Back to Journals » Journal of Asthma and Allergy » Volume 15
Management of Uncontrolled Asthma: A Framework for Novel and Legacy Biologic Treatments
Authors Tan LD , Nguyen N, Alismail A , Castro M
Received 13 April 2022
Accepted for publication 15 June 2022
Published 29 June 2022 Volume 2022:15 Pages 875—883
DOI https://doi.org/10.2147/JAA.S369836
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Luis Garcia-Marcos
Laren D Tan,1,2 Nolan Nguyen,3 Abdullah Alismail,2 Mario Castro4
1Department of Internal Medicine, School of Medicine, Loma Linda University Health, Loma Linda, CA, USA; 2Department of Cardiopulmonary Sciences, School of Allied Health Professions, Loma Linda, CA, USA; 3Department of Biological Sciences, University of California, San Diego, CA, USA; 4Department of Medicine, University of Kansas School of Medicine, Kansas City, KS, USA
Correspondence: Laren D Tan, Email [email protected]
Abstract: Asthma continues to be a complex respiratory disease to control for many despite optimal standard inhaler therapy. The increased dependence on steroid-sparing biologic treatments in the 21st century has created a dilemma between identifying the patient’s intrinsic biomarkers and their “life markers.” With Tezepelumab being the most recent FDA-approved biologic for asthma, it is even more critical for asthma specialists to better understand and establish a framework to determine which biologic would work best for their patients. While cost and payor approvals limit access to certain asthma biologics, medical decisions on which biologic to select should be centered around shared decision-making, the rationale for biologic initiation, and critical biologic education to help achieve successful asthma control.
Keywords: severe asthma, Tezepelumab, asthma biologic, shared decision making, asthma
Introduction
Asthma is a heterogeneous disease with varying degrees of airway inflammation and airflow limitation that affects 1–18% of the population in different countries.1,2 Of those with asthma, refractory disease can range from 5% to 10%. Unfortunately, this group of refractory asthmatics also disproportionately contributes significantly to the overall economic and healthcare burden on the total cost of asthma.3 To address this disparity and provide best practice evidence-based recommendations and guidelines, NAEPP (National Asthma Education and Prevention Program) and GINA (Global Initiative for Asthma) were formed.2,4,5 With the help of NAEPP and GINA, most asthmatics are well controlled with conventional pharmacotherapy of inhaled corticosteroids combined with long or short-acting B-agonist bronchodilators (LABA or SABA) or long-acting muscarinic antagonists with the occasional addition of leukotriene antagonists (LTRAs). However, for those with severe refractory disease, these current modalities are not sufficient to control their asthma. Gaps in medical knowledge, deficiencies in the identification of severe asthma, barriers to clinical restraints in coordination, integration, resources, and access to bronchial thermoplasty (BT) as well as advanced treatments in legacy and novel asthma biologics (ie, Tezepulumab)6 could all be contributing factors as to why control of severe asthmatics continues to be problematic. Therefore, a framework for understanding, discussing, and determining the best treatment options is critical, especially for biologics. While there are many new biologics in the pipeline for asthma, the same framework should hold true for other novel biologics as they become available. In addition to the framework, in this paper, we will review the prospects for treating severe refractory asthmatics with a novel biologic agent (ie, Tezepelumab).
Understanding Phenotyping and Endotyping in Asthma
Asthma historically was thought to manifest as two major phenotypes, non-atopic or “non-allergic” asthma, and atopic or “allergic” asthma. Most prevalent was early-onset atopic asthma which was typically seen during childhood and into young adulthood. Evidence has shown that non-atopic asthma predominates among older age groups.7 Additional asthma phenotypes were also identified based on age of onset, asthma triggers, disease severity, exacerbations, and airflow limitation.8 A significant limitation with this approach arose because distinguishing groups based on observation was complex, overlap exists between clinical phenotypes and it does not address the underlying pathobiology.
While the historical idiom of “what you see is what you get” is great to help identify the variable clinical presentations of asthmatics (phenotype), it still falls short of defining the specific mechanistic pathway (endotype) that leads to the phenotypic presentation. Understanding these mechanistic pathways is critical to asthma management due to their therapeutic and prognostic implications.9
Endotyping in asthma was once deep-rooted in understanding that CD4+ T-cell responses are heterogenous and promote many inflammatory pathways. More importantly, within the subsets of T-helper1 (Th1) and T-helper2 (Th2) subpopulations, it was once accepted that Th2 cells were the principal driver of airway inflammation by generating interleukins (IL), more specifically IL-4, IL-5, and IL-13.10
It is now better understood as Type 2 inflammation since various cell types can also produce the characteristic and key cytokines (IL-4, IL-5, IL-13), which are often produced by the adaptive immune system on recognizing allergens.2
In addition to Th2-driven airway inflammation, group 2 innate lymphoid cells (ILC2) have also been found to play a critical role in type 2 immune responses that do not participate in the classic allergen-specific activation. ICL2 also produces vast amounts of prototypic type 2 cytokines, IL-5 and IL-13, which are found to be widespread within lung tissue and are mediated by alarmins. ILC2 mediated inflammation occurs when airway epithelial cells release alarmins in response to stressors (ie, infection, noxious irritation) or inflammation. ILC2 appears to play an early and critical role in augmenting asthma’s type 2 (T2) responses. This mechanistic pathway highlights the intimate connection between the adaptive and innate immunity.2,10
The body’s first line of defense in asthma or innate immune pathway is the airway epithelium, which is complex and dynamically orchestrates the immune responses in T2 asthma. Previous studies have shown that asthmatic airways have dysregulated airway epithelial barriers.11,12 Dysfunction in barrier integrity caused by damage from various inhaled noxious stimuli results in access to stromal tissue by allergens and microbes, which is integral to asthma pathogenesis. Damaged airway epithelium also releases innate immune cytokines known as alarmins (TSLP, IL-25, IL-33). These airway epithelium cytokines initiate multiple T2 pathways in response to allergen and infection-driven inflammation. IL-33 and IL-25 are known to mainly activate ILC2s. Thymic stromal lipoproteins (TSLP), on the other hand, have been shown to promote antigen-presenting cells (APCs) that lead to the activation of T cells and B cells.13
In addition to the more commonly mentioned T2 cytokines, IL-25, IL33 and TSLP should also be recognized in the pathogenesis of inflammatory airway diseases. Elevated bronchial mucosal expression of TSLP could be found in some subsets of asthma and COPD, both heterogeneous conditions with the overlapping features, with increased expression correlating with severity.13 Understanding asthma phenotype and endotype is essential as asthma biologic treatments continue to grow even though commercially available endotype testing is not yet readily available. Therefore, it is foundational for asthma specialists to comprehend the inflammatory pathophysiology and the pathway targeted by each asthma biologic (see Table 1).
 |
Table 1 Available Asthma Biologic Therapies |
Why is Shared Decision-Making Critical for All Asthmatic Patients? First, Identify the “Life Markers” of All Asthmatics
Shared decision-making (SDM) is important in the treatment plan for all asthmatics (ie, understanding asthma, inhaler technique, and adherence to treatment plan). This is especially crucial since the severity of the disease is defined by the level of treatment required to maintain asthma control and depends on the patient’s understanding and adherence to therapy.2,14 In a survey of 300 patients who attended an allergy clinic, 53% indicated they had searched online for allergy information before their consultation.15 Asthmatic patients are becoming more aware of their therapy regimens. In the era of personalized medicine, patient-centered asthma care should not be implemented without going beyond the phenotypic biomarkers and exploring a patient’s needs, values and preferences.16 The combination of the aforementioned has been previously published as “life-markers” and should be addressed with all asthmatics early and throughout their asthma journey.17
When and Why Should the Discussions About Biologics Occur, and What Should the Biologic Discussion Entail?
While the majority of asthmatics can be managed with inhalers that contain ICS with LABA, long-acting anti-muscarinic (LAMA) and SABA.2,4 Some continue to be “difficult to control” despite optimal standard therapy and may benefit significantly from the treatment of biologic agents that target IgE, IL-4 receptor, IL-5, IL-5 receptor, and most recently, TSLP.18,19 Existing biologic therapies have been found to have a greater response among those with eosinophilic asthma (ie, blood eosinophil count of ≥300 cells/mL).20,21 Unfortunately, approximately 50% of patients with severe asthma are non-eosinophilic and some patients with eosinophilic asthma respond suboptimally to standard therapy and the available biologic therapies or lose control of their asthma after an initial response.18,22 Because of these reasons, discussions with patients on biologic options should occur very early on when first establishing care with asthma specialists. Then, it should continue throughout their lifelong asthma journey, especially if their symptoms are not well uncontrolled.
Asthmatic Patients Should Understand the Rationale for Biologics
Since the 1950s, oral corticosteroids (OCS) have been the preferred treatment for acute asthma exacerbations.23 Previous publications have demonstrated the effectiveness of OCS for treating asthma exacerbations, reducing relapses, lowering short‐acting beta2‐agonist (SABA) use, and decreasing hospital admissions.24 There are also overwhelming data that show repeated and continued OCS use is a major cause of serious drug‐related adverse effects (AE), contributes to significant morbidity and substantially increases healthcare costs.25–28
Patients who cannot achieve asthma control despite being on maximal ICS combination therapy and LTRAs often experience multiple asthma exacerbations requiring continued or repeated courses of OCS.29 Over the past decade, an improved understanding of the complex pathophysiology of asthma has led to the development of new treatment options for asthma. Today, patients with uncontrolled severe asthma are routinely considered for steroid-sparing treatments such as biologic therapies as well as bronchial thermoplasty.30,31
It is also important to highlight that the majority of the randomized controlled trials on biologics in patients with uncontrolled severe asthma have demonstrated a significant response to placebo with reductions in exacerbations, improvement in lung function, and improvement in patient-reported outcomes. It suggests that some asthmatics are not intrinsically severe but often poorly controlled and would benefit from the following: Improving affordability, availability, and accessibility to ICS combination therapy, as well as emphasizing the principles of asthma management, such as shared decision-making, encouraging adherence, good inhaler technique, and allergen avoidance, are sufficient to control symptoms and prevent asthma exacerbations.32
Critical Biologic Administration Information for the Asthma Specialist and Patient
Understanding Types of Injectors (Prefilled vs Autoinjector)
A prefilled syringe is a single dose disposable syringe that contains medication to which a needle has been fixed by the manufacturer. Autoinjector syringes are similar to prefilled syringes but are designed to help self-administration with the assistance of a spring-loaded syringe. With the advent of 6 asthma biologics, preference for injector type can vary among the available options. It is difficult to conclude which injection type is superior, but there is growing evidence to support that patients prefer autoinjector syringes, in addition to minimal errors occurring when using autoinjectors33–35 (See Table 2).
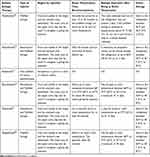 |
Table 2 Critical Biologic Administration Information |
Injection Site
Injection sites for asthma biologics can vary (typically in the arms, abdomen, and thighs). Still, it ultimately depends on the patients’ preference for injection and the route of administration (ie, intravenous, subcutaneous). These are all critical points for education and could also assist in achieving better compliance and tolerance.36 Asthma specialists should also be familiar with the various approved regions for subcutaneous biologic injections (see Table 2). Arm injections can be challenging to perform without the help of a family member, especially in rheumatologic/arthritic or neurologic conditions (ie, tremor). These conditions cause impediment to choosing a self-administration option and will need to be considered in the overall choice of an asthma biologic. This is a key “life marker” point that is often missed without exploring if someone is available to assist the patient with arm injections.
Biologic Temperature Considerations and Expiration Countdown
Additional critical information that asthma specialists and patients should be aware of are the varying recommended time for asthma biologic medications to warm up before administration, maximum storage temperature after being taken out of the refrigerator, and how long a biologic medication can be stored at room temperature. Air conditioning may be scarce in high-temperature regions with low socioeconomic status, and during hot months room temperatures may exceed asthma biologics’ recommended maximum room temperature.37 While it is not clear on the effectiveness of a biologic that has exceeded the manufacturer’s recommended room temperature (see Table 2), this may result in suboptimal biologic responders. Another knowledge gap of high importance is the manufacturer time for how long a biologic can be stored at room temperature before injection. This time frame can range from 2 to 30 days. Finally, with the growing number of approved asthma biologics, asthma specialists and patients will need to focus more on when a biologic medication will expire after being taken out of the refrigerator. Although, the majority of patients should be instructed to keep their biologic medication in the fridge until it is time for their injection, there will likely be some patients that will take their biologic medication on trips, may have prematurely taken their biologic medications out of the refrigerator or forgot to administer their biologic medication. Asthma specialists and clinic staff will also likely receive questions from patients seeking advice on whether their biologic medication is effective after so many days of being outside the refrigerator. Patients may inadvertently inject biologic medications that have gone past the recommended time after being thawed to room temperature. This will be especially problematic for patients during biologic switches. The importance of proper inhaler technique and adherence is still a priority in asthma care, but so should biologic knowledge, technique, and adherence. Without asthma specialists knowing these nuances which will likely be complicated further due to payor and coverage on the route of biologic administration, it will be much more difficult for patients to understand and adhere to proper biologic techniques.
Novel Asthma Biologic: Tezepelumab
Mode of Action of Tezepelumab
Thymic stromal lymphopoietin (TSLP) is a commonly overexpressed cytokine in asthmatics responsible for the inflammatory response seen in asthma. Predominant in lung epithelial cells, TSLP exerts its biological effects through binding high-affinity heteromeric receptor complexes of TSLPR and IL-7 receptor ⍺. Once expressed, TSLP induces the release of cytokines and chemokines, activation of functional T-helper type 2 cells, and eosinophils, mediating a broad range of immunological pathologies pertaining to asthma. As TSLP has been shown to play a relevant role in mediating the asthma response, Tezepelumab was developed as a human monoclonal antibody anti-TSLP that advantageously binds to TSLP. Upon the binding of Tezepelumab to TSLP, Tezepelumab, while not necessarily resulting in a conformational change of TSLP, does result in the blockage of the required binding site relevant to asthma pathologies. This high-affinity binding of Tezepelumab to TSLP occurs via its variable heavy-chain domain. Consequently, it inhibits the formation of the TSLP receptor complex necessary for the pathological responses prevalent in asthmatics. More specifically, Tezepelumab actively inhibits the dendritic cell maturation as well as chemokine production responsible for inflammatory cell trafficking into tissue.38
Tezepelumab Clinical Trials
As Tezepelumab becomes more prevalent as a potential biologic therapy to combat severe uncontrolled asthma, there have been various Phase 2 and 3 trials to evaluate the safety and efficacy of Tezepelumab in patients. First, the PATHWAY study was established as a phase 2b trial to analyze patient-reported outcomes after administration of a subcutaneous dosage of Tezepelumab. Patient-reported outcomes (PROs) were assessed through reports of asthma control from the Asthma Control Questionnaire-6, health-related quality of life from the Asthma Quality of Life Questionnaire (standardized) for patients aged 12 years or older, as well as daily asthma-related health issues from the Asthma Daily Diary questionnaire. An analysis of responses from PROs, the PATHWAY study indicates that treatment with Tezepelumab reduced the exacerbation median time to first well-controlled or partially controlled asthma and improved daily asthma-related health issues compared to baseline. However, due to the nature of this study, the PROs may not be reflective of the true treatment benefit.39
In response, a pivotal Phase 3 trial was designed to investigate further and build on the observations gained from the previous studies. This study, NAVIGATOR, is a phase 3 trial with the primary objective of assessing Tezepelumab's effect on asthma exacerbations via the annualized asthma exacerbation rate (AAER). Additionally, the effects on PROs, type 2 inflammatory biomarkers, asthma control, and patient health status were assessed. Overall, the NAVIGATOR study demonstrated that administration of Tezepelumab resulted in a significantly reduced annualized rate of asthma exacerbations as well as significant improvements in forced expiratory volume in one second, PROs, and reductions in exacerbations resulting in hospitalization.19 Thus, confirming the findings established in the PATHWAY study.39 In further examinations, Tezepelumab shows promise in reducing blood eosinophil count and levels of FENO and IgE, indicating inhibition of the inflammatory response and suppression of TSLP. There were no significant reported difference in the frequency or type of adverse events compared to the placebo groups in both the NAVIGATOR and PATHWAY studies. With regard to the safety profile of Tezepelumab, there were no reports of treatment-related anaphylactic reactions or development of neutralizing antibodies.19,40,41
SOURCE is yet another phase 3 trial analyzing the effects of Tezepelumab in asthma patients. However, this trial focuses on using oral corticosteroids (OCS) to treat severe asthma and asthma exacerbations and how Tezepelumab may lead to OCS dose reduction, and determining Tezepelumab’s efficacy in reducing asthma exacerbations via AAER. An analysis of data found that although the use of Tezepelumab in treatment for patients diagnosed with OCS-dependent asthma did reduce AAER, it did not result in a significant reduction in OCS dosage compared to placebo.42 Additionally, one other Phase 4 extension trial (initially started as a phase 3 trial prior to Tezepelumab FDA approval), DESTINATION, aims to examine the long-term effect and safety of Tezepelumab. With the primary criteria of having completed the NAVIGATOR and SOURCE studies, patients enrolled in DESTINATION will continue to receive administration of Tezepelumab over an additional one-year period. The long-term effects of Tezepelumab will be assessed via adverse events (AEs), AEs of particular interest, and serious AEs. Subjects previously randomized in one of the predecessor studies to Tezepelumab will be assigned and remain on Tezepelumab dosing in the DESTINATION study. Subjects randomized to the placebo arm in the predecessor studies will be re-randomized in a 1:1 ratio to either Tezepelumab or placebo. Given the randomization scheme of subjects in the predecessor studies, this will result in an overall subject distribution of 3:1 (Tezepelumab: placebo), assuming a similar number of subjects rollover from each arm in the predecessor studies. An additional extended follow-up period will be assessed following treatment cessation to investigate any improvements in lung function and changes in blood eosinophil count, FENO levels, and IgE. At this time, there are no results available for DESTINATION.43
Where Does Tezepulumab Fit in the Biologic Treatments for Asthma?
Prior to the approval of Tezepelumab, various asthma biologics have been used to downregulate the pathological effects of asthma. These have included, but are not limited to, anti-IgE, anti-IL5, anti-IL4, and anti-IL13 biologics. On the other hand, Tezepelumab directly binds and inhibits upstream TSLP, thus preventing the induction of numerous downstream targets. In reviewing the National Asthma Education and Prevention Program (NAEPP) step guidelines for asthma management, asthma biologics should be considered in a treatment plan during steps 5 and 6 for individuals ages 12 and older, with discussions about biologics occurring much sooner. In addition to NAEPP, Global Initiative for Asthma (GINA) step guidelines for asthma management and prevention, it is noted that anti-IgE, anti-IL5, anti-IL4, and anti-IL13 are recommended to be added to the treatment plan during step 5, which would also apply for Tezepelumab.1,2,5
Conclusion
Patients who suffer from asthma typically respond well to standard inhaler therapy and do not need asthma biologics to control their asthma. Unfortunately, some asthmatics continue to suffer despite being adherent to their inhaler regimen that should be considered for biologic therapy. Informing and educating patients about biologic treatments early in their asthma journey is essential and can help identify “life markers” that could be barriers to specific biologic therapy. In recognizing patients who have severe uncontrolled asthma with luminal obstruction and asthma severity predominantly mediated by eosinophils, anti–IL-5 mAbs are the therapy of choice. In moderate-to-severe asthmatic patients with severity driven by mucus production, eosinophils, and smooth muscle contraction and remodeling, an anti–IL-4R mAb may be the therapy of choice. Severe asthmatic patients who are clearly atopic driven by elevated IgE are candidates for anti-IgE therapy; however, anti–IL-5 mAbs have also been effective in these patients as well and have beneficial effects on lung function.32 In severe asthmatics that have luminal obstruction with non-eosinophilic or T2-low disease, anti-TSLP mAb should be considered as a therapy of choice.40 The early identification of biomarkers, “life-markers,” and biologic discussions will help facilitate an earlier initiation of biologics in hopes of altering the disease progression of asthma. In addition to the aforementioned, the success of biologics in controlling asthma will depend not only on adherence to inhaler therapy and biologic therapy but also on asthma specialists and patients having an in-depth understanding of proper biologic administration and the nuances of each biologic (see Table 2).
Disclosure
Dr Mario Castro reports grants from NIH, grants from ALA, grants from PCORI, grants, personal fees from AstraZeneca, grants, personal fees from GSK, grants, personal fees from Novartis, grants from Pulmatrix, grants, personal fees from Sanofi-Aventis, grants from Shionogi, personal fees from Elsevier, personal fees from Genentech, personal fees from Teva, personal fees from Amgen, personal fees from Regeneron, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Tan LD, Alismail A, Ariue B. Asthma guidelines: comparison of the national heart, lung, and blood institute expert panel report 4 with global initiative for asthma 2021. Curr Opin Pulm Med. 2022;28(3):234–244. doi:10.1097/MCP.0000000000000867
2. Reddel HK, Bacharier LB, Bateman ED, et al. Global Initiative for Asthma Strategy 2021: executive summary and rationale for key changes. Eur Respir J. 2021;59(1):2102730.
3. Yaghoubi M, Adibi A, Safari A, FitzGerald JM, Sadatsafavi M. The projected economic and health burden of uncontrolled asthma in the United States. Am J Respir Crit Care Med. 2019;200(9):1102–1112. doi:10.1164/rccm.201901-0016OC
4. Chipps BE, Murphy KR, Oppenheimer J. 2020 NAEPP guidelines update and GINA 2021-asthma care differences, overlap, and challenges. J Allergy Clin Immunol Pract. 2022;10(1S):S19–S30. doi:10.1016/j.jaip.2021.10.032
5. Cloutier MM, Baptist AP, Blake KV; Expert Panel Working Group of the National Heart L, Blood Institute a, coordinated National Asthma E, et al. 2020 focused updates to the asthma management guidelines: a report from the national asthma education and prevention program coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146(6):1217–1270. doi:10.1016/j.jaci.2020.10.003
6. Tan LD, Bratt JM, Godor D, Louie S, Kenyon NJ. Benralizumab: a unique IL-5 inhibitor for severe asthma. J Asthma Allergy. 2016;9:71–81. doi:10.2147/JAA.S78049
7. Settipane GA, Greisner WA
8. Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113(1):101–108. doi:10.1016/j.jaci.2003.10.041
9. Kuruvilla ME, Lee FE, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56(2):219–233. doi:10.1007/s12016-018-8712-1
10. Wenzel SE, Schwartz LB, Langmack EL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160(3):1001–1008. doi:10.1164/ajrccm.160.3.9812110
11. Heijink IH, Kies PM, Kauffman HF, Postma DS, van Oosterhout AJ, Vellenga E. Down-regulation of E-cadherin in human bronchial epithelial cells leads to epidermal growth factor receptor-dependent Th2 cell-promoting activity. J Immunol. 2007;178(12):7678–7685. doi:10.4049/jimmunol.178.12.7678
12. Sweerus K, Lachowicz-Scroggins M, Gordon E, et al. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J Allergy Clin Immunol. 2017;139(1):72–81 e71. doi:10.1016/j.jaci.2016.02.035
13. Hong H, Liao S, Chen F, Yang Q, Wang DY. Role of IL-25, IL-33, and TSLP in triggering united airway diseases toward type 2 inflammation. Allergy. 2020;75(11):2794–2804. doi:10.1111/all.14526
14. Boulet LP, FitzGerald JM, Reddel HK. The revised 2014 GINA strategy report: opportunities for change. Curr Opin Pulm Med. 2015;21(1):1–7. doi:10.1097/MCP.0000000000000125
15. Carpio-Escalona LV, Gonzalez-de-Olano D. Use of the Internet by patients attending allergy clinics and its potential as a tool that better meets patients’ needs. J Allergy Clin Immunol Pract. 2018;6(3):1064–1066. doi:10.1016/j.jaip.2017.10.034
16. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US); 2001. PMID: 25057539.
17. Tan L, Chupp G, Castro M, Kraft M. Going beyond “Bio-markers,” Think “Life-markers”. Chest. 2020;157(3):503–505. doi:10.1016/j.chest.2019.08.2210
18. Reibman J, Tan L, Ambrose C, et al. Clinical and economic burden of severe asthma among US patients treated with biologic therapies. Ann Allergy Asthma Immunol. 2021;127(3):318–325 e312. doi:10.1016/j.anai.2021.03.015
19. Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384(19):1800–1809. doi:10.1056/NEJMoa2034975
20. Busse W, Spector S, Rosen K, Wang Y, Alpan O. High eosinophil count: a potential biomarker for assessing successful omalizumab treatment effects. J Allergy Clin Immunol. 2013;132(2):485–486 e411. doi:10.1016/j.jaci.2013.02.032
21. Hammad H, Lambrecht BN. The basic immunology of asthma. Cell. 2021;184(6):1469–1485. doi:10.1016/j.cell.2021.02.016
22. Patel SS, Casale TB, Cardet JC. Biological therapies for eosinophilic asthma. Expert Opin Biol Ther. 2018;18(7):747–754. doi:10.1080/14712598.2018.1492540
23. Christie L, Scadding J, Boyd J. CONTROLLED trial of effects of cortisone acetate in status asthmaticus; report to the medical research council by the subcommittee on clinical trials in asthma. Lancet. 1956;271(6947):803–806.
24. Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2001;1:CD000195. doi:10.1002/14651858.CD000195
25. Sweeney J, Patterson CC, Menzies-Gow A, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the optimum patient care research database and the British thoracic difficult asthma registry. Thorax. 2016;71(4):339–346. doi:10.1136/thoraxjnl-2015-207630
26. Dalal AA, Duh MS, Gozalo L, et al. Dose-response relationship between long-term systemic corticosteroid use and related complications in patients with severe asthma. J Manag Care Spec Pharm. 2016;22(7):833–847. doi:10.18553/jmcp.2016.22.7.833
27. Price DB, Trudo F, Voorham J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204. doi:10.2147/JAA.S176026
28. Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018;141(1):110–116 e117. doi:10.1016/j.jaci.2017.04.009
29. Chung LP, Upham JW, Bardin PG, Hew M. Rational oral corticosteroid use in adult severe asthma: a narrative review. Respirology. 2020;25(2):161–172. doi:10.1111/resp.13730
30. Carr TF, Kraft M. Management of severe asthma before referral to the severe asthma specialist. J Allergy Clin Immunol Pract. 2017;5(4):877–886. doi:10.1016/j.jaip.2017.04.027
31. Tan LD, Yoneda KY, Louie S, Hogarth DK, Castro M. Bronchial thermoplasty: a decade of experience: state of the art. J Allergy Clin Immunol Pract. 2019;7(1):71–80. doi:10.1016/j.jaip.2018.08.017
32. McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433–445. doi:10.1164/rccm.201810-1944CI
33. Bernstein D, Pavord ID, Chapman KR, et al. Usability of mepolizumab single-use prefilled autoinjector for patient self-administration. J Asthma. 2020;57(9):987–998. doi:10.1080/02770903.2019.1630641
34. Vermeire S, D’Heygere F, Nakad A, et al. Preference for a prefilled syringe or an auto-injection device for delivering golimumab in patients with moderate-to-severe ulcerative colitis: a randomized crossover study. Patient Prefer Adherence. 2018;12:1193–1202. doi:10.2147/PPA.S154181
35. Zheng Y, Abuqayyas L, Megally A, et al. Tezepelumab pharmacokinetics, safety, and tolerability after administration via vial-and-syringe, accessorized prefilled syringe, or autoinjector: a randomized trial in healthy volunteers. Clin Ther. 2021;43(1):142–155 e145. doi:10.1016/j.clinthera.2020.11.014
36. Jin JF, Zhu LL, Chen M, et al. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer Adherence. 2015;9:923–942. doi:10.2147/PPA.S87271
37. WHO Housing and Health Guidelines. Geneva: World Health Organization; 2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535293/
38. Marone G, Spadaro G, Braile M, et al. Tezepelumab: a novel biological therapy for the treatment of severe uncontrolled asthma. Expert Opin Investig Drugs. 2019;28(11):931–940. doi:10.1080/13543784.2019.1672657
39. Corren J, Garcia Gil E, Griffiths JM, et al. Tezepelumab improves patient-reported outcomes in patients with severe, uncontrolled asthma in PATHWAY. Ann Allergy Asthma Immunol. 2021;126(2):187–193. doi:10.1016/j.anai.2020.10.008
40. Menzies-Gow A, Colice G, Griffiths JM, et al. NAVIGATOR: a phase 3 multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the efficacy and safety of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir Res. 2020;21(1):266. doi:10.1186/s12931-020-01526-6
41. Hoy SM. Tezepelumab: first approval. Drugs. 2022;82(4):461–468. doi:10.1007/s40265-022-01679-2
42. Wechsler ME, Colice G, Griffiths JM, et al. SOURCE: a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel group trial to evaluate the efficacy and safety of tezepelumab in reducing oral corticosteroid use in adults with oral corticosteroid dependent asthma. Respir Res. 2020;21(1):264. doi:10.1186/s12931-020-01503-z
43. Menzies-Gow A, Ponnarambil S, Downie J, Bowen K, Hellqvist A, Colice G. DESTINATION: a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the long-term safety and tolerability of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir Res. 2020;21(1):279. doi:10.1186/s12931-020-01541-7
44. NUCALA (mepolizumab) - HIGHLIGHTS OF PRESCRIBING INFORMATION. Food and Drug Administration. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/103976s5225lbl.pdf. Accessed June 27, 2022.
45. XOLAIR® (omalizumab) - HIGHLIGHTS OF PRESCRIBING INFORMATION. Food and Drug Administration. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761122s000lbl.pdf. Accessed June 27, 2022.
46. CINQAIR® (reslizumab) - HIGHLIGHTS OF PRESCRIBING INFORMATION. Food and Drug Administration. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761033lbl.pdf. Accessed June 27, 2022.
47. Fasenra (benralizumab) - HIGHLIGHTS OF PRESCRIBING INFORMATION. Food and Drug Administration. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761070Orig1s000TOC.cfm. Accessed June 27, 2022.
48. DUPIXENT® (dupilumab) - HIGHLIGHTS OF PRESCRIBING INFORMATION. Food and Drug Administration. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761055s014lbl.pdf. Accessed June 27, 2022.
49. TEZSPIRE™ (tezepelumab-ekko) - HIGHLIGHTS OF PRESCRIBING INFORMATION. Food and Drug Administration. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761224s000lbl.pdf. Accessed June 27, 2022.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
