Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Malnutrition-Modulated Diabetes Mellitus in Children, Rare Disease with Atypical Presentation: Case Report
Authors Haftu H , Gebrearegay H, Berhane A
Received 4 June 2020
Accepted for publication 3 August 2020
Published 25 August 2020 Volume 2020:13 Pages 3069—3074
DOI https://doi.org/10.2147/DMSO.S263229
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Hansa Haftu,1 Hailemariam Gebrearegay,2 Alemseged Berhane2
1Mekelle University, College of Health Science, Department of Pediatrics and Child Health, Mekelle, Ethiopia; 2Mekelle University, College of Health Science, Department of Pediatrics and Child Health, Tigray, Ethiopia
Correspondence: Hansa Haftu
Mekelle University, College of Health Science, Department of Pediatrics and Child Health, Mekelle, Ethiopia
Tel +251948487877
Email [email protected]
Aim:: Atypical diabetes is commonly reported in Africa. The objective of this case report is to highlight an unusual case of thin, severely hyperglycemic and ketone resistant teenager with history and signs of chronic under-nutrition to raise the awareness of clinicians on the existence of atypical phenotype of diabetes not fitting the current classification of diabetes.
Case Presentation: A 17-year-old male patient, known diabetic, was diagnosed in the health center as type1 diabetes for 8 months. He was on insulin for 3 months and discontinued for 5 months. He presented with polydipsia, polyuria, and weight loss since he discontinued the drug. On examination, he was severely wasted and underweight with unexplained bilateral parotid enlargement. On investigations;, he had hyperglycemic, glucosuria but no ketonuria. The patient was admitted with the diagnosis of type 1 diabetes and severe acute malnutrition. He started insulin (1IU/Kg/day) subcutaneously and nutritional management. On follow-up, RBS and FBS remained high and insulin was escalated to 3.32 IU/kg/day. Subsequently, serial RBS and FBS, the measurements were in the acceptable range and the patient was gaining weight. As the weight increases, his demand for insulin was decreased and the dose of insulin was de-escalated to 1.2 IU/Kg/day over 3 months. Finally, the patient was discharged with 1.2 IU/Kg/day with a weight of 44 kg over 4 months of hospital course with the diagnosis of malnutrition-modulated diabetes. Now, the patient is in diabetic clinic follow-up with good glycemic control.
Conclusion: Though there are unclear and uncertainties in malnutrition-modulated diabetes mellitus, clinicians need a high index of suspicion to reach the diagnosis especially in those countries where malnutrition is common. Early diagnosis and appropriate management of the patients demand are important in patient care and outcome.
Keywords: malnutrition, diabetes mellitus, children, insulin
Introduction
Diabetes Mellitus (DM) is a public health problem and is progressively increasing in developing countries, especially the atypical ones.1 In 1985, WHO classified diabetes into three; Insulin-dependent, insulin-independent, and malnutrition-related diabetes (MRDM). Later MRDM further classified into two called Fibrocalculous pancreatic DM and Protein deficient DM (which was later renamed to Malnutrition -modulated DM).2 MRDM was previously known as ‘tropical diabetes’ and patients present at a young age, resistant to ketosis in undernourished individuals with high subsequent insulin requirements.3 The implication of malnutrition as a possible factor in the genesis and atypical features of this form of diabetes was first explained by Kar and Tripathy (1963),4–6 Patients with MRDM characterized by a socioeconomic setting of poverty and undernutrition, young age (less than 30 years), clinical evidence of malnutrition, a high dose of insulin to control the hyperglycemia, and they are not prone to ketosis. However, patients with MMDM, they do not develop ketosis even after stopping insulin for prolonged periods.6,7 Protein-deficient pancreatic diabetes (PDPD) or protein-deficient diabetes mellitus (PDDM) has the same characteristics, but differs from FCPD in the absence of clinical and radiological evidence of pancreatic dysfunction and relative resistance to insulin.5 Most of the patients, typically, they are lean even before the onset of symptoms and appear poorly nourished. Patients with this syndrome commonly present with excessive eating, excessive drinking, excessive urination, and significant weight loss, fatigue, weakness, and abdominal cramp, often leads to prostration in course of time.8–10 Since 1985, the World Health Organization (WHO) incorporated the category of malnutrition-related diabetes mellitus (MRDM) in the classification of diabetes. WHO had set criteria for possible diagnosis of MRDM. These criteria’s were: Blood sugar >200 mg/dl, the onset of symptoms before the age of 30 years, BMI of <19 kg/m2, absence of ketosis on insulin withdrawal, poor socioeconomic status, history of malnutrition, and insulin requirements >2 IU/Kg/d.11 Despite the criteria proposed for its diagnosis, MRDM continues to be an elusive and controversial entity.11 Fifteen years ago, the well-known Kenyan dialectologist, described the atypical DM as it is “a syndrome seeking clarity”. But, this elusion of this disease is very much true today and remained unexplained.10 As there are limited data and reports on the frequency of MMDM, there are diagnostic challenges. We are going to present a patient with MMDM but initially diagnosed with type 1 DM. This is very much true in children and rarely considered and diagnosed which may affect the management and long term quality of life. So, this case report may increase awareness about MRDM and may create the fertile ground for the further triggering of another study to state clearly the relation of Malnutrition and DM.
Case Presentation
A 17-year-old male patient known diabetic, diagnosed in the health center as type1 diabetes for 8 months. He was on insulin for 3 months and discontinued the medication for 5 months because he cannot afford to buy insulin. He presented with polydipsia, polyuria, and weight loss of 5 months duration (Since he discontinued insulin). He had a complaint of intermittent abdominal pain and bloating since 3 years back, but he has no vomiting, diarrhea, shortness of breath, cough, and fever. He is an orphan (both parents died in his early childhood with unknown cause, and he was living with his relatives. On examination: He was chronically sick looking but normal vital signs. Anthropometry: Wt-25 kg, Ht-159 cm, BMI-10 kg/m2, WFH- severely wasted, WFA- severely underweight. On LGS-, bilateral parotid enlargement with no discharge, tenderness, and no color change of skin. The patient was conscious and oriented.
On investigation; he had hyperglycemia, glucosuria but no ketonuria. Otherwise, Complete blood count (CBC), renal function test, Liver enzymes, Lipid profile, Plain-x-ray of the abdomen, and abdominal U/S were normal (Table 1). The patient was admitted with the diagnosis of Type 1 Diabetes and Severe acute malnutrition. He started insulin (1 IU/Kg/day) SQ and nutritional management. On subsequent follow up of RBS (three times per day) and FBS (one times per day), both remained high and insulin was escalated to 3.5 IU/kg/day (Table 2). After the insulin dose was reached to 3.5 IU/kg/day, Serial RBS and FBS were in the acceptable range and the patient was gaining Weight progressively. As the weight increases, his demand for insulin was decreased and the dose of insulin was de-escalated to 1.2 IU/Kg/day over four months. The blood sugar (both RBS and FBS) remains within the acceptable ranges. Because of this atypical presentation, the findings of the investigation, different management (high insulin demand to control hyperglycemia), and his responses for the treatment, the patient was evaluated meticulously for other types of DM. Based on the diagnostic criteria suggested by Bajaj et al,5 for MRDM (Malnutrition -Modulated Diabetes previously called PDPD), our patient fulfilled the criteria (scored −11) and he was diagnosed after 6 weeks of hospital stay (Table 3). Finally, the patient was discharged with 1.2 IU/Kg/day with a weight of 44 kg over 4 months of total hospital course. On the subsequent follow-ups, the patient is taking 1.6 IU/kg/day with good glycemic control in the endocrinology clinic.
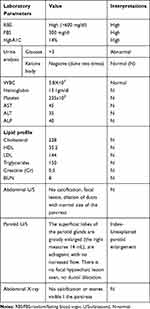 |
Table 1 Summary of Initial Investigations |
 |
Table 2 Serial RBS and FBS with Insulin Requirement During Hospital Stay |
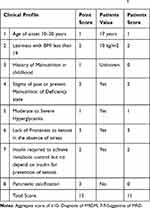 |
Table 3 Diagnostic Criteria: For the Diagnosis of MRDM, a Defined Set of Criteria Was Suggested by Bajaj et al5 |
Discussion and Conclusion
Diabetes mellitus (DM) is a a heterogeneous metabolic disorder characterized by long-standing hyperglycemia. Like our case, DM patients may present with characteristic symptoms such as, excessive thirst, excessive urination, excessive eating and sometimes blurring of vision due to hyperglycemia.12 Clinically, MRDM resembles typical insulin-dependent ketosis-prone type Ⅰ DM in acuteness and the age of the patient, severity of symptoms, unresponsiveness to sulphonylurea, and requirement of insulin to control metabolic status. But both (MRDM and Type-1) are different in two aspects; One, resistance to develop ketosis in typical young diabetes despite a prolonged period of insulin withdrawal, which can be explained by a better-preserved beta-cell function. Second, high insulin requirement which is considered as an indicator of insulin resistance.13 Malnutrition-related diabetes mellitus is characterized by young-onset diabetes, but not prone to ketosis in those patients with evidence of chronic malnutrition like stunting or bilateral parotid enlargement or recent evidence of undernutrition like the low BMI and emaciation with very high subsequent insulin requirements. There are reports of protein-energy malnutrition (PEM) causing persistent insulin deficiency as well as insulin resistance leading to glucose intolerance.10,12,14 Clinical criteria for the diagnosis of atypical DM (MRDM) were suggested by Ahuja and Tripathy. These include young age at onset of diabetes (<30 years), lean body mass (<BMI<19kg/m2), the absence of ketosis on withdrawal of insulin-requiring more than 60 IU/day or greater than 1.5 IU/Kg/day.13 Based on this diagnostic criteria, our patient fulfills, which includes; he is 17 years old with body mass index (BMI) <10 kg/m2, he did not develop ketosis despite he stopped insulin for five-month and his demand for insulin reaches 3.5 IU/kg/day. Our patient had longstanding painless bilateral parotid enlargement with low BMI, which goes with characteristics of the preceding or the recent presence of malnutrition.
In 1985, the World Health Organization (WHO) incorporated category of malnutrition-related diabetes mellitus (MRDM) in the classification of diabetes and set criteria for the diagnosis of MMDM.10,15 Based on The WHO criteria, our patient had all the criteria; his RBS was greater than 600 mg/dl, his age is17 years old, his BMI is 10 kg/m2, he did not develop ketosis despite the discontinuation of insulin for five-month and he needs insulin 3.5 IU/kg/day to control hyperglycemia. The clinical features of MMDM than FCPM are, fasting blood sugar greater than 200mg/dl, age at onset is less than 30 years, emaciated, BMI<18kg/m2, no ketosis when they stop insulin, poor socioeconomic status, history of childhood malnutrition, they need insulin more than 60IU/day (>1.5 to 2 IU/Kg/day), Of rural origin, Absence of radiographic or ultrasound findings of pancreatic stone, dilatation, and fibrosis; laboratory evidence of exocrine pancreatic dysfunction.5 From the subclass of MRDM, because of the symptom and sign, he is having, upon the investigations of Ultrasound and X-ray of the abdomen (he was not having any stones or typical changes of the pancreas for calculi) and the absences of the symptoms of exocrine insufficiency with a normal lipid profile, our patient is having PDDM. There was another diagnostic criterion of MMDM suggested by Bajaj et al.5 Based on this, our patient fulfilled the criteria (scored −11) and he was diagnosed after 6 weeks of hospital stay (Table 2). Typically patients with MMDM are wasted before the onset of symptoms and appear poorly nourished. The onset of symptoms and the progression of the disease are subacute, but some patient’s progress may be relatively fast. Common sign and symptoms of such patients are, excessive eating, excessive drinking, excessive urination, and wasted, fatigue, and cramps often lead to prostration in the course of time.8,9 Our patient had polyphagia, polydipsia, polyuria with emaciation, and abdominal pain. The preceding data gives a view of the pancreatic changes associated with protein-energy malnutrition. Macroscopically, there is atrophy of the pancreas during malnutrition which is rapidly reversed on recovery, as evidenced by an adequate insulin response in the latter stage.16 Patients with MRDM, their high insulin requirement tend to fall as weight stabilizes and blood glucose becomes more normal. Improvement in blood glucose and symptoms usually takes weeks to stabilize and an increase in weight is especially rapid in the first 3–4 weeks which is very common to gain 3 to 5 kg. One of the reasons for the fall in insulin requirements once they start management is the improvement of B-cell function with improved glycemic-metabolic status.11 This was very much true in our patient, as the weight progressively increases and stabilizes, his demand for insulin was decreased and glycemic control was good (Table 3). After the patient started insulin with the usual dose for his age per standard treatment for DM in children (1–1.2 IU/kg/day), glycemic control was very poor and insulin was escalated to 3.5 IU/kg/day. Our patient glycemic control was achieved at 3.5 IU/kg/day. And with all the managements (insulin and nutritional), our patient progressively gained weight. As he gains weight, his demand for insulin progressively declined, and finally, our patient is taking 1.6 IU/kg/day with good glycemic control. However, certain irreversible microscopic changes could have occurred which predisposed the patient to an increased echogenicity. The pathogenesis of beta1-cell damage in undernutrition is highlighted by a recent report.16 The most important thing in such kind of patients is, to achieve good glycemic control, most patients’ need a large amount of insulin which is generally beyond their body means. Most patients with MMDM, their demand for insulin may go up to 120–200 IU without any complications, and this shows the presence of insulin resistance.5 This was true for our patient, taking a high dose of insulin above 120 IU/day with no complications (no episodes of hypoglycemia).
Conclusion
Though there are unclear and uncertainties in Malnutrition-modulated Diabetes Mellitus, it is very rarely diagnosed and reported in children. Few studies in a young adult with the atypical DM in tropical areas remain unclear about MRDM and need further clarification. This case report demonstrates a Malnutrition-Modified Diabetes mellitus previously called protein-deficient diabetes mellitus (PDDM). As we discussed in our case, there was a diagnostic challenge and his management is different from the type 1 DM. So clinicians need a high index of suspicion to reach the diagnosis especially in those countries where malnutrition is common like in Africa. This case and others may lead scientists and clinicians to study further. Because this was elusive for many years and still remains unclear but not under the classification of the common types of DM.
Abbreviations
SAM, severe acute malnutrition; Wt, weight; WFH, weight for height; Ht, height; HFA, height for age; LGS, lyphoglandular system; U/S, ultrasound; MRDM, malnutrition-related diabetes mellitus; MMDM, malnutrition-modulated diabetes mellitus; PDDM, protein-deficient diabetes mellitus; FCPD, fibrocalculous pancreatic diabetes; FBS, fasting blood sugar; RBS, random blood sugar; BMI, body mass index; WHO, World Health Organization; IU, international unit; SQ, subcutaneous.
Data Sharing Statement
Readers can contact the main author for data requests.
Consent for Publication
Written informed consent was obtained from the patient’s his uncle for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief. Based on the University there is no need for ethical clearance for the case report.
Acknowledgment
The authors thank all staff in Ayder hospitals who were involved in the hospital management of this patient. They also thank the patient and his uncle for their willingness for the publication. Special thanks to Dr. Hailemariam and Dr. Alemseged for their reviewing and editing of the case report.
Author Contributions
This work was carried out in collaboration between all authors. All authors contributed to data analysis, drafting and revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
No conflict of interest for any of the authors.
References
1. AkadiriYessoufou K, Moutairou AH, et al. Does tropical or malnutrition-related diabetes mellitus (MRDM) still exists? State Rev Int J Biol Chem Sci. 2008;2(2):91.
2. Sanjeevi CB, Kanungo A, Samal KC. Immunogenetic studies on malnutrition-modulated diabetes mellitus. Ann N Y Acad Sci. 2002;958:144–147. doi:10.1111/j.1749-6632.2002.tb02957.x
3. Abdulkadir J, Mengesha B, Welde Gabriel Z, et al. The clinical and hormonal (C-peptide and glucagon) profile and liability to ketoacidosis during nutritional rehabilitation in Ethiopian patients with malnutrition-related diabetes mellitus. Diabetologia. 1990;33:222–227. doi:10.1007/BF00404800
4. TahminurRuhman M, Kabir Y. HajeraMehtab, anetiopathology of malnutrition related diabetes mellitus (MRDM), conference paper, research gates.ACPN Publication; 2005;244(72).
5. Taksande A, Taksande B, Kumar A, et al. Malnutrition-related diabetes mellitus. J MGIMS. 2008;13(2):19–24.
6. Dissanayake M, Kushalee P, Ekanayake M. Fibrocalculous pancreatic diabetes: a case report; 2015; Ralapanawa et al. BMC Res Note. 2005;8(175):1–4.
7. Dabadghao P, Bhatia E, Bhatia V, et al. Islet-cell antibodies in malnutrition-related diabetes mellitus from North India. Diab Res Clin Pract. 1996;34:73–78. doi:10.1016/S0168-8227(96)01336-8
8. Pai KN, Soman CR, Varghese R. Pancreatic diabetes.
9. Mohan V, Ramchandran A, Viswanathan M. Diabetes mellitus science, and practice. Diab Res Centre. 1984. Madras.
10. Gill GV, Mbanya J-C, Ramaiya KL, et al. A sub-Saharan African perspective of diabetes. Diabetologia. 2009;52::8–16. doi:10.1007/s00125-008-1167-9
11. Yajnik CS. Treatment of malnutrition related diabetes mellitus. K E M Hospital Res Center. 1985;78:1–20.
12. World Health Organization Department of non-communicable disease surveillance Geneva. Diagnosis and classification of Diabetes Mellitus;WHO/NCD, 99 and its complication. 1999:1–40.
13. Garge MK, Shah P, Tripathy DK. Patients with malnutrition diabetes mellitus is as insulin sensitive as insulin-dependent diabetes. JAPI. 1999;47(12):1145–1148.
14. Elamin A, Altahir H, Ismail B, et al. Clinical patterns of Childhood Type 1 (Insulin-dependent) diabetes mellitus in Sudan. Diabetologia. 1992;35::645–648. doi:10.1007/BF00400256
15. Soula HA, Géloën A, Soulage CO. Model of adipose tissue cellularity dynamics during food restriction. J Theor Biol. 2015;364:189–196. doi:10.1016/j.jtbi.2014.08.046
16. A. Morrison EY
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
