuates enhanced calcium influx and inflammatory cytokine production in activated microglia. Glia. 2012;60(12):2050–2064. doi:10.1002/glia.22419
54. Dolga AM, Culmsee C. Protective roles for potassium SK/Kca2 channels in microglia and neurons. Front Pharmacol. 2012;3:1–9. doi:10.3389/fphar.2012.00196
55. Mizoguchi Y, Ohgidani M, Haraguchi Y, Murakawa-Hirachi T, Kato TA, Monji A. ProBDNF induces sustained elevation of intracellular Ca2+ possibly mediated by TRPM7 channels in rodent microglial cells. Glia. 2021;69(7):1694–1708. doi:10.1002/glia.23996
56. Tang W, Li B, Chen S, et al. Increased GSK-3β expression in DRG microglia in response to sciatic nerve crush. Acta Biochim Biophys Sin. 2016;48(6):581–583. doi:10.1093/abbs/gmw027
57. Cai M, Zhuang W, Lv E, et al. Kaemperfol alleviates pyroptosis and microglia-mediated neuroinflammation in Parkinson’s disease via inhibiting p38MAPK/NF-κB signaling pathway. Neurochem Int. 2022;152:105221. doi:10.1016/j.neuint.2021.105221
58. Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron. 2018;100(6):1292–1311. doi:10.1016/j.neuron.2018.11.009
59. Yu C, Li P, Wang YX, Zhang KG, Zheng ZC, Liang LS. Sanguinarine attenuates neuropathic pain by inhibiting P38 MAPK activated neuroinflammation in rat model. Drug Des Devel Ther. 2020;14:4725–4733. doi:10.2147/DDDT.S276424
60. Choi DC, Lee JY, Lim EJ, Baik HH, Oh TH, Yune TY. Inhibition of ROS-induced p38MAPK and ERK activation in microglia by acupuncture relieves neuropathic pain after spinal cord injury in rats. Exp Neurol. 2012;236(2):268–282. doi:10.1016/j.expneurol.2012.05.014
61. Zhou LF, Chen QZ, Yang CT, et al. TRPC6 contributes to LPS-induced inflammation through ERK1/2 and p38 pathways in bronchial epithelial cells. Am J Physiol. 2018;314(3):C278–C288. doi:10.1152/ajpcell.00117.2017
62. Chen Q, Zhou Y, Zhou L, et al. TRPC6-dependent Ca2+ signaling mediates airway inflammation in response to oxidative stress via ERK pathway. Cell Death Dis. 2020;11(3). doi:10.1038/s41419-020-2360-0
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.