Back to Journals » Journal of Pain Research » Volume 17
Management of Post-Dural Puncture Headaches in Pediatric Patients with Epidural Blood or Saline Patch: An Educational Focused Review
Authors Elhamrawy A, Syed A, Smith T, Veneziano G , Tobias JD
Received 30 October 2023
Accepted for publication 15 March 2024
Published 19 March 2024 Volume 2024:17 Pages 1197—1207
DOI https://doi.org/10.2147/JPR.S444381
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Andrea Tinnirello
Amr Elhamrawy,1 Ahsan Syed,1,2 Timothy Smith,1,2 Giorgio Veneziano,1,2 Joseph D Tobias1,2
1Department of Anesthesiology & Pain Medicine, Nationwide Children’s Hospital, Columbus, OH, USA; 2Department of Anesthesiology & Pain Medicine, The Ohio State University College of Medicine, Columbus, OH, USA
Correspondence: Amr Elhamrawy, Department of Anesthesiology & Pain Medicine, Nationwide Children’s Hospital, 700 Children’s Drive, Columbus, OH, 43205, USA, Tel +1 614-722-4200, FAX +1 614-722-4203, Email [email protected]
Abstract: Post-dural puncture headache (PDPH) is a common adverse outcome following puncture of the dura. It can occur after inadvertent dural puncture during epidural catheter placement or following diagnostic or therapeutic LP. The incidence of PDPH in pediatric patients has been estimated at 1– 15% depending on patient factors (age, gender, body mass index) and needle factors (size and needle bevel/point type). The larger the needle gauge, the higher the incidence of PDPH. Various options have been proposed to treat PDPH including observation, bed rest, hydration, caffeine, and epidural blood/saline patch. The current manuscript provides a review of the use of epidural blood/saline patch in pediatric-aged patients with PDPH.
Keywords: headache, epidural blood patch, epidural saline patch, dural puncture, epidural catheter
Introduction
Post-dural puncture headache (PDPH) is a common adverse outcome following puncture of the dura. It occurs after accidental dural puncture during epidural catheter placement or less commonly, following diagnostic or therapeutic LP. According to the International Headache Society (IHS) definition, PDPH is a headache attributed to low cerebrospinal fluid (CSF) pressure occurring within 5 days of a lumbar puncture caused by CSF leakage through the dural puncture. Headache is usually accompanied by neck stiffness and/or subjective hearing symptoms, remitting spontaneously within 2 weeks or after sealing of the leak with an epidural patch.1 The incidence of PDPH in the pediatric population has been estimated at 1–15% depending on patient factors (age, gender, body mass index) and needle factors (size and needle bevel/point type). The larger the needle gauge size used, the higher the incidence of PDPH. Although the incidence is approximately 4% for 25–27-gauge needles, the incidence increases to 15% with a 22-gauge needle.2 As the pediatric population has a lower compliance of epidural space with relatively low cerebrospinal fluid (CSF) pressure, the probability of a PDPH is much lower in younger children, less than 12 years of age. Female gender and low body mass index (BMI) are risk factors to develop PDPH.
PDPH generally manifests at 3 days up to 2 weeks following lumbar LP with clinical signs and symptoms of fronto-occipital non-throbbing postural headache, as it improves when lying flat and worsens in the upright position.3 The influence of positional changes is one of the most important diagnostic criteria for PDPH. Other symptoms include nausea, vomiting, hearing impairment, photophobia, low back pain, and neck stiffness. Rarely, there is a paradoxical response to positional changes with headache worsening when supine and improving when upright position and while sitting upright.4 The primary pathophysiology of PDPH is persistent CSF leakage through the dural puncture site, which leads to a reduction in both CSF volume and intracranial pressure (ICP). Therefore, as the patient stands up, the reduction of ICP allows the brain to shift in a caudal direction, resulting in stretching of pain sensitive fibers of the dura as well as dilatation of cerebral blood vessels leading to headache. One of the important differential diagnoses is meningitis or meningo-encephalitis from infectious agents (bacterial, viral, or fungal). Headache (diffuse or localized), fever, mental status changes, meningeal signs (eg, nuchal rigidity), photophobia, vomiting, positive Kernig’s and Brudzinski’s signs may be suggestive of an infectious process. In these cases, meningitis should be confirmed by CSF analysis.
Etiology of PDPH
As the optimal way to manage PDPH is the prevention, it should be appreciated that various factors have been reported to impact its incidence. Patient-related, needle-related, and procedure-related factors may impact the incidence of PDPH. Patient-related factors that increase the incidence of PDPH include older age (>10 years), female gender (2–3 times higher incidence), higher BMI, higher opening pressure, and a history of prior PDPH or chronic headache disorders (pseudotumor cerebri). Procedural related factors focus primarily on the needle type and its trajectory through the dural fibers.5 The incidence of PDPH is lower with a smaller, pencil point needle such as a Whitacre or Sprotte needle which has a closed tip shaped like that of the end of a pencil with a hole on the side of the needle near the tip. These needles are different from a Quincke cut needle where the hole is at the end of the needle. The Whitacre and Sprotte needle may reduce the incidence of PDPH by theoretically splitting or separating instead of cutting dural fibers (high level of certainty). The incidence is also lower with needle placement using a steeper angle while orienting the bevel parallel to the longitudinal neural axis so that the dural fibers are separated and not cut. Replacement of the stylet before withdrawing the non-cutting needle may also be beneficial. Additional procedure-related factors include the number of attempts and the level of experience of the provider (moderate level of certainty). There remains conflicting evidence regarding the impact of the volume of CSF removed, the position of the patient during the procedure (sitting versus lateral), the use of saline or air for loss of resistance during epidural catheter insertion, preoperative fluid administration and hydration, and duration of bed rest after dural puncture. However, regardless of the technique, type of needle, and associated risk factors, PDPH may still occur, especially in situations where repeated LPs, which may be necessary such as oncological patients who need monitoring for potential central nervous system (CNS) involvement or intrathecal chemotherapy.3,6–8
Treatment of PDPH
Management of PDPH is essential as if left untreated, it may result in persistent backache, chronic headache, cranial nerve dysfunction, subdural hematoma, or cortical vein and cerebral venous sinus thrombosis.9 The first step in the treatment of PDPH includes conservative modalities, as it is generally a self-limiting complication. Conservative management strategies include bed rest, supine positioning, oral or intravenous (IV) fluid administration to avoid dehydration, caffeine, and oral non-opioid analgesic agents as acetaminophen or non-steroid anti-inflammatory drugs (NSAIDs).10 Although bed rest and supine positioning decrease the intensity of PDPH, there is limited evidence-based medicine to demonstrate its efficacy in decreasing the actual duration or PDPH or increasing the chances of resolution.11 The same is true for bed rest following LP and its impact on the incidence of PDPH. Similarly, there is a lack of evidence-based medicine to demonstrate the efficacy of hydration on shortening the duration or decreasing the incidence of PDPH.
Since 1949, different doses of methylxanthine derivatives or caffeine have been used for the treatment of PDPH. Caffeine has 2 postulated mechanisms of action in treating PDPH as it is an adenosine antagonist and thereby causes cerebral vasoconstriction that leads to a reduction in cerebral blood flow (CBF) and brain blood volume. Additionally, it stimulates the sodium-potassium pump and increases CSF production.12 Both oral and IV caffeine sodium benzoate formulations may result in a transient reduction in pain scores. Oral caffeine is well absorbed, more convenient, and less expensive. Although generally safe, the adverse effect profile of caffeine (oral and IV) includes restlessness, palpitations, cardiac dysrhythmias, seizures, and irritation of gastrointestinal tract. Caffeine dosing is weight-based and dependent on the patient’s ability to tolerate oral medications. For patients that weigh more than 40 kg, dosing is 200 mg PO every 12 hours. For patients that weight 20 to 40 kg, the dose is 100 mg PO every 12 hours. If the patient is unable to take oral medications, IV caffeine sodium benzoate can be administered in a single dose of 10 mg/kg (maximum dose of 500 mg), repeat times one in 4 hours if needed.13–15 Spontaneous resolution or the therapeutic effects of the above-mentioned measures, including conservative management, result in headache resolution in up to 85% of cases.16
Epidural Blood Patch to Treat PDPH
If the above-mentioned measures fail to improve the symptoms within 2–3 days, epidural blood patch (EBP) may be considered. Although the ideal time to wait prior to EBP remains controversial, several retrospective studies reported an increased success rate when EPB is performed within 24–48 hours after dural puncture. In a cohort of 24 patients with inadvertent dural puncture with an 18-gauge Tuohy needle, Loeser et al reported failure of EBP in 71% of patients when EBP was placed within 24 hours of dural puncture versus only a 4% failure rate when the EBP was placed after 24 hours.17,18 In adults, the recommended volume to be used for an EBL is a maximum of 20 mL. However, less is used, and injection stopped if the patient complains of back pain, tightness or a paresthesia in the lower extremity. In pediatric population, placement of an EBP is generally performed in a sedated or anesthetized patient. Therefore, the recommended volume of blood is 0.2–0.3 mL/kg with the added recommendation to stop injection if there is increased resistance.19,20 Despite its therapeutic role in treating an established PDPH, there is no documented role of EBP as prophylaxis against PDPH. It appears that previous reports that were supportive of a prophylactic EBP (placed at the time of dural puncture prior to the onset of headache) were influenced by selective bias as well as a non-randomized observational study design.21
Placement of an EBP follows the same processes as lumbar puncture or epidural catheter placement. Following explanation to patient/family and informed consent, standard American Society of Anesthesiologists’ monitors are placed and a large bore intravenous cannula placed. As both the identification of the epidural space and obtaining autologous blood are to be performed using sterile technique, the anesthesia provider creates two sterile fields: one for drawing autologous blood and the other for identification of the epidural space. The sitting position may be feasible for adolescents who can tolerate the procedure without either aggravating his/her headache or the need for general anesthesia or deep sedation. Prone positioning is an option as well if fluoroscopic guidance will be used. Once sedation or general anesthesia has been administered, the epidural space is identified using the loss of resistance technique, the needle is left in place, and autologous blood is drawing using sterile technique from an available vein (Figure 1). Venipuncture and phlebotomy can be performed by an assistant or second anesthesia provider to facilitate the procedure. As the injected blood spreads more cephalad than caudad, the epidural needle is inserted at the lowest interspace that was entered or one interspace below the lowest puncture site if there were multiple needle insertion sites during the previous procedure. Generous use of local anesthesia is recommended given the frequent presence of paravertebral muscle spasm and sensitized spinal segments from the prior procedure. The epidural procedure will be initiated using loss of resistance to saline in order to identify the epidural space. Once the procedure is completed, the patient lies supine with minimal movement for at least two hours in order to give time for clot formation at the desired level. Symptoms specifically headache should improve within minutes of procedure; however, in complex or resistant cases, the EBP may need to be repeated, but only if some improvement in symptoms is noted with the first EBP. If the first EBP has no effect and the procedural technique was sound, other etiologies of the headache must be considered, perhaps in consultation with other specialists such as pain physicians or neurologists. Additionally, neuroimaging may be needed to investigate other etiologies or confirm intracranial hypotension. Imaging at the level of the LP may be needed to identify the level of the dural tear, the presence of single or multiple tears, and to perform targeted or high volume EBP.22,23
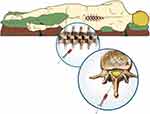 |
Figure 1 Demonstration of technique of epidural blood patch with placement of autologous blood into the epidural space. |
Epidural Blood Patch: History, Mechanism of Action, Contraindications, and Adverse Effects
In 1960, Dr. James Gormley, a general surgeon in Pennsylvania, was the first to report preliminary experience with the injection of small volumes (2–3 mL) of autologous blood in the epidural space to treat PDPH.24 The theory behind the potential efficacy of EBL was based on the observation that a bloody tap is associated with a decreased incidence of PDPH. He believed that replacement of intra-arachnoid fluid volume would result only in temporary relief of headache. However, to achieve permanent relief, he postulated that an EBL should be placed after restoration of CSF volume. Within 4 days of the original LP for diagnostic purpose of placement of spinal anesthesia, a Tuohy needle was advanced through the same intervertebral level and 15 mL of sterile 0.9% saline was injected to theoretically restore intra-arachnoid fluid. The Tuohy needle was then withdrawn into the epidural space and 2 mL of autologous blood injected. Patients were kept recumbent and intravenous hydration provided (1 liter of fluid). Headache relief occurred in 7 patients including the author himself, who had an LP myelographic examination of a herniated intervertebral disc. Despite the successes reported, this novel therapeutic intervention was not widely applied. In 1970, Dr. Anthony DiGiovanni, an anesthesiologist, adopted and refined the concept of EBP, reporting the injection of 10 mL of autologous blood into the epidural space.25 Forty-five patients were treated with resolution of the headache in 41 patients. An additional 5 patients received a prophylactic EBP following lumbar puncture with none of the 5 developing a headache.
The primary goal of EBP is to seal the puncture site, allow the re-accumulation of CSF, restoration of ICP, and reversal of cerebral vasodilatation. There are two hypotheses postulated to explain the mechanism of action of an EBP. The first is the “pressure patch” hypothesis in which blood spreads in both directions preferably cranially over several segments within the epidural space. This blood compresses the thecal sac, increasing both epidural and subarachnoid (ICP) pressure. The increase in ICP, epidural, and subarachnoid pressure reduces the caudad movement of the brain when the patient assumes the upright position and hence relieves traction on pain sensitive fibers of the dura, thereby relieving the headache.26 The other hypothesis is the “plug” theory which postulates that the injected blood in the epidural space coagulates, forming a gelatinous plug, sealing the dural hole and preventing further CSF leakage into the epidural space. Regeneration of CSF restores CSF pressure and alleviates the headache, in the absence of continued loss.27
There are a limited number of absolute contraindications to EBP including coagulation disturbances, local infection at the insertion site, anatomic abnormalities, and sepsis.28 The success rate of EBP has been reported to be as high as 70–98%. The procedure can be repeated if the first attempt fails to relieve the symptoms. Approximately 30% of patients require a second EBP, following which 50% experience complete relief, 36–38% experience partial relief, and 12–14% experience no relief.29 Adverse effects related to EBP include neck stiffness, vertigo, tinnitus, and transient paresthesia in the lower extremities during injection.16,30
Aside from blood, various other fluids have been injected into the epidural space of when the use of autologous blood is refused, contraindicated, or has failed. This has included saline, hydroxyethyl starch (dextran), fibrin glue, and dexamethasone.31–35 In 1949, Flowers et al were the first to report treatment of PDPH by the epidural injection of saline.31 Subsequently, McCord et al reported their experience with the injection of normal saline (70–100 mL) into the epidural space to treat PDPH in a cohort of 15 obstetrical patients.32 A second injection of normal saline was offered to 3 of the 15 patients who experienced a recurrence of their symptom within 24–48 hours. All patients reported permanent relief of their headache. Mehl reported a novel maneuver to prevent the development of PDPH using the potential tamponading effect of epidural saline (10–15 mL).33 After the intrathecal injection of the local anesthetic agent through a 22-gauge spinal needle, the needle was withdrawn into the epidural saline and normal saline injected. Three patients of 100 (3%) in the study cohort developed a PDPH during the post-partum period. Two achieved immediate relief of symptoms after the injection of 40 mL of normal saline into epidural space through a caudal approach. The third patient was treated with epigastric pressure as well as small doses of both codeine and aspirin.
Dextran has also been injected into the epidural space to treat PDPH. With a higher molecular weight and viscosity than normal saline, it remains longer in the epidural space before it is absorbed, which may enhance healing of the dura. Barrios-Alarcon et al reported the successful treatment of PDPH in 56 adults following the epidural administration of dextran 40 (average volume of 20 mL) after failure of other conservative measures including bed rest, intravenous hydration, oral corticosteroids, opioid and non-narcotic analgesics as well as invasive measures (EBP or epidural saline patch).34 No recurrence of symptoms was observed. However, the most recent evidence does not support routine use of epidural dextran, gelatin, or hydroxyethyl starch to treat PDPH.1
Although off-label, anecdotal evidence has also suggested the potential utility of the epidural injection of fibrin sealant or glue to treat PDPH.35–38 Although anecdotal, success has generally been reported in these studies even when fibrin glue was used after failed EBP. Fibrin sealant is a common neurosurgical adjunct administered to enhance a watertight dural closure during open procedures as it has a high tensile strength. The fibrin plug theory hypothesizes that adhesion created at the dural tear promotes healing by stimulation of fibroblasts. It also has a direct tamponading effect, which provides an additional therapeutic role. To avoid the potential complications of inadvertent intrathecal injection including a chemical meningitis, epidural positioning of the needle before injection of the fibrin glue can be documented by contrast injection.
Although the epidural injection of corticosteroids is a well-established treatment modality for pain, its use in the prevention or treatment of PDPH remains controversial.39,40 Its postulated analgesic mechanisms relate to its local anti-inflammatory effects with suppression of the synthesis of inflammatory mediators. Various non-randomized and retrospective trials have suggested the utility of epidural corticosteroids in preventing PDPH.39–41 To date, there are no data regarding the use or potential efficacy of epidural corticosteroids in the prevention or treatment of PDPH in pediatric-aged patients.
In severe refractory headache, sphenopalatine ganglion block has been used successfully in both adult and pediatric-aged patients.42–44 Sphenopalatine ganglion blockade is easy to perform using a cotton tipped applicator and an intranasal approach, minimally invasive, and provide a safer alternate to an EPB. Bhettay A et al reported a 3-year-old boy, 16 kg with complicated Hirschsprung’s disease, who was scheduled for redo endorectal pull-through. The anesthetic plan was a combined general-epidural anesthesia technique. While performing placement of the thoracic epidural catheter placement (T11), unintentional dural puncture with backflow of CSF was encountered. The second attempt at T12 was successful. Postoperatively, the revised FLACC pain score was 1/10. At 28-hours following the dural puncture, his mother reported that the patient had a headache with pain score 8/10. Conservative treatment included supine position, IV fluids, and ibuprofen. After failure of conservative treatment, bilateral sphenopalatine ganglion blocks were placed. The patient’s headache resolved, and he remained comfortable. At his 2-week follow-up, his mother reported that his headache never returned.44
Epidural Blood for PDPH in Children and Adolescents
In addition to the reports in the adult population, both EBP and ESP have been used to control pediatric PDPH resistant to conservative measures (Tables 1–2).45–60 The first of these reports was published in 1984. Purtock RV et al reported a 9-year-old, 39 kg boy who developed severe bifrontal postural headache with nausea and vomiting after diagnostic lumbar puncture.40 Conservative treatment at home using oral propoxyphene napsylate and trimethobenzamide was unsuccessful. An EBP was performed at L4-5, under general anesthesia, using 5 mL of autologous blood. His headache was completely resolved after recovery from general anesthesia.
 |
Table 1 Case Reports of Successful First Trial Epidural Blood Patch for PDPH in Pediatric Patients |
 |
Table 2 Miscellaneous Reports of Repeat EBP, ESP, or Epidural Fibrin Glue |
The literature contains a total of 17 published reports regarding the use of an EBP in pediatric-aged patients.2,45–60 All of these reports are relatively anecdotal with 15 being single patient case reports and two being retrospective case series including 41 and 7 patients. The first and largest series to date was reported by Kokki et al, who evaluated the effectiveness of EPB in 41 pediatric-aged patients over a 10-year period at two different hospitals. They used standard diagnostic criteria for diagnosing PDPH including a headache, which develops within 5 days of an LP, worsens within 15 minutes after assuming an upright position, and improves within 15 minutes after resuming a recumbent position. The patient should also have at least one associated symptom such as neck stiffness, tinnitus, hypacusia, photophobia or nausea. Conservative treatment before EBP included analgesic agents, caffeine, and bed rest. Complete and persistent resolution of symptoms were noted after EBP were noticed in 35 of 41 patients. Although it did not reach statistical significance, 23 of 24 patients with an EBP using a volume ≥0.25 mL/kg reported complete and persistent resolution of the symptoms compared to 12 of 17 patients with less than 0.25 mL/kg of autologous blood for the EBP. There was no correlation with duration of bed rest after EBP and its efficacy. The authors also suggested the potential to use gelatin instead of autologous blood in a patient who was febrile to avoid the potential risk of spreading an infection or oncologic processes.
The remaining literature includes a total of 23 patients from 16 reports. The patients varied in age throughout the use of pediatric range from 2 to 17 years old. The etiology of the PDPF included diagnostic or therapeutic LP, inadvertent dural puncture during attempted epidural catheter placement, lumbar drain for CSF removal during sinus surgery, and postoperative CSF following a neurosurgical procedure. The size of the needle resulting in the dural puncture most commonly included a 22-gauge needle for LP or a 17–18-gauge epidural needle. In addition to headache (generally postural), signs and symptoms included nausea/vomiting, neck pain, neck stiffness, refusal to ambulate, and visual changes. In the majority of cases, radiologic imaging was not obtained and conservative treatment measures failed. Although not reported in all cases, the amount of autologous blood generally varied 0.15–0.5 mL/kg with the majority of cases using approximately 0.3 mL/kg. In the reported cases, the response was favorable with immediate resolution of headache and other symptoms. No recurrences or treatment failures were noted in these patients.
Aside from previous reports of successful primary EBP, there are additional anecdotal reports involving repeat EBP, ESP, and epidural fibrin glue (Table 2).61–65 These reports include a total of 7 patients from 4 case reports and one case series. Similar to the reports outlined in Table 1, the patients varied in age from 4 to 14 years old. The etiology of the PDPF included diagnostic LP, inadvertent dural puncture during epidural catheter for postoperative analgesia following a surgical procedure, spinal anesthesia for inguinal hernia repair, and lumbar drain placement for CSF decompression during and following transnasal endoscopic encephalocele repair. The size of the needle resulting in the PDPH varied from 14 to 25 gauge. The primary complaint was postural headache associated with nausea and vomiting. One of the single case reports discussed the efficacy of a repeat EBP with a larger volume of autologous blood (0.76 mL/kg versus 0.4 mL/kg) for treatment of PDPH.61 Two of the reports outlined the use of epidural saline.62,63 One reported successful headache resolution after a 48-hour continuous infusion of epidural saline when 4 previous EBPs had failed, while the other reports a single case of a primary ESP. The remaining two reports outlined presented anecdotal experience with the use of epidural fibrin glue when EBP failed to resolve PDPH in a total of 4 pediatric patients.64,65
Summary
EBP remains a time-honored therapy for PDPH when conservative treatment fails. It was first reported in adults in 1960. To date, all reports regarding the efficacy of EBP in both adult and pediatric patients remain retrospective, thereby limiting the availability of true evidence-based medicine. As these cases are retrospective, there is likely to be reporting bias with reports generally focusing on positive clinical outcomes. The reports of failed EBP from the literature are limited to those, which report the use of other treatment modalities including repeat EBP, ESP, and epidural fibrin glue. The failed cases of EBP are reported to show the potential benefit of other treatment modalities (ESP, epidural fibrin glue) when EBP fails.
Reports have included various etiologies of the PDPH with varying sizes of needles including diagnostic/therapeutic LP, LP for spinal anesthesia, inadvertent dural puncture during epidural catheter placement for surgical or postoperative care, lumbar CSF drain placement, or a surgical procedure with a postoperative CSF leak. The diagnosis is made on clinical grounds with limited need for imaging unless the diagnosis is in doubt. Clinical signs and symptoms including a postural headache with pain localized to frontal or occipital region. Other signs and symptoms may include nausea/vomiting, neck pain, neck stiffness, refusal to ambulate, and visual changes. EBP is generally recommended after failure of conservative measures with limited evidence-based medicine to suggest the efficacy of caffeine. The retrospective literature generally suggests improved outcomes when the EBP is placed within 24–48 hours of the onset of symptoms with no recommendations for prophylactic EPB. Resolution of symptoms, especially postural headache, is generally immediate. Recommendations for the amount of autologous blood to be used include 20 mL in adults and 0.2–0.3 mL/kg in pediatric-aged patients. Anecdotal evidence supports that success may be increased by the use of a larger volume of autologous blood (≥0.2 mL/kg). Following the procedure, the patient should remain recumbent for 12–24 hours. Although even more anecdotal, ESP or epidural fibrin glue may be considered if there is a contraindication to the use of autologous blood.
Acknowledgement
The authors would like to thank Nema Shaltoot for her assistance in creating the image for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Uppal V, Russell R, Sondekoppam R, et al. Consensus practice guidelines on postdural puncture headache from a multisociety, international working group: a summary report. JAMA Network Open. 2023;6(8):e2325387. doi:10.1001/jamanetworkopen.2023.25387
2. Kokki M, Sjövall S, Kokki H. Epidural blood patches are effective for postdural puncture headache in pediatrics--a 10-year experience. Paediatr Anaesth. 2012;22(12):1205–1210. doi:10.1111/pan.12034
3. Morgan KJ, Mohan R, Karol SE, Flerlage J. Epidural blood patch for post-dural puncture headaches in adult and paediatric patients with malignancies: a review. Br J Anaesth. 2021;126(6):1200–1207. doi:10.1016/j.bja.2020.11.041
4. Liu H, Kaye AD, Comarda N, Li M. Paradoxical postural cerebrospinal fluid leak-induced headache: report of two cases. J Clin Anesth. 2008;20(5):383–385. doi:10.1016/j.jclinane.2008.01.011
5. Buddeberg BS, Bandschapp O, Girard T. Post-dural puncture headache. Minerva Anestesiol. 2019;85(5):543–553. doi:10.23736/S0375-9393.18.13331-1
6. Strupp M, Brandt T, Muller A. Incidence of post‐lumbar puncture syndrome reduced by reinserting the stylet: a randomised prospective study of 600 patients. J Neurol. 1998;245(9):589–592. doi:10.1007/s004150050250
7. Ahmed SV, Jayawarna C, Jude E. Post lumbar puncture headache: diagnosis and management. Postgrad Med J. 2006;82(973):713–716. doi:10.1136/pgmj.2006.044792
8. Bandatmakur M, Bench C, Ngwa N, et al. Factors predisposing to post dural puncture headache in children. J Child Neurol. 2021;36(10):831–840. doi:10.1177/08830738211007699
9. Chordas C. Post–dural puncture headache and other complications after lumbar puncture. J Pediatric Oncology Nurs. 2001;18(6):244–259. doi:10.1053/jpon.2001.28454
10. Raiger LK, Naithani U, Gupta M, Pareek SK. Post dural puncture headache in children: a report of two cases. Anaesth Pain Intens Care. 2019;(16):67–70.
11. Thoennissen J, Herkner H, Lang W, Domanovits H, Laggner AN, Müllner M. Does bed rest after cervical or lumbar puncture prevent headache? A systematic review and meta-analysis. CMAJ. 2001;165(10):1311–1316.
12. Shahriari A, Nataj-Majd M, Khooshideh M, Salehi-Vaziri S. The comparison of post-dural puncture headache treatment with Acetaminophen-caffeine capsule and intravenous mannitol infusion: a randomized single-blind clinical trial. Curr J Neurol. 2021;20(2):95–101. doi:10.18502/cjn.v20i2.6745
13. Camann WR, Murray RS, Mushlin PS, Lambert DH. Effects of oral caffeine on postdural puncture headache. A double-blind, placebo-controlled trial. Anesth Analg. 1990;70(2):181–184. doi:10.1213/00000539-199002000-00009
14. Choi A, Laurito CE, Cunningham FE. Pharmacologic management of postdural puncture headache. Ann Pharmacother. 1996;30(7–8):831–839. doi:10.1177/106002809603000722
15. Janssens E, Aerssens P, Alliet P, Gillis P, Raes M. Post-dural puncture headaches in children. A literature review. Eur J Pediatr. 2003;162(3):117–121. doi:10.1007/s00431-002-1122-6
16. Turnbull DK, Shepherd DB. Post‐dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth. 2003;91(5):718–729. doi:10.1093/bja/aeg231
17. Safa-Tisseront V, Thormann F, Malassiné P, et al. Effectiveness of epidural blood patch in the management of post-dural puncture headache. Anesthesiology. 2001;95(2):334–339. doi:10.1097/00000542-200108000-00012
18. Loeser EA, Hill GE, Bennett GM, Sederberg JH. Time vs. success rate for epidural blood patch. Anesthesiology. 1978;49(2):147–148. doi:10.1097/00000542-197808000-00024
19. McHale J, O’Donovan FC. Postdural puncture symptoms in a child. Anaesthesia. 1997;52(7):688–690. doi:10.1111/j.1365-2044.1997.az0158b.x
20. Ylönen P, Kokki H. Epidural blood patch for management of postdural puncture headache in adolescents. Acta Anaesthesiol Scand. 2002;46(7):794–798. doi:10.1034/j.1399-6576.2002.460707.x
21. Vasdev GM, Southern PA. Postdural puncture headache: the role of prophylactic epidural blood patch. Curr Pain Headache Rep. 2001;5(3):281–283. doi:10.1007/s11916-001-0044-8
22. Zetlaoui PJ, Buchheit T, Benhamou D. Epidural blood patch: a narrative review. Anaesth Crit Care Pain Med. 2022;41(5):101138. doi:10.1016/j.accpm.2022.101138
23. Kawaguchi M, Hashizume K, Watanabe K, Inoue S, Furuya H. Fluoroscopically guided epidural blood patch in patients with postdural puncture headache after spinal and epidural anesthesia. J Anesth. 2011;25(3):450–453. doi:10.1007/s00540-011-1135-2
24. Gormley JB. Treatment of post spinal headache. Anesthesiology. 1960;21:565–566.
25. DiGiovanni AJ, Galbert MW, Wahle WM. Epidural injection of autologous blood for postlumbar-puncture headache. II. Additional clinical experiences and laboratory investigation. Anesth Analg. 1972;51(2):226–232.
26. Harrington BE. Postdural puncture headache and the development of the epidural blood patch. Reg Anesth Pain Med. 2004;29(2):136–163. doi:10.1097/00115550-200403000-00014
27. Usubiaga JE, Usubiaga LE, Brea LM, Goyena R. Effect of saline injections on epidural and subarachnoid space pressures and relation to postspinal anesthesia headache. Anesth Analg. 1967;46(3):293–296.
28. Duffy PJ, Crosby ET. The epidural blood patch. Resolving the controversies. Can J Anaesth. 1999;46(9):878–886. doi:10.1007/BF03012979
29. Shin HY. Recent update on epidural blood patch. Anesth Pain Med. 2022;17(1):12–23. doi:10.17085/apm.21113
30. Olsen KS. Epidural blood patch in the treatment of post-lumbar puncture headache. Pain. 1987;30(3):293–301. doi:10.1016/0304-3959(87)90017-0
31. Flowers CE, Hellman LM, Hingson RA. Continuous peridural anesthesia and analgesia for labor, delivery and cesarean section. Curr Res Anesth Analg. 1949;28(4):181–189. doi:10.1213/00000539-194901000-00048
32. McCord JM, Epperson JW, Jacoby JJ. Headache following spinal anesthesia in obstetrics. Curr Res Anesth Analg. 1951;30(6):354–357. doi:10.1213/00000539-195101000-00066
33. Mehl LB. Epidural injection of normal saline as a means of prevention of spinal headache. Am J Obstet Gynecol. 1954;68(4):1105–1108. doi:10.1016/S0002-9378(16)38406-X
34. Barrios-Alarcon J, Aldrete JA, Paragas-Tapia D. Relief of post-lumbar puncture headache with epidural dextran 40: a preliminary report. Reg Anesth. 1989;14(2):78–80.
35. Crul BJ, Gerritse BM, van Dongen RT, Schoonderwaldt HC. Epidural fibrin glue injection stops persistent postdural puncture headache. Anesthesiology. 1999;91(2):576–577. doi:10.1097/00000542-199908000-00039
36. Mammis A, Agarwal N, Mogilner AY. Alternative treatment of intracranial hypotension presenting as postdural puncture headaches using epidural fibrin glue patches: two case reports. Int J Neurosci. 2014;124(11):863–866. doi:10.3109/00207454.2014.880436
37. Wong K, Monroe BR. Successful treatment of postdural puncture headache using epidural fibrin glue patch after persistent failure of epidural blood patches. Pain Pract. 2017;17(7):956–960. doi:10.1111/papr.12541
38. Dupoiron D, Narang S, Seegers V, et al. Preventing post dural puncture headache after intrathecal drug delivery system implantation through preventive fibrin glue application: a retrospective study. Pain Physician. 2021;24(2):E211–20.
39. Gharibo C, Koo C, Chung J, Moroz A. Epidural steroid injections: an update on mechanisms of injury and safety. Reg Anesth Pain Med. 2009;13(4):266–271.
40. Najafi A, Emami S, Khajavi M, et al. Is epidural dexamethasone effective in preventing postdural puncture headache? Acta Anaesthesiol Taiwan. 2014;52(3):95–100. doi:10.1016/j.aat.2014.07.001
41. De Matteis C, Pisana G. Prevenzione della cefalea da rachi-anestesia con uso di cortisone epidurale [prevention of headache from spinal anesthesia with the use of epidural cortisone]. Minerva Anestesiol. 1991;57(4):137–140.
42. Li H, Wang Y, Oprea AD, Li J. Postdural puncture headache—risks and current treatment. Curr Pain Headache Rep. 2022;26(6):441–452. doi:10.1007/s11916-022-01041-x
43. Stalls C, Zatochill M, Petersen TR, et al. Transnasal sphenopalatine ganglion block for postdural puncture headache in an adolescent: a case report. AA Pract. 2019;13(5):185–187. doi:10.1213/XAA.0000000000001029
44. Bhettay A, Burger R. Sphenopalatine ganglion blocks for post-dural puncture headache: a case report in a 3-year-old child. Paediatr Anaesth. 2024;34(2):182–184. doi:10.1111/pan.14785
45. Purtock RV, Buhl JL, Abram SE. Epidural blood patch in a nine year old boy. Reg Anesth. 1984;9:154–155.
46. Robbins KB, Prentiss JE. Prolonged headache after lumbar puncture. Successful treatment with an epidural blood patch in a 12-year-old boy. Clin Pediatr. 1990;29(6):350–352. doi:10.1177/000992289002900614
47. Roy L, Vischoff D, Lavoie J. Epidural blood patch in a seven-year-old child. Can J Anaesth. 1995;42(7):621–624. doi:10.1007/BF03011882
48. Ylönen P, Kokki H. Management of postdural puncture headache with epidural blood patch in children. Paediatr Anaesth. 2002;12(6):526–529. doi:10.1046/j.1460-9592.2002.00863.x
49. Cassady JF, Lederhaas G, Turk WR, Shanks DE. Unusual presentation and treatment of postlumbar puncture headache in an 11-yr-old boy. Anesthesiology. 2000;92(6):1835–1837. doi:10.1097/00000542-200006000-00047
50. Liley A, Manoharan M, Upadhyay V. The management of a postdural puncture headache in a child. Paediatr Anaesth. 2003;13(6):534–537. doi:10.1046/j.1460-9592.2003.01116.x
51. Nafiu OO, Monterosso D, Walton SR, Bradin S. Post dural puncture headache in a pediatric patient with idiopathic intracranial hypertension. Paediatr Anaesth. 2005;15(9):778–781. doi:10.1111/j.1460-9592.2004.01529.x
52. Gök F, Apilioğulları S. Baş ağrılı çocukta epidural kan yaması ve epidural salin uygulaması: iki olgu sunumu. Türk Anestezi ve Reanimasyon Dergisi. 2009;37(5):324–327.
53. Haruna J, Yamanaka H, Tachibana K, et al. Epidural blood patch for postdural puncture headache in a five-year-old child. Masui. 2010;59(10):1273–1275.
54. Lee DH, Kim EJ. Management of postdural puncture headache with epidural blood patch in a child. Korean J Anesthesiol. 2011;61(4):344–345. doi:10.4097/kjae.2011.61.4.344
55. Hunyady AI, Anderson CT, Kuratani JD, Kundu A. Fever following an epidural blood patch in a child. Case Rep Anesthesiol. 2012;2012:753875. doi:10.1155/2012/753875
56. Franklin AD, Hays SR. Successful management of a thoracic cerebrospinal fluid cutaneous fistula in a two year old child using a thoracic epidural blood patch. J Clin Anesth. 2013;25(4):331–334. doi:10.1016/j.jclinane.2012.11.012
57. Cornman-Homonoff J, Schweitzer A, Chazen JL. CT-guided epidural blood patch for treatment of CSF leak and pseudomeningocele following tethered cord release in a 3-year-old. Clin Imaging. 2016;40(6):1191–1194. doi:10.1016/j.clinimag.2016.08.010
58. Heine CL, Furse CM. A safe method for performing an epidural blood patch in a pediatric patient requiring deep sedation for epidural catheter placement: a case report. AA Pract. 2019;13(9):356–357. doi:10.1213/XAA.0000000000001086
59. Al Wosaibai A, Alfaraj A, Alshabeb AK. Management of postdural puncture headache in pediatric using an epidural catheter for an epidural blood patch. Saudi J Anaesth. 2020;14(3):394–396. doi:10.4103/sja.SJA_779_19
60. Silva R, Oliveira M, Abreu F, Vaz MJ. Epidural blood patch for the treatment of liquor hypotension after intrathecal chemotherapy in a 10-year-old: case report. Braz J Anesthesiol. 2021;71(4):458–460. doi:10.1016/j.bjane.2021.02.024
61. Borges BC, Wong G, Isaac L, Hayes J. Unusual presentation of postdural puncture headache requiring repeat epidural blood patch in a 4-year-old child. Paediatr Anaesth. 2014;24(5):541–543. doi:10.1111/pan.12330
62. D’Souza G, Seidel FG, Krane EJ. Management of a ventral cerebrospinal fluid leak with a lumbar transforaminal epidural blood patch in a child with persistent postdural puncture headache: a case report. Reg Anesth Pain Med. 2017;42(2):263–266. doi:10.1097/AAP.0000000000000562
63. Kara I, Ciftci I, Apiliogullari S, Arun O, Duman A, Celik JB. Management of postdural puncture headache with epidural saline patch in a 10-year-old child after inguinal hernia repair: a case report. J Pediatr Surg. 2012;47(10):e55–7. doi:10.1016/j.jpedsurg.2012.07.055
64. Roberts JM, Peterson VE, Heran MKS. Percutaneous CT-guided epidural fibrin sealant injection for refractory pediatric post-dural puncture headache. Pediatr Radiol. 2020;50(8):1156–1158. doi:10.1007/s00247-020-04691-4
65. Armstrong SA, Nguyen HTN, Rebsamen SL, Iskandar B, Stadler JA. Epidural fibrin sealant injection for the management of cerebrospinal fluid leak following dural puncture in children. Cureus. 2020;12(2):e6940. doi:10.7759/cureus.6940
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
